Abstract
Background
Little is known about pregnancy patterns and levels of HIV RNA in HIV-infected women conceiving on highly-active antiretroviral therapy (HAART) with non-suppressed viral load (VL), nor their therapeutic management.
Methods
Linear mixed models were fitted to study changes in VL and potential associated factors including HAART type/duration and immune status among 127 women receiving HAART at conception with detectable VL enrolled in the prospective European Collaborative Study.
Results
Median duration of HAART at conception was 10 months. Seventy-eight (61%) women conceived on PI-based HAART. Seventy-two (57%) women remained on the same HAART regimen throughout pregnancy, 24 (19%) switched regimens and 31 (24%) interrupted HAART during early pregnancy. The intention-to-treat model indicated constant VL up to 10 gestational weeks; thereafter levels decreased significantly, by 0.06 log10 copies/ml weekly until delivery. At baseline, immune status was significantly associated with HIV RNA levels. Excluding treatment-interrupters, there was no significant difference in VL slope between women who did and did not modify their HAART regimens (p=0.14); women conceiving on NNRTI-based HAART had consistently lower VL throughout pregnancy than those on PI-based HAART (p=0.02). Most (64/103, 62%) women had detectable VL within four weeks of delivery (median 2.40 log10 copies/ml). The MTCT rate overall was 1.72% (95%CI 0.21-6.1%).
Conclusion
Practices regarding management of women conceiving on HAART with detectable VL vary in Western Europe. The existence of this group of pregnant women highlights the need for improved monitoring of and support for treated women before they become pregnant, as well as during pregnancy itself.
Keywords: HIV, pregnancy, HIV RNA, HAART
Introduction
In developed countries, widespread use of highly-active antiretroviral therapy (HAART) has resulted in decreases in HIV-related morbidity and mortality [1-3] and mother-to-child transmission (MTCT) rates [4-7]. Consequently, increasing numbers of HIV-infected women are becoming pregnant or planning pregnancies [8, 9], with many on HAART at conception [6, 10]; in the UK and Ireland, 24% of HIV-infected women on HAART delivering in 2000-2006 conceived on HAART, around 40% of the total diagnosed with HIV infection before pregnancy [6]. Of women conceiving on HAART, those with detectable HIV RNA levels at their first antenatal visit are of particular interest as control of viral replication is key to reducing MTCT risk [4,11,12] and preventing disease progression [13,14]. In terms of MTCT risk, viral load during the later stages of pregnancy are of most importance; we have previously shown that NNRTI-based HAART initiated in pregnancy in treatment-naive women was associated with more rapid VL decline than PI-based HAART [15]. Little is known about treatment or likelihood, or timing, of achieving undetectable viral load (VL) in women who conceive while on HAART with detectable VL. In particular, no studies to date have been carried out to describe the virological patterns in pregnancy in this group of women.
Using data from a European multicentre prospective cohort study, this analysis was conducted to assess the pattern and levels of HIV RNA in pregnancy in HIV-infected women on HAART with non-suppressed VL at conception taking into account treatment modifications and to identify factors which may affect these levels and patterns, including type of HAART and maternal factors.
Methods
The study population was selected from women enrolled in the European Collaborative Study (ECS), a prospective cohort study in which HIV-infected pregnant women were enrolled and followed in pregnancy, and their children followed from birth [15, 16]. Informed consent and ethical approval were obtained according to local guidelines. Maternal information routinely collected included socio-demographic characteristics, obstetrical history and HIV-specific information, including ART use, CD4 counts and plasma HIV RNA VL. HIV RNA quantification was performed using commercially available assays. Classification of undetectable viral load was based on the lower limit of quantification of the assay used. Amplicor HIV-1 Monitor Tests (Standard version 1.5 and ultrasensitive, Roche Diagnostic Systems Inc., Branchburg, NJ, USA) was used for most (73%) measurements, of which 97% were measured with the ultrasensitive assay with a quantification limit of <50 copies/ml.
