Abstract
In the endeavor to understand how our brains enable our multifaceted memories, much controversy surrounds the contributions of the hippocampus and perirhinal cortex (PrC). Here we recorded functional magnetic resonance imaging (fMRI) in healthy controls and intracranial Electroencephalography (EEG) in patients during the same recognition memory paradigm. Although conventional fMRI analysis showed indistinguishable roles of the hippocampus and PrC in familiarity-based item recognition and recollection-based source retrieval, event-related fMRI and EEG time-courses revealed a clear temporal dissociation of memory signals within and across these regions. Whereas an early source retrieval effect was followed by a late, post-decision item novelty effect in hippocampus, an early item novelty effect was followed by a sustained source retrieval effect in PrC. Although factors like memory strength were not experimentally controlled, the temporal pattern across regions suggests that a rapid item recognition signal in PrC triggers a source retrieval process in the hippocampus, which in turn recruits PrC representations/mechanisms – evidenced here by increased hippocampal-PrC coupling during source recognition.
Our memories range from a vague feeling of familiarity for a face in a crowd to recollecting specific details of a previous encounter. While the critical role of the medial temporal lobes (MTL) for these different expressions of memory is well established1, 2, much controversy surrounds the precise contributions of different MTL subregions, most notably the hippocampus and the adjacent perirhinal cortex. Considerable evidence points to a role of the hippocampus in associative memory processes 3, 4, also referred to as source memory 5, relational memory 6 or recollection-based memory 7, 8. The common tenet of these theories is that the hippocampus is needed for the retrieval of multiple event details, but not for simple old/new identification of individual items (also referred to as item- or familiarity-based recognition). However, a series of neuropsychological and fMRI studies has challenged the claim for an exclusive role of the hippocampus in source memory, demonstrating that patients with selective hippocampal damage can also show impaired item memory 9, 10, and that hippocampal fMRI responses can be explained by memory strength alone 11, 12. One way to resolve this issue is to investigate the temporal profiles of item effects in the hippocampus. In particular, theoretical accounts 13-15, behavioral data 15, 16 as well as electrophysiological scalp recordings17 suggest that a familiarity-based item signal occurs rapidly, whereas recollection-based source memory occurs later, requiring more sustained engagement.
A similar controversy surrounds the role of the perirhinal cortex in memory. Whereas some theories suggest that this region is dedicated specifically to familiarity-based item recognition (just as the hippocampus is dedicated specifically to recollection-based source memory) 7, 8, 18, others suggest that perirhinal cortex can support both item- and source memory, as long as the critical information is object-related 3, 19, 20. One approach to reconcile these views has been to postulate that perirhinal involvement in source memory tasks actually reflects enhanced familiarity for object-related associations 21. If so, perirhinal source effects should occur at the same latency as simple old/new effects (both reflecting familiarity).
Here we assessed the roles of the hippocampus and perirhinal cortex by examining the temporal profiles of item- and source memory signals in these regions during recognition memory. To this end, we employed the same memory paradigm to probe both item recognition and source memory during fMRI recordings in healthy participants, as well as during intracranial EEG (iEEG) recordings obtained directly from the hippocampus and perirhinal cortex of presurgical epilepsy patients (Fig. 1). Strikingly, both methods converged on the same temporal pattern of item- and source effects within and across these regions, supporting a role of the hippocampus specifically in recollection-based source retrieval, and a role of the perirhinal cortex not only in rapid familiarity-based item recognition, but also in later recollection-based source retrieval.
Figure 1.
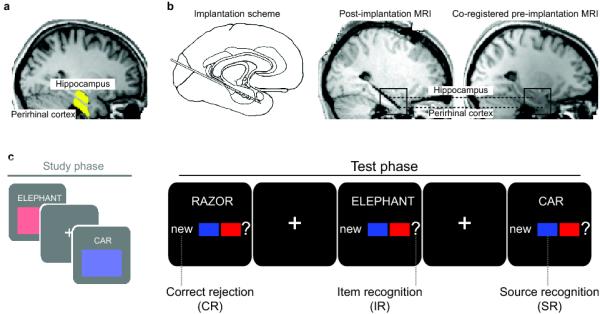
Methods and design. a. fMRI regions of interest (highlighted in yellow) for hippocampus and perirhinal cortex of an example participant. b. iEEG electrode locations. Left: Medial temporal lobe implantation scheme used for all participants. Middle: Hippocampus and perirhinal cortex contacts of an example participant, shown on the post-implantation MRI scan. Right: Same contacts shown on the co-registered pre-implantation MRI scan. Anatomical images in a and b are normalized for comparability. c. Schematized experimental design (iEEG version). During the Study phase, participants saw concrete nouns together with an associated “source” (a color in half the runs and a scene in the other half of the runs) and indicated whether or not the combination was plausible. During the Test phase, from which the present data were analyzed, studied (old) words were shown along with unstudied (new) words, and participants indicated, with one button press, whether they remembered the word from the study phase and whether they remembered its associated source. Conditions of interest were correct rejection of new items (CR), correct identification of old items, without remembering the associated source (item recognition, IR) and correct identification of old items plus remembering the associated source (source recognition, SR). Note that procedural details differed slightly between fMRI and iEEG versions of the experiment (see Methods) to allow for the different signal characteristics and to match behavioral performance across controls and patients.