Overall, a total of 569 women conceived on HAART with date of HAART initiation reported: of these, 255 had undetectable VL at conception and 180 did not have VL or CD4 counts available. Of the remaining 134 women, we excluded seven women who conceived whilst taking HAART containing both a protease inhibitor (PI) and a non-nucleoside reverse transcriptase inhibitor (NNRTI). Our study population therefore comprised of 127 women on HAART at conception (i.e. receiving at least three drugs including either a PI or a NNRTI), with known initiation date, detectable VL at first antenatal visit and ≥1 CD4 count reported by June 2007.
Statistical methods
The pattern of log10 transformed HIV RNA VL over pregnancy was described using a supersmoother (an adaptive running mean in which the sampling window size varies according to local density of measurements). Linear mixed effects models were used to explore log10 HIV RNA VL over pregnancy; this approach allows inclusion of random effects and is appropriate for analysis of repeated measures data. Left-censoring patterns of VL due to undetectable measurements were dealt with using parametric censored regression [17, 18]. To account for between-woman variability in VL, we fitted individual random effects for VL at the time of the change point (model intercept) and the subsequent slope; this incorporated the association between the value of VL at the time of change-point and the slope which models changes in VL thereafter.
Covariates considered in the models included time of VL measurement (gestational weeks), ethnic group stratified by history of injecting drug use (IDU) into white with IDU history, white without IDU history and black (as no black woman had an IDU history), type (PI-containing or NNRTI-containing) and duration of HAART, time period of delivery (to account for changes over time in HIV management), baseline CD4 count and HIV RNA assay type (Roche or other) [19]. An intention-to-treat approach was taken for the first model, which included all women (model 1). A two-phase linear mixed model best described the functional form of VL, with the change-point of the slope taken as 10 gestational weeks, the time at which the model Akaike Information Criteria (a goodness of fit criterion that allows comparison of non-nested models) was minimised. The model was then refitted, firstly excluding women who interrupted treatment (on the basis that this group were potentially at risk of viral rebound [13]) with adjustment for whether or not the HAART regimen at conception was modified later in pregnancy for the remainder (model 2). A third model was fitted for the sub-group continuing on the same regimen from conception to delivery (i.e. without interruption or modifications) (model 3). For the models excluding women who interrupted treatment, the VL decline was linear and therefore models fitted for this group required only one slope to describe the change in VL over pregnancy (See Figure 1a). Statistical analyses were performed with SAS software, version 9.1 (SAS Institute, Cary, NC, USA) and with R version 2.6.1 (R Foundation for Statistical Computing, Vienna, Austria) in a Windows environment.
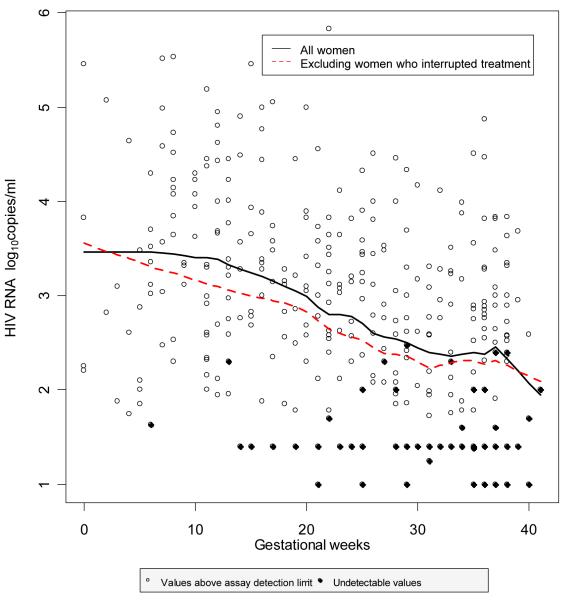
Figure 1. Scatterplot of HIV RNA measurements over pregnancy, with a supersmoother to summarize the trend.