Results
Behavioral Results
Our attempts to match memory performance across iEEG (patient) and fMRI (control) groups (by varying the number of sources, retention interval and encoding list length; see methods) were successful (Table 1). A “Pr” measure (probability of correct minus probability of incorrect “old” decisions to studied items) showed that overall recognition memory was significantly above chance (0), (fMRI: Pr = 70% (+/− 4), t19 = 16.18, P < .001; iEEG: Pr = 62% (+/− 9), t4 = 6.56, P = .003). Pr for source recognition (probability of correct minus incorrect source decisions to studied items excluding “don’t know” responses) was also significantly above chance (fMRI: Pr = 74% (+/− 6), t19 = 13.46, P < .001; iEEG: Pr = 52% (+/− 8), t4 = 6.15, P = .004). No significant differences were observed in item- or source Pr measures across fMRI vs. iEEG groups (both t23 < 1.63, P > .11). For further analyses, trials in which an item was recognized (“hit”), but either a “don’t know” response or an incorrect source response was given, were collapsed, and are referred to as “item recognition” (IR). Mean reaction times (RTs) for correct rejection (CR), item recognition (IR) and source recognition (SR) are shown in Table 1. All pairwise comparisons were significant (fMRI: all t19 > 3.47, P < .01; iEEG: all t4 > 3.93, P < .05).
Table 1.
Behavioral results for fMRI and iEEG version of the experiment. Memory performance is expressed in percentage of old or of new items. Standard errors are shown in parentheses. CR = correct rejection, IR = item recognition, SR = source recognition.
| studied items |
unstudied items | source memory out of hits | reaction times (sec) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| hit | miss | CR |
IR | SR | CR | IR | SR | |||
|
|
|
|
|
|||||||
|
correct
rejectio n |
false
alarm |
“?” respons e |
source
incorrec t |
source
correct |
||||||
|
fMR
I |
87 (3) |
13 (3) | 82 (3) | 18 (3) | 35 (3) | 9 (2) | 56 (4) | 1.72 (.08) |
2.27 (.06) |
1.90 (.07) |
|
iEE
G |
82 (6) |
18 (6) | 80 (10) | 20 (10) | 42 (11) | 14 (4) | 43 (8) | 1.65 (.19) |
2.10 (.17) |
1.86 (.20) |
Imaging Results
For all imaging analyses, we defined two memory effects of interest: (i) an “item effect”, i.e., the difference between CR and IR, which emphasizes processes related to simple item recognition or novelty detection, while reducing the impact of target source retrieval; (ii) a “source effect”, i.e., the difference between SR and IR, which emphasizes processes related to retrieval of target source details, while reducing the impact of item recognition/novelty.
For our fMRI data, we first queried item- and source memory effects via a conventional analysis of the blood-oxygenation-level-dependent (BOLD) response based on an assumed haemodynamic response function (HRF). We extracted the mean parameter estimates across voxels within each region of interest (ROI), where ROIs were defined anatomically and separately for each participant, based on their structural MRI (Fig. 2). No hemispheric differences were seen in this or any subsequent analysis (see supplementary material), so the data reported here are averaged across left and right ROIs. We found that both hippocampus and perirhinal cortex exhibited a significant item effect (both t19 > 2.41, P < .05) as well as a significant source effect (both t19 > 2.18, P < .05), resulting in a “U-shaped” pattern across CR-IR-SR.
Figure 2.
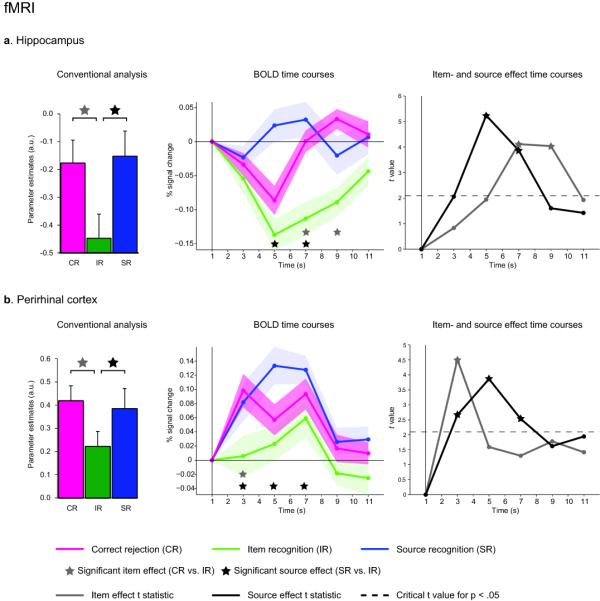
fMRI results for hippocampus (a) and perirhinal cortex (b). Left: Results from modeling conditions in a conventional analysis using an assumed HRF. Bar graphs represent mean (+ s.e.m.) parameter estimates. Note that, though differing with respect to baseline, item effects (CR vs. IR) and source effects (SR vs. IR) are indistinguishable within or across regions. Middle: Average (+/− s.e.m.) fMRI BOLD time courses versus baseline for the three conditions of interest. Right: Statistical development of the item effect (differential evoked response for CR vs. IR) and source effect (SR vs. IR), showing t-values for each effect across time. Points above the dashed line correspond to P < .05, two-tailed. Note the temporal dissociation of item- and source effects within and across regions.
Next we assessed whether item- and source effects might show different temporal BOLD profiles. As would be expected based on the above results, the integrated BOLD signal (averaged from 3-9 sec post stimulus onset) in both hippocampus and perirhinal cortex revealed a significant item effect (both t19 > 2.91, P < .01) as well as a significant source effect (both t19 > 3.27, P < .005). More critically, however, our data showed that these effects have different temporal characteristics within and across regions (Fig. 2). In the hippocampus, the item effect was delayed relative to the source effect, whereas in the perirhinal cortex, there was an early and transient item effect, together with a more sustained source effect. These temporal dissociations within regions were confirmed by a repeated-measures ANOVA with the factors Effect (item, source) and Time (TR1-4), which revealed significant Effect x Time interactions in both regions (both F3,57 > 4.61, P < .01). Moreover, the BOLD data suggested that the sequence of item- and source effects differs across the two regions, as evidenced by a significant Region x Effect x Time interaction (F3,57 = 3.83, P = .01). Comparing the latencies of each participant’s effect peak (using a non-parametric Wilcoxon test), we observed that the perirhinal cortex item effect peaked significantly earlier than the hippocampal source effect (P = .02), whereas the perirhinal cortex source effect peaked significantly earlier than the hippocampal item effect (P < .005). Nonetheless, inferring the latency of neural activity from the temporal characteristics of the BOLD response is difficult (given potential nonlinear neural-vascular mappings 22). Thus, to better explore the temporal profiles of memory signals in the hippocampus and perirhinal cortex, we capitalized on the real-time resolution of the iEEG data.
In a first step, we defined two time windows of interest: An early window ranging from 250-750ms post stimulus onset that encompassed the initial peak responses in both hippocampus and perirhinal cortex when collapsing across conditions (Fig. S1a), and a late window from 800-2000ms post stimulus onset that captured a sustained, second component in both regions (Fig. S1b). (As in the fMRI data, no hemispheric differences were seen; supplementary material.)
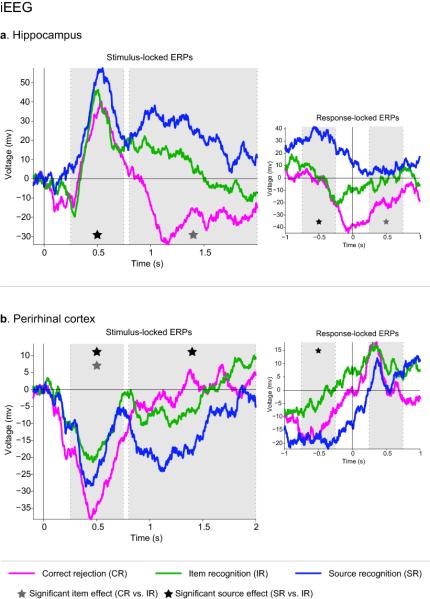
In the hippocampus (Fig. 3a), the early analysis window (250-750ms) showed a significant source effect (t4 = 4.88, P = .008), but no evidence of an item effect (t4 = 0.05, P = .960). In the late analysis window (800-2000ms), the item effect was now significant (t4 = 3.17, P = .034), whereas the source effect no longer reached significance (t4 = 1.82, P = .142). The late onset of the item effect suggests that this hippocampal response might reflect post-retrieval processes for new items, rather than the fast identification of old items that is expected by the behavioral evidence for rapid familiarity-based recognition. To assess more directly whether the hippocampal item effect reflects post-retrieval processes, we compared item- and source effects in response-locked (instead of stimulus-locked) ERPs. More specifically, we compared a 500ms time window from −750ms to −250ms with that from +250ms to +750ms, defined relative to the participants’ key-press on each trial. Results revealed an interaction between Time Window (pre, post) and Effect (item, source) (F1,4 = 11.07, P = .029), due to the pre-response window showing a source effect (t4 = 3.49, P = .025), but no item effect (t4 = 1.09, P = .339), and the post-response window showing an item effect (t4 = 3.33, P = .029), but no source effect (t4 = 0.95, P = .398). This result suggests that the hippocampal source effect precedes the memory judgment, whereas the item effect only occurs after the memory judgment, and thus likely reflects processes such as incidental encoding of experimentally novel information (see Discussion).
Figure 3.
iEEG results for hippocampus (a) and perirhinal cortex (b). Left: Stimulus-locked ERPs. Right: Response-locked ERPs. Shaded areas show the two time windows used for statistical analysis.
In the perirhinal cortex (Fig. 3b), the early time window (250-750ms) showed both an item effect (t4 = 5.82, P = .004) and a source effect (t4 = 3.20, P = .033). In the late time window (800-2000ms), the source effect was still significant (t4 = 5.30, P = .006), whereas the item effect was not (t4 = 0.34, P = .752). For the response-locked analysis, there was - unlike in the hippocampus - no differential size of source- vs. item effects with respect to pre- vs. post-response time windows (F1,4 = 0.12, P = .743); only a main effect of Time Window (F1,4 = 12.12, P = .025), reflecting the fact that the combined item- and source effects were stronger in the pre- than in the post-response time window. However, only the source effect reached significance in the pre-response time window (t4 = 7.46, P = .002), whereas the item effect did not (t4 = 2.24, P = .088), suggesting that the source effect was sustained and terminated with the memory response compared to a rapid and transient item effect.
To further investigate the latency of these effects, we calculated the earliest timepoint to show a reliable item and source effect within each region. The first effect was an item effect in perirhinal cortex at 200ms post-stimulus onset. Next, we observed a source effect in the hippocampus at 250ms, followed by a source effect in perirhinal cortex at 400ms. A hippocampal item effect was first observed at 1050ms (Fig. 4).