Results
The characteristics of the 127 women are summarized in Table 1. Most women were on PI-containing regimens (mostly unboosted PIs) and had been on HAART for nearly a year before conception. Of the 49 women conceiving on NNRTI-containing HAART, 40 were on nevirapine-based regimens and nine on efavirenz; most of those on PI-containing HAART received non-boosted PIs, mostly nelfinavir (n=35). Only one woman with a history of IDU was known to be an active user. All the black women were born in Africa. Women had a median of three antenatal VL measurements (IQR 2-3), with the first VL and CD4 count measured at a median of 12 gestational weeks (IQR 8-20); less than a quarter of all VL measurements were undetectable (Table 1) and overall, 36% (n=46) women achieved a VL <50 copies/ml at least once during pregnancy. Figure 1 shows a scatterplot of HIV RNA measurements, with a supersmoother to summarize the trend over pregnancy.
Table 1. Characteristics of women receiving HAART at conception and the available measurements on these women during pregnancy.
| Women (n=127) | |
|---|---|
|
| |
| n (%)† | |
| Type of HAART regimen | |
| Pi-containing | 78 (61) |
| NNRTI-containing | 49 (39) |
| Race by IDU status | |
| Black non-IDU | 37 (32) |
| White non-IDU | 47 (41) |
| White IDU | 31 (27) |
| Other | 12 |
| Age at delivery (years) | |
| Median (IQR) | 33 (30-37) |
| 18-25 | 10 (8) |
| 26-34 | 63 (53) |
| ≥35 | 46 (39) |
| Unknown | 8 |
| Time period of delivery | |
| 1998-1999 | 21 (16) |
| 2000-2001 | 49 (38) |
| 2002-2003 | 49 (38) |
| 2004-2006 | 8 (6) |
| Duration of HAART at time of conception | |
| Median months (IQR) | 10 (5, 20) |
| ≥ 15 months | 42 (33) |
| 6-15 months | 41 (32) |
| < 6 months | 44 (35) |
|
| |
| Measurements (n=371) | |
|
| |
| n (%)† | |
|
| |
| Median baseline VL* (log10 copies/ml) (IQR) | 3.22 (2.61-3.92) |
| No. of undetectable measurements | |
| Total | 79/363 (22) |
| First trimester | 1/74 (1) |
| Second trimester | 23/140 (16) |
| Third trimester | 36/100 (35) |
| Delivery | 21/50 (42) |
| Median VL at delivery (log10 copies/ml) (IQR) | 2.45 (1.45-2.99) |
| Median VL at delivery among detectable | 2.89 (2.57-3.48) |
| HIV RNA assay | |
| Roche | 270 (73) |
| Other | 107 (27) |
| Median baseline* CD4 count (cells/mm3) (IQR) | 380 (288-517) |
- unless other stated
at first pregnancy measurement
Abbreviations: IQR, interquartile range; VL, VL; PI, protease inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor
Overall, 72 (57%) women remained on the same HAART regimen from conception and throughout pregnancy, 24 (19%) switched HAART regimens and 31 (24%) interrupted HAART during early pregnancy (Table 2). Among the 31 women who interrupted therapy, this lasted a median of 9 weeks (IQR 7-14); they re-started at a median of 17 gestational weeks (IQR 14-21), in 18 cases on the same regimen. The 24 women who switched HAART regimens had higher HIV RNA levels at first antenatal measurement (p=0.07) and lower median CD4 count (p=0.019) than those who stayed on the same HAART regimen (Table 2). Of these 24 women, six switched from efavirenz (two to nevirapine and four to a PI) and three from nevirapine (all to a PI); of the 15 conceiving on PIs, 10 switched to another PI-containing regimen and five to nevirapine. There was no difference in HAART type at conception between the women who switched and those who continued regimens (χ2=0.06, p=0.49) (Table 2).