Figure 4.
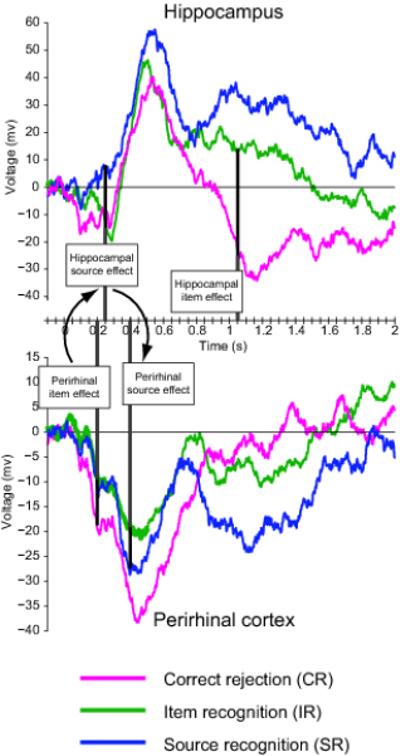
Temporal sequence of iEEG item- and source effects in perirhinal cortex (bottom) and hippocampus (top). ERPs are identical to Fig. 3 (left). Vertical lines demarcate the onset of the first statistically reliable item- and source effect in each region. Darker portion of vertical lines highlights the relevant condition differences.
In summary, although item- and source effects were indistinguishable within and across regions via conventional fMRI analysis, time-resolved BOLD data showed that these effects can be temporally dissociated. This dissociation was confirmed by the iEEG data: In the hippocampus, we observed an early source effect that terminated with the participants’ memory response, and an item effect that onset much later in the trial, after the memory decision had been made. In perirhinal cortex, our data suggest that there might be two independent processes: A fast and transient item effect and a later-onsetting source effect that is sustained throughout the retrieval period and terminates with participants’ memory decision. Thus, while both regions seem to conjointly support source retrieval (see below), the item effect appears to reflect different processes in each case: a fast novelty signal in perirhinal cortex and a late, post-retrieval encoding process in the hippocampus.
To test the notion that successful source retrieval is accompanied by increased functional coupling across hippocampus and perirhinal cortex, we conducted connectivity analyses in both fMRI and iEEG data. For the fMRI data, we used a Psychophysiological interaction (PPI) analysis (see Methods). Using the individually- and anatomically-defined hippocampi as seed regions, a set of bilateral perirhinal cortex clusters emerged showing greater functional coupling with the hippocampus during SR vs. IR (Pcorrected = .042; Fig. 5a). No differences in hippocampal-perirhinal coupling were observed for the item effect (CR vs. IR) at P < .001, uncorrected.
Figure 5.
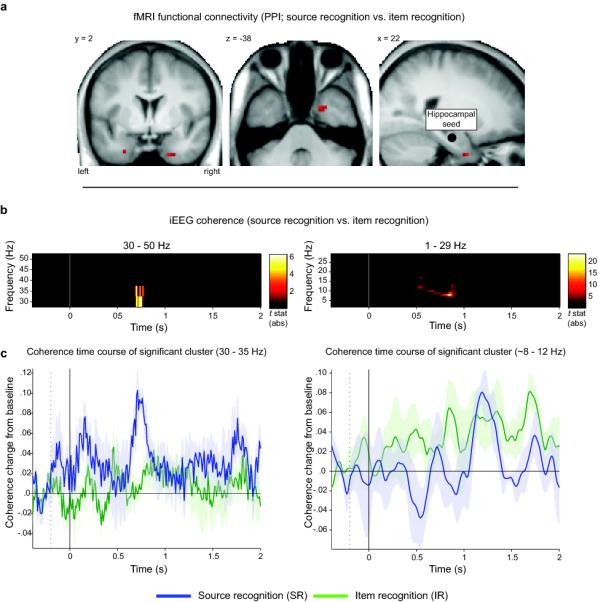
Functional coupling between hippocampus and perirhinal cortex increases during source recognition (SR vs. IR). a. MTL regions showing a psychophysiological interaction (PPI) using participants’ individually drawn hippocampi as seed regions (schematized in the sagittal view, right panel). Results are shown at P < .001 (uncorrected) for display purposes. Left peak: x = −27, y = 2, z = −35; right peak: x = 21, y = 2, z = −38. b. Time/frequency clusters of significant iEEG coherence differences between SR vs. IR (P < .05, corrected for multiple comparisons). Color reflects absolute t values for SR vs. IR (only significant t values shown). c. Average (+/− s.e.m.) time course of coherence in the significant clusters. Left: Coherence in the low gamma band (30-35 Hz) is enhanced for SR relative to IR between ~700 to 800 ms. Right: Coherence in the alpha band (8-12 Hz) is reduced for SR relative to IR between ~500 to 900 ms.
For the iEEG data, we conducted spectral coherence analysis (see Methods for details). For the comparison of SR vs. IR, this analysis revealed two clusters (P < .05, corrected via a cluster-based statistic across time and frequencies23; see Methods) in which coherence differed significantly across conditions (Fig. 5 b,c). First, SR showed significantly greater coherence between hippocampus and perirhinal cortex in the low gamma band (30-35 Hz) from ~700 to 800 ms. Second, we observed increased coherence for IR – or, conversely, increased decoupling for SR - between hippocampus and perirhinal cortex in the alpha band (8-12 Hz) from ~500 to 900 ms. Because the temporal resolution of spectral analysis is inferior to that of ERPs we refrain from making strong conclusions about the timing of these gamma- and alpha-coupling effects, but it is worth noting that both effects overlap in time and coincide with the period in which both regions show differential ERPs for SR vs. IR. The increase in low gamma coupling for successful source recognition is consistent with previous findings during successful relative to unsuccessful memory encoding 24, 25, and the inverse alpha coupling effect (greater for unsuccessful source recognition) is interesting in light of the emerging role of alpha oscillations in functional inhibition of brain regions 26. Again, no changes in coherence were observed for CR vs. IR (using the same statistical threshold as for SR vs. IR).
Together, the fMRI and iEEG connectivity results provide strong support for the notion that successful source retrieval is accompanied by an increase in functional coupling between hippocampus and perirhinal cortex.
Discussion
Using the same memory paradigm during fMRI recordings in healthy participants and iEEG in patients (Fig. 1), our study was designed to elucidate the functional contributions of the hippocampus and perirhinal cortex to recognition memory. Consistent with previous fMRI studies11, 27, item- and source memory signals were indistinguishable within and across regions when applying a conventional fMRI analysis (Fig. 2). Importantly, however, a more fine-grained latency analysis revealed a temporal dissociation of item- and source memory effects within and across hippocampus and perirhinal cortex, both in BOLD time course data (Fig. 2) and, with much superior temporal resolution, in iEEG recordings (Fig. 3). Most critically, the latency of the perirhinal item/novelty effect was short enough to provide, consistent with behavioral 16 and neural 17, 28 evidence, a rapid familiarity/novelty signal. The hippocampal item/novelty effect, on the other hand, emerged too late to be likely to contribute to the recognition decision – indeed, was only reliable after rather than before that decision – and thus more likely reflects post-retrieval encoding of new items 29, 30.
We operationalized the “item effect” as the difference between correct rejection of experimentally novel items (CR) and item recognition without remembering the associated source (IR). Nonetheless, it is conceivable that IR trials were also accompanied by retrieval of non-target source details or by increased source retrieval effort4. Similarly, though our “source effect” was operationalized as the difference between item recognition and item recognition along with successful source memory (SR), it is conceivable that SR trials also exceeded IR in terms of overall memory strength11. While we did not obtain continuous measures of source memory or memory strength, and so cannot rule out the contribution of either to our results, we note that neither is sufficient as the sole explanation of the complete pattern of condition effects across perirhinal and hippocampal regions. In other words, although any contrast of experimental conditions is unlikely to be process-pure, our pattern of data is inconsistent with a single, common factor (such as memory strength) driving activity in both hippocampus and perirhinal cortex. We discuss the data for each region in more detail below, focusing on the temporal characteristics revealed via the iEEG recordings.
The hippocampus showed an early (onset at 250ms) source effect (increase for SR relative to IR), which is in agreement with this region’s established role in associative/contextual/recollection-based memory3, 6-8, 18, 31. Interestingly, the hippocampus also showed a strong response to novel items (CR vs. IR), but critically this effect did not emerge until 1050ms post-stimulus onset. This “late negative component” (LNC) for new items relative to old items has been observed in iEEG recordings before32, but has never been related to item- vs. source memory. Given the inverted polarity of the iEEG item effect relative to the earlier source effect, one possibility is that the LNC might be mediated by a different cell population in the hippocampus. Indeed, single unit recordings from human hippocampus during recognition memory paradigms have identified two cell types - one type producing greater firing rates for old items (“old-selective”), and the other type producing greater firing rates for new items (“new-selective”)33, 34. Importantly, our current findings extend these single-unit data in two ways: First, regarding the “new-selective” response, this signal seems unlikely to support novelty detection per se, considering the latency of the LNC (~800ms after the source effect). That is, the “new-selective” response is unlikely to be a purely stimulus-driven response. Rather, the hippocampal response to (correctly rejected) unstudied items seems likely to support incidental, episodic encoding processes (that occur even during a recognition memory test29, 30), which is corroborated by our finding that this item effect/novelty response unfolds after the memory decision has been made (Fig. 3a). Note that while these results argue against a role of the hippocampus in rapid, familiarity-based item recognition, they are not incompatible with the established role of the hippocampus in detection of other types of (contextual) novelty, such as context deviancy 35, configural novelty 36 or prediction error37. Second, regarding the hippocampal “old-selective” response, our data suggest that this response may largely reflect associative/source retrieval rather than simple item recognition. That is, although the hippocampal peak response would resemble a basic item-recognition response when collapsing IR and SR into a single “old” bin (as in previous iEEG studies32, 38), separation of IR and SR suggests that this response is in fact driven by events in which successful associative/source retrieval occurs.