Table 2. Immunological and virological characteristics and HAART type, by treatment sub-group.
| HAART type at conception |
First antenatal CD4 count Median (IQR) cells/mm3 |
First antenatal HIV RNA Median (IQR) log10 copies/ml |
Timing of first antenatal measurement Median (IQR) Gest weeks |
Gestational age at interruption or switch Median (IQR) |
N (%) with undetectable HIV RNA levels in 4 weeks up to delivery |
||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| PI-based N (%) |
NNRTI- based N (%) |
||||||
| No change to regimen n=72 |
49 (68) | 23 (32) | 421 (304-560) |
2.77 (2.44-3.65) |
12 (8-22) | N/A | 24/60 (40) |
| Interruption of HAART n=31 |
14 (45) | 17 (55) | 360 (270-412) |
4.08 (3.37-4.45) |
12 (9-16) | 7 weeks (5-8) | 10/24 (42) |
| Switch to new regimen n=24 |
15 (63) | 9 (38) | 306 (208-447) |
3.12 (2.91-3.48) |
10 (6-16) | 21 weeks (15-24) | 5/19 (26) |
The 31 VL measurements on four Asian women and nine women with missing information on ethnicity and IDU (Table 1) were excluded from the regression analyses. Median VL for these women was not significantly different to the remaining 114 women (median 3.00 [IQR 2.58-3.86] vs. median 2.76 [IQR 2.00-3.50] log10 copies/ml; Wilcoxon test p=0.15).
The initial mixed model for HIV RNA levels over pregnancy was an intention-to-treat analysis including all 114 women. The inclusion of any interaction terms did not result in significant improvements in the model. VL in pregnancy was estimated to remain constant at the beginning of pregnancy with a minor increase of 0.06 log10 copies/ml per week up to 10 gestational weeks, at which time the mean VL was estimated to be 3.19 log10 copies/ml (Table 3). HIV RNA levels thereafter decreased significantly, by an estimated 0.06 log10 copies/ml per week until delivery, an approximate 13% weekly decrease in HIV RNA copies/ml. In the 4 weeks up to delivery, 27 (29%) achieved VL <50 copies/ml and the median in the remainder was 2.71 log10 copies/ml (IQR 2.32-3.24). Black non-IDU women had an estimated mean VL 0.28 log10 copies/ml higher than white non-IDU women and white women with an IDU history had levels 0.31 log10 copies/ml lower than white non-IDU (Table 3), but these differences did not reach statistical significance. Women with baseline CD4 counts <500 cells/mm3 had significantly higher antenatal HIV RNA levels than those without such immunosuppression.
Table 3. Adjusted coefficients of change for log10 HIV RNA levels from intention-to-treat model (n=114, N=342).
| Coefficient (95%CI) |
p | |
|---|---|---|
| Mean at 10 gestational weeks | 3.19 (2.55, 3.83) | <0.0001 |
| Initial slope (<10weeks) | 0.06 (−0.0, 0.13) | 0.07 |
| Slope to delivery (≥10 weeks) | −0.06 (−0.08, −0.05) | <0.0001 |
| Race by IDU | ||
| White non-IDU | 0.00 | |
| Black non-IDU | 0.28 (−0.08, 0.65) | 0.12 |
| White IDU | −0.31 (−0.73, 0.11) | 0.24 |
| Time period | ||
| 1998-1999 | 0.00 | |
| 2000-2001 | −0.03 (−0.53, 0.47) | 0.90 |
| 2002-2006 | −0.26 (−0.74, 0.23) | 0.23 |
| Type of HAART regimen at conception | ||
| PI-containing | 0.00 | |
| NNRTI-containing | −0.25 (−0.56, 0.056) | 0.11 |
| Duration of HAART by conception | ||
| ≥ 15 months | 0.00 | |
| 6-15 months | 0.17 (−0.21, 0.55) | 0.37 |
| < 6 months | 0.12 (−0.23, 0.46) | 0.51 |
| HIV RNA assay | ||
| Roche | 0.30 (−0.02, 0.62) | 0.07 |
| Other | 0.00 | |
| Baseline CD4 count (cells/mm3) | ||
| ≥ 500 | 0.00 | |
| 200-499 | 0.37 (0.02, 0.73) | 0.04 |
| <200 | 0.73 (0.25, 1.21) | 0.003 |
When the model was refitted, excluding the 28 women who interrupted HAART during pregnancy (model 2), HIV RNA levels declined significantly from baseline by an estimated 0.05 log10 copies/ml per week (Table 4). There was no significant difference in the slopes of VL between women who did and did not undergo modification to their HAART regimens, although women who were receiving NNRTI-based HAART at conception had consistently lower estimated HIV RNA levels than those on PI-based HAART. In the model excluding women who interrupted or modified their HAART (model 3), the significant decline in HIV RNA levels was of the same magnitude as seen in model 2 (Table 4). In both models, CD4 count significantly predicted HIV RNA levels over pregnancy, but only severely immunosuppressed women (ie CD4 counts <200 cells/mm3) had significantly higher levels than women with CD4 counts ≥500 cells/mm3. In model 3, the ethnic group difference noted in model 1, with black women having higher estimated HIV RNA levels compared with white non-IDU women, was apparent and marginally significant. The HAART-type effect on HIV RNA levels seen in model 2, that is lower levels associated with NNRTI versus PI, remained in model 3.
Table 4. Adjusted coefficients of change for log10 HIV RNA levels from model excluding women who interrupted HAART (Model 2) (n=86, N=249) and from model excluding women who interrupted or modified HAART (Model 3) (n=65, N=181).
| Model 2 | Model 3 | |||
|---|---|---|---|---|
|
| ||||
| Coefficient# (95%CI) |
P | Coefficient# (95%CI) |
P | |
| Slope to delivery | −0.046 (−0.06, −0.03) | <0.0001 | −0.047 (−0.06, − 0.03) |
<0.0001 |
| Modified HAART regimen in pregnancy |
||||
| No | 0.00 | - | ||
| Yes | 0.27 (−0.09, 0.63) | 0.14 | - | |
| Race by IDU | ||||
| White non-IDU | 0.00 | |||
| Black non-IDU | 0.29 (−0.09, 0.67) | 0.14 | 0.49 (0.02, 0.95) | 0.04 |
| White IDU | −0.21 (−0.65, 0.23) | 0.34 | −0.09 (−0.62, 0.42) | 0.71 |
| Type of HAART regimen at conception |
||||
| PI-containing | 0.00 | 0.00 | ||
| NNRTI-containing | −0.41 (−0.76, −0.06) | 0.02 | −0.48 (−0.88, −0.07) | 0.02 |
| Duration of HAART at conception |
||||
| ≥ 15 months | 0.00 | 0.00 | ||
| 6-15 months | 0.08 (−0.36, 0.52) | 0.72 | 0.12 (−0.39, 0.64) | 0.63 |
| < 6 months | 0.16 (−0.22, 0.54) | 0.40 | 0.29 (−0.18, 0.76) | 0.22 |
| Baseline CD4 count (cells/mm3) |
||||
| ≥ 500 | 0.00 | 0.00 | ||
| 200-499 | 0.072 (−0.31, 0.46) | 0.72 | −0.02 (−0.44, 0.41), | 0.94 |
| <200 | 0.52 (0.014, 1.03) | 0.04 | 0.78 (0.16, 1.41) | 0.01 |
Adjusted for by time period and type of assay used
Overall, 64 (62%) of 103 women with reported HIV RNA levels within four weeks up to delivery had detectable VL at this time, with a median of 2.40 log10 copies/ml (range 1.40-2.95). None of these 64 women transmitted infection to their infants, although the exact binomial 95% confidence limit was 5.6%. Overall, the MTCT rate for the whole study population was 1.27% (2/116, 95%CI 0.21-6.1%).