Prevalent models of the perirhinal cortex postulate a role of this region in familiarity-based recognition 7, 8 or in coding for weak rather than for strong memories 11. To the extent that both familiarity and memory strength increase from correct rejection of an unstudied item (CR) to IR, and further from IR to SR, the early effect pattern we observed in our iEEG data (IR < SR < CR) is partially incompatible with both views. An alternative scenario, however, is that perirhinal cortex independently supports both (i) item recognition via decreased neural activity for studied (experimentally-familiar) relative to unstudied (experimentally-novel) stimuli (IR < CR), and (ii) source memory via enhanced activity for successful relative to unsuccessful retrieval of associated event details (SR > IR). The first role (item recognition through decreased activity, or conversely, increased activity for experimentally-novel stimuli) has received considerable support from a variety of methods. For instance, neurons in the primate perirhinal cortex have been shown to decrease their firing rates as a function of stimulus repetition 28, and lesions to the primate perirhinal cortex result in marked object recognition deficits 39, 40. Moreover, human fMRI studies have reported reduced BOLD signals for old relative to new items 41-43, while previous iEEG studies in human epilepsy patients have reported reduced N400 components for old relative to new items 32, 44. Thus, our finding of a reduced perirhinal response for IR relative to CR is compatible with this region’s established role in (familiarity-based) item recognition. Importantly, however, a more recent series of findings also suggest a role of perirhinal cortex in memory processes beyond simple item recognition. For example, single neurons in primate perirhinal cortex have been shown to code for object-object associations (pair-coding neurons45, 46). Also, recent fMRI studies in humans have shown that perirhinal cortex can support, in conjunction with the hippocampus, successful associative encoding 47 as well as associative retrieval 27, 48. Thus, converging evidence suggests that the role of perirhinal cortex in episodic memory may encompass both item- and source memory. However, if item- and source memory are indeed separate functions of perirhinal cortex, one might expect these functions to be mediated by separate cell populations and/or to show different temporal/dynamic profiles. Indeed, we found that the item recognition effect (IR vs. CR) lasted from 200ms until ~800ms post-stimulus onset, whereas the associative recognition effect (SR vs. IR) started at 400ms (Figs. 3b and 4) and was sustained throughout the remainder of the trial period, terminating with the memory response. Thus, not only do the current data show both item- and source effects in perirhinal cortex, but they suggest that item recognition and source retrieval may be partially-separable processes supported by this region (possibly in conjunction with the hippocampus; see below).
How do hippocampus and perirhinal cortex interact during recognition memory? Our data reveal that the item effect in perirhinal cortex precedes the onset of the source effect in the hippocampus, which in turn precedes the onset of the source effect in perirhinal cortex (Fig. 4). This temporal pattern is suggestive of a signaling loop between perirhinal cortex and hippocampus, in which a rapid item recognition signal in perirhinal cortex triggers source retrieval processes in the hippocampus, which in turn entrain the perirhinal cortex in the service of retrieving and/or maintaining an associated source detail. A recent study obtaining intralaminar recordings from primate perirhinal cortex during a paired associate task showed a reversal of the signal flow from (i) a forward signal during the cue period (item processing) to (ii) a backward signal during the delay period (when retrieving the paired associate) 49. Although that study did not record from the hippocampus, our current data suggest that this reversal might be triggered by the hippocampus. Indeed, a critical role of the hippocampus in initiating source retrieval is consistent with single unit data showing that successful free recall is preceded by a gradual increase of hippocampal firing rates 50. This idea that hippocampus and perirhinal cortex need to interact during successful source retrieval is further substantiated by our finding of enhanced functional coupling among these regions during SR relative to IR (Fig. 5).
Together, the temporal sequence of an early perirhinal item effect/familiarity signal followed by a hippocampal source effect/recollection signal lends strong support to current models that emphasize a functional separation between hippocampus and perirhinal cortex during episodic memory processes 3, 4, 7, 8, 18. Importantly, however, the subsequent source effect in perirhinal cortex, along with increases in functional coupling between hippocampus and perirhinal cortex, also highlight the intricate interaction of these regions that underlies the retrieval of episodically rich memory traces.
Supplementary Material
Acknowledgements
We thank Andrea Greve for helpful discussion. This work was supported by a Sir Henry Wellcome Postdoctoral Fellowship to B.P.S., the UK Medical Research Council Program MC_A060_5PR10 to R.N.H., and the German Research Foundation (DFG FE 366/5-1 to A.D.L.)
Footnotes
Author contributions B.P.S. and R.N.H. designed research. B.P.S., N.A., J.F. and R.N.H. wrote manuscript. B.P.S. conducted experiments and analyzed data. A.D.L. assisted in conducting iEEG experiments.