Discussion
This analysis was based on a group of HIV-infected pregnant women on HAART at conception but with detectable VL. Most of our study population could be considered as having sub-optimal viral suppression [12], as nearly two-thirds had been on HAART for at least 6 months, theoretically sufficient time for achievement of viral suppression [20]; however, three-quarters had baseline VL below 3.93 log10 and could thus be classified as having partial control of HIV replication (complete suppression of VL is the aim for PMTCT). Although most women remained on their pre-pregnancy HAART regimens throughout pregnancy, a quarter interrupted treatment in early pregnancy and a further fifth changed their HAART regimens. Our initial ITT analysis indicated a constant VL for the first 10 weeks of pregnancy, with a subsequent significant decrease until delivery of around 13% per week. As the ITT model included women who switched or interrupted HAART, potentially resulting in improved virological control or virological rebound respectively [13,14], we ran further models excluding the HAART modification group and/or the interruption group, with estimated significant declines of between 7% and 15% log10 copies/ml per week. In all models, NNRTI-based HAART at conception was associated with significantly lower VL throughout pregnancy.
Guidelines [12,21] recommend that HIV-infected women planning a pregnancy are offered pre-conception counselling, which provides the opportunity to switch from antiretroviral drugs that may be associated with toxicity [12,22] and to optimize treatment through adherence assessment/support and/or regimen modification in order to attain a stable, maximally suppressed VL prior to conception. However, a considerable proportion of pregnancies among HIV-infected women are unintended. European studies have indicated prevalences of unplanned pregnancy of 51% to 58% [10,23] and pre-conception counselling rates of only 25% among HIV-positive women [24]; in the USA, underuse of effective contraception has been documented among HIV-positive women [25] and 83% of pregnancies in a study of HIV-positive youth were unplanned [26]. It was not recorded whether the women in our study had planned their pregnancies, but it is likely that a substantial proportion were unintended, consistent with their detectable VL.
We showed a significantly declining VL during pregnancy, both for women remaining on their conception regimens and for those switching HAART regimens. We have previously documented declining VL in untreated women from our study, which may in part be due to pregnancy-related haemodilution, which could also explain some of the VL decline seen here [19]. For the women remaining on the same HAART, the VL decline may be partly explained by improved adherence after awareness of the pregnancy, but this could not be verified as adherence data were unavailable. Three-quarters of pregnant women in the American PACTG 1025 study reported perfect adherence to HAART (no missed doses 4 days before all study visits), with those antiretroviral-naïve before the pregnancy more than twice as likely to be perfectly adherent compared with antiretroviral-experienced women [27]. Women in our study with existing adherence problems may have been motivated to improve adherence once aware of their pregnancy and/or may have received more intensive/effective adherence support from their care providers compared with that received before pregnancy [28-30].
For the fifth of women who switched HAART regimens, their HIV physicians may have determined the need for a switch through adherence assessment and resistance testing, in accordance with clinical guidelines which state that women on failing HAART should have their regimens changed to maximize the likelihood of achieving undetectable VL by delivery [12,13,21]. This group may also include women for whom the caring physician may have had more concerns regarding viremia control and immunosuppression, suggested by higher VL in women with lower CD4 counts. Of note, treatment modifications took place at a median of 21 weeks gestation, possibly due to delayed notification of pregnancy to a woman’s HIV physician and/or testing lag times. In the non-pregnant HIV-positive population, delays between detection of virological failure and switching to a new regimen are not uncommon, with a six month delay reported for a third of such patients in a recent UK national audit [31]. The lack of a significant difference between the switching and the continuing HAART groups in terms of HIV RNA slopes in model two was confirmed by the almost identical slope in the model which excluded the modifying HAART group (model three). The fact that treatment change did not occur until around half-way through pregnancy may have contributed to this finding. Women undergoing treatment modifications had significantly lower baseline CD4 counts than those continuing with the same HAART regimen (on average over 100 cells/mm3 lower), most likely reflecting treatment guidelines and an example of confounding by indication [13,14]. Although we adjusted for baseline CD4 count in the model, we lacked data on drug resistance and could not investigate whether women switching treatment not only had poorer immune status, but also more resistance than those remaining on the same regimens.