References
- 1.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annual review of neuroscience. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 3.Davachi L. Item, context and relational episodic encoding in humans. Current opinion in neurobiology. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 4.Mayes A, Montaldi D, Migo E. Associative memory and the medial temporal lobes. Trends Cogn Sci. 2007;11:126–135. doi: 10.1016/j.tics.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Johnson MK, Hashtroudi S, Lindsay DS. Source monitoring. Psychological bulletin. 1993;114:3. doi: 10.1037/0033-2909.114.1.3. [DOI] [PubMed] [Google Scholar]
- 6.Cohen NJ, Eichenbaum HE. Memory, Amnesia, and the Hippocampal System. MIT Press; Cambridge, MA: 1993. [Google Scholar]
- 7.Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn Sci. 2007;11:379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annual review of neuroscience. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manns JR, Hopkins RO, Reed JM, Kitchener EG, Squire LR. Recognition memory and the human hippocampus. Neuron. 2003;37:171–180. doi: 10.1016/s0896-6273(02)01147-9. [DOI] [PubMed] [Google Scholar]
- 10.Stark CE, Bayley PJ, Squire LR. Recognition memory for single items and for associations is similarly impaired following damage to the hippocampal region. Learning & memory (Cold Spring Harbor, N.Y. 2002;9:238–242. doi: 10.1101/lm.51802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: a new perspective. Nature reviews. 2007;8:872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wais PE, Squire LR, Wixted JT. In search of recollection and familiarity signals in the hippocampus. Journal of cognitive neuroscience. 2010;22:109–123. doi: 10.1162/jocn.2009.21190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacoby LL. A process dissociation framework: Separating automatic from intentional uses of memory* 1. Journal of memory and language. 1991;30:513–541. [Google Scholar]
- 14.Mandler G. Recognizing: The judgment of previous occurrence. Psychological review. 1980;87:252. [Google Scholar]
- 15.Yonelinas AP. The Nature of Recollection and Familiarity: A Review of 30 Years of Research* 1. Journal of memory and language. 2002;46:441–517. [Google Scholar]
- 16.McElree B, Dolan PO, Jacoby LL. Isolating the contributions of familiarity and source information to item recognition: A time course analysis. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1999;25:563. doi: 10.1037//0278-7393.25.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curran T. Brain potentials of recollection and familiarity. Memory & Cognition. 2000;28:923–938. doi: 10.3758/bf03209340. [DOI] [PubMed] [Google Scholar]
- 18.Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behavioral and Brain Sciences. 1999;22:425–444. [PubMed] [Google Scholar]
- 19.Duarte A, Henson RN, Graham KS. Stimulus content and the neural correlates of source memory. Brain Research. 2010 doi: 10.1016/j.brainres.2010.11.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Staresina BP, Davachi L. Object unitization and associative memory formation are supported by distinct brain regions. The Journal of Neuroscience. 2010;30:9890. doi: 10.1523/JNEUROSCI.0826-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diana RA, Yonelinas AP, Ranganath C. The effects of unitization on familiarity-based source memory: Testing a behavioral prediction derived from neuroimaging data. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2008;34:730. doi: 10.1037/0278-7393.34.4.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henson RN, Shallice T, Josephs O, Dolan RJ. Functional magnetic resonance imaging of proactive interference during spoken cued recall. NeuroImage. 2002;17:543–558. [PubMed] [Google Scholar]
- 23.Maris E, Oostenveld R. Nonparametric statistical testing of EEG-and MEG-data. Journal of Neuroscience Methods. 2007;164:177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 24.Fell J, et al. Human memory formation is accompanied by rhinal-hippocampal coupling and decoupling. Nature neuroscience. 2001;4:1259–1264. doi: 10.1038/nn759. [DOI] [PubMed] [Google Scholar]
- 25.Jutras MJ, Fries P, Buffalo EA. Gamma-band synchronization in the macaque hippocampus and memory formation. The Journal of Neuroscience. 2009;29:12521–12531. doi: 10.1523/JNEUROSCI.0640-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haegens S, Nácher V, Luna R, Romo R, Jensen O. α-Oscillations in the monkey sensorimotor network influence discrimination performance by rhythmical inhibition of neuronal spiking. Proceedings of the National Academy of Sciences. 2011;108:19377–19382. doi: 10.1073/pnas.1117190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirwan CB, Stark CE. Medial temporal lobe activation during encoding and retrieval of novel face-name pairs. Hippocampus. 2004;14:919–930. doi: 10.1002/hipo.20014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiang JZ, Brown MW. Differential neuronal encoding of novelty, familiarity and recency in regions of the anterior temporal lobe. Neuropharmacology. 1998;37:657–676. doi: 10.1016/s0028-3908(98)00030-6. [DOI] [PubMed] [Google Scholar]
- 29.Buckner RL, Wheeler ME, Sheridan MA. Encoding processes during retrieval tasks. Journal of cognitive neuroscience. 2001;13:406–415. doi: 10.1162/08989290151137430. [DOI] [PubMed] [Google Scholar]
- 30.Stark CE, Okado Y. Making memories without trying: medial temporal lobe activity associated with incidental memory formation during recognition. J Neurosci. 2003;23:6748–6753. doi: 10.1523/JNEUROSCI.23-17-06748.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Konkel A, Cohen NJ. Relational memory and the hippocampus: representations and methods. Frontiers in Neuroscience. 2009;3:166. doi: 10.3389/neuro.01.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ludowig E, et al. Intracranially recorded memory-related potentials reveal higher posterior than anterior hippocampal involvement in verbal encoding and retrieval. Journal of cognitive neuroscience. 2008;20:841–851. doi: 10.1162/jocn.2008.20507. [DOI] [PubMed] [Google Scholar]