Two key factors predictive of HIV RNA levels in pregnancy were baseline CD4 count and type of HAART at conception. In the ITT model, women with CD4 counts <500 cells/mm3 had significantly higher VL than other women, although in subsequent models this lost significance for the women with moderate CD4 counts (200-499 cells/mm3). Women conceiving on NNRTI-containing regimens had consistenly lower VL than those on PI-containing HAART. We previously showed among antiretroviral-naïve women that those initiating HAART with a NNRTI-based regimen reached an undetectable VL more rapidly than those starting with a PI-based regimen (mainly non-boosted) [15]. Several studies have indicated that very high adherence levels are required to maintain virological suppression on PI-containing regimens (above 90%), higher than those required for NNRTI-containing HAART [32-34]; this could help explain our finding here that women conceiving on PI-based HAART had significantly reduced slope of VL decline versus those on NNRTI-based HAART. A further potential explanation of the differences by HAART type could relate to pharmacokinetics: some studies have reported low concentrations of nelfinavir and other PIs in the third trimester [35-37], while studies on plasma nevirapine concentrations have generally shown no significant differences between pregnant and non-pregnant women [38].
Treatment interruption in pregnancy is not recommended by current guidelines as it may result in viral rebound and subsequent increased risk of immune and/or clinical deterioration, in addition to an increased MTCT risk [12,21,39]. A recent study from Italy examining the impact of interruption of therapy during pregnancy, found that interruption in the first trimester was associated with a 10-fold increased risk of MTCT, after adjusting for maternal VL, type of therapy and other factors [39]. Most women who interrupted therapy in our study population did so in the earlier years of the study [data not shown] and it is likely that this practice is becoming increasingly rare, underscored by the recent Italian results. Switches away from efavirenz due to concerns regarding terato-embryogenic toxicity risk are often advocated for pregnant women [12] and were also seen here.
Study limitations include the observational nature of the data and lack of information on adherence, drug resistance, HIV subtype and immunological and virological patterns before pregnancy. Our ability to explore potential reasons behind the similar VL declines in the women who switched treatment and in those who remained on their conception regimens was therefore constrained. A limitation of our analyses was the inability to adjust for baseline VL, as women had their first measurement at different gestational ages. However the statistical approach used was able to account for the variability in intercepts between women which should reduce some of the bias from not adjusting for the baseline VL implicitly. However we modelled appropriately the left-censored HIV RNA measurements and avoided imputation with midpoints, which can result in biased parameter estimates and their standard errors [18]. An additional limitation was a relative overestimation of the effects of drugs that were used in HAART regimens in pregnant women during earlier years (e.g. nelfinavir), which have been largely substituted by more potent PIs today. Although antiretroviral-experienced, our study population had not yet accumulated long durations of treatment and most had partial control of viremia and thus the generalizability of our findings, for example, to highly treatment experienced pregnant women with high VL at conception, remains uncertain.
Our findings come from a “real life” setting, and indicate a variety of practices regarding the management of women conceiving on HAART with detectable VL in this Western European setting. The existence of this group of pregnant women highlights the need for improved monitoring of and support for treated women before they become pregnant, as well as during pregnancy itself. Clinical concerns during pregnancy include attempts at improving virological control to avoid MTCT and improve maternal health, whilst minimizing potential adverse effects on the fetus and mother, including the risk of exposure to potentially teratogenic drugs. The MTCT rate in our study population was just over 1%; concern regarding the risk of pregnant women on failing HAART developing drug resistance, which could potentially be vertically transmitted [40,41] is a key factor behind recommendations for switching regimens as soon as virological failure is determined. However, there is a lack of consensus regarding when to change HAART for virological failure in non-pregnant adults [13]; few studies are aimed at evaluating optimal antiretroviral management during pregnancy for women on HAART at conception, while clinical trial data on efficacy of different HAART regimens in pregnancy are lacking. This context may partly explain the finding here that only a fifth of women underwent a treatment switch in pregnancy. Future controlled studies are needed to obtain information on mechanisms for VL decrease and on optimal clinical management of HAART in pregnant women, who are already on treatment at conception, particularly those with accumulated resistance.