- 33.Rutishauser U, Schuman EM, Mamelak AN. Activity of human hippocampal and amygdala neurons during retrieval of declarative memories. Proceedings of the National Academy of Sciences. 2008;105:329. doi: 10.1073/pnas.0706015105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Viskontas IV, Knowlton BJ, Steinmetz PN, Fried I. Differences in mnemonic processing by neurons in the human hippocampus and parahippocampal regions. Journal of cognitive neuroscience. 2006;18:1654–1662. doi: 10.1162/jocn.2006.18.10.1654. [DOI] [PubMed] [Google Scholar]
- 35.Axmacher N, et al. Intracranial EEG correlates of expectancy and memory formation in the human hippocampus and nucleus accumbens. Neuron. 2010;65:541–549. doi: 10.1016/j.neuron.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 36.Köhler S, Danckert S, Gati JS, Menon RS. Novelty responses to relational and non relational information in the hippocampus and the parahippocampal region: A comparison based on event related fMRI. Hippocampus. 2005;15:763–774. doi: 10.1002/hipo.20098. [DOI] [PubMed] [Google Scholar]
- 37.Kumaran D, Maguire EA. Novelty signals: a window into hippocampal information processing. Trends Cogn Sci. 2009;13:47–54. doi: 10.1016/j.tics.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 38.Grunwald T, et al. Dissecting out conscious and unconscious memory (sub)processes within the human medial temporal lobe. NeuroImage. 2003;20(Suppl 1):S139–145. doi: 10.1016/j.neuroimage.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 39.Meunier M, Bachevalier J, Mishkin M, Murray EA. Effects on visual recognition of combined and separate ablations of the entorhinal and perirhinal cortex in rhesus monkeys. J Neurosci. 1993;13:5418–5432. doi: 10.1523/JNEUROSCI.13-12-05418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murray EA, Richmond BJ. Role of perirhinal cortex in object perception, memory, and associations. Current opinion in neurobiology. 2001;11:188–193. doi: 10.1016/s0959-4388(00)00195-1. [DOI] [PubMed] [Google Scholar]
- 41.Gonsalves BD, Kahn I, Curran T, Norman KA, Wagner AD. Memory strength and repetition suppression: multimodal imaging of medial temporal cortical contributions to recognition. Neuron. 2005;47:751–761. doi: 10.1016/j.neuron.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 42.Henson R, Cansino S, Herron J, Robb W, Rugg M. A familiarity signal in human anterior medial temporal cortex? Hippocampus. 2003;13:301–304. doi: 10.1002/hipo.10117. [DOI] [PubMed] [Google Scholar]
- 43.Montaldi D, Spencer TJ, Roberts N, Mayes AR. The neural system that mediates familiarity memory. Hippocampus. 2006;16:504–520. doi: 10.1002/hipo.20178. [DOI] [PubMed] [Google Scholar]
- 44.Grunwald T, Lehnertz K, Heinze HJ, Helmstaedter C, Elger CE. Verbal novelty detection within the human hippocampus proper. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:3193–3197. doi: 10.1073/pnas.95.6.3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buckley MJ, Gaffan D. Perirhinal cortex ablation impairs configural learning and paired-associate learning equally. Neuropsychologia. 1998;36:535–546. doi: 10.1016/s0028-3932(97)00120-6. [DOI] [PubMed] [Google Scholar]
- 46.Higuchi S, Miyashita Y. Formation of mnemonic neuronal responses to visual paired associates in inferotemporal cortex is impaired by perirhinal and entorhinal lesions. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:739–743. doi: 10.1073/pnas.93.2.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Staresina BP, Davachi L. Differential encoding mechanisms for subsequent associative recognition and free recall. J Neurosci. 2006;26:9162–9172. doi: 10.1523/JNEUROSCI.2877-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diana RA, Yonelinas AP, Ranganath C. Medial temporal lobe activity during source retrieval reflects information type, not memory strength. Journal of cognitive neuroscience. 2010;22:1808–1818. doi: 10.1162/jocn.2009.21335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takeuchi D, Hirabayashi T, Tamura K, Miyashita Y. Reversal of Interlaminar Signal Between Sensory and Memory Processing in Monkey Temporal Cortex. Science. 2011;331:1443. doi: 10.1126/science.1199967. [DOI] [PubMed] [Google Scholar]
- 50.Gelbard-Sagiv H, Mukamel R, Harel M, Malach R, Fried I. Internally generated reactivation of single neurons in human hippocampus during free recall. Science. 2008;322:96. doi: 10.1126/science.1164685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10:433–436. [PubMed] [Google Scholar]
- 52.Stark CE, Squire LR. When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proc Natl Acad Sci U S A. 2001;98:12760–12766. doi: 10.1073/pnas.221462998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dale AM. Optimal experimental design for event-related fMRI. Human brain mapping. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Insausti R, et al. MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. Ajnr. 1998;19:659–671. [PMC free article] [PubMed] [Google Scholar]
- 55.Brett M, Anton J-L, Valabregue R, Poline J-B. Region of interest analysis using an SPM toolbox [abstract]. 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan. June 2-6.2002. [Google Scholar]
- 56.McCarthy G, Nobre AC, Bentin S, Spencer DD. Language-related field potentials in the anterior-medial temporal lobe: I. Intracranial distribution and neural generators. J Neurosci. 1995;15:1080–1089. doi: 10.1523/JNEUROSCI.15-02-01080.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith ME, Stapleton JM, Halgren E. Human medial temporal lobe potentials evoked in memory and language tasks. Electroencephalography and clinical neurophysiology. 1986;63:145–159. doi: 10.1016/0013-4694(86)90008-8. [DOI] [PubMed] [Google Scholar]
- 58.Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Computational Intelligence and Neuroscience. 2011;2011:1. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jokisch D, Jensen O. Modulation of gamma and alpha activity during a working memory task engaging the dorsal or ventral stream. The Journal of Neuroscience. 2007;27:3244–3251. doi: 10.1523/JNEUROSCI.5399-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meeuwissen EB, Takashima A, Fernández G, Jensen O. Increase in posterior alpha activity during rehearsal predicts successful long-term memory formation of word sequences. Human brain mapping. 2010 doi: 10.1002/hbm.21167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.