Acknowledgements
We thank Prof L Chieco-Bianchi, Prof F Zacchello, Dr E Ruga, Dr AM Laverda, Dr R D’Elia and Mrs S Oletto (Padua); Dr T Schmitz, Dr R Weogel, Dr Karen Seel and Dr S Casteleyn (Berlin); Dr S Burns, Dr N Hallam, Dr PL Yap, and Dr J Whitelaw (Edinburgh); Dra B Sancho, and Dr G Fontan-Casanego (Madrid); Dr A Gonzalez Molina, Dr M Gobernado, Dr JL Lopez, and Dr J Cordoba (Valencia); A van der Plas, E.M. Lepoole (Amsterdam); Dr E Belfrage, Dr L Navér, Dr A Kaldma and Dr AC Lindholm (Sweden); Dr A Ferrazin, Dr R Rosso, G Mantero, Prof S Trasino, Dr J Nicoletti (Genoa); Dr E Mur (Barcelona); Dr B Martinez de Tejada, Dr L Zamora, Dr R Vidal (Barcelona); Dr G Zucotti (Milan); Dr M Carla Re (Bologna); Prof PA Tovo, Dr C Gabiano (Turino); Dr A Maccabruni, (Pavia); Dr G Ferraris, (Clinica Mangiagalli, Milano); Dr T Bruno (Naples), The Regional Health Office and RePuNaRC (Naples); G Mantero, Dr A Nicoletti, Dr B Bruzzone, Dr R Rosso and Dr M Setti (Genoa); M Kaflik (Medical University of Warsaw, Poland).
The ECS is a coordination action of the European Commission (PENTA/ECS 018865). Claire Thorne is supported by a Wellcome Trust Research Career Development Fellowship. Some of this work was undertaken at GOSH/UCL Institute of Child Health which received a proportion of funding from the UK Department of Health’s NIHR Biomedical Research Centres funding scheme. The Centre for Paediatric Epidemiology and Biostatistics also benefits from funding support from the Medical Research Council in its capacity as the MRC Centre of Epidemiology for Child Health. The centre at Universita degli Studi di Padova is supported by Progetto di Ricerca sull AIDS - Istituto Superiore di Sanità – 2006.
European Collaborative Study Collaborators
Dr C Thorne, Dr D Patel, Dr K England, Prof ML Newell (ECS coordinating Centre, UCL Institute of Child Health, UK), Dr C Giaquinto, Dr O Rampon, Dr A Mazza and Prof A De Rossi (Universita degli Studi di Padova, Italy); Prof I Grosch-Wörner (Charite Virchow-Klinikum, Berlin, Germany); Dr J Mok (Royal Hospital for Sick Children, Edinburgh); Dr Ma I de José, Dra B Larrú Martínez, Dr J Ma Peña, Dr J Gonzalez Garcia, Dr JR Arribas Lopez and Dr MC Garcia-Rodriguez (Hospital Infantil La Paz, Madrid); Prof F Asensi-Botet, Dr MC Otero, Dr D Pérez-Tamarit (Hospital La Fe, Valencia, Spain); Dr H J Scherpbier, M Kreyenbroek, Dr MH Godfried, Dr FJB Nellen and Dr K Boer (Academisch Medisch Centrum, Amsterdam, The Netherlands); Prof A Ehrnst, Dr AB Bohlin, Dr S Lindgren, Dr B Anzén and Dr K Lidman (Karolinska University Huspital, Huddinge and Solna, Sweden); Prof J Levy, Dr P Barlow, Dr Y Manigart, Dr M Hainaut and Dr T Goetghebuer (Hospital St Pierre, Brussels, Belgium); Prof B Brichard, J De Camps, N Thiry, G Deboone, H Waterloos (UCL Saint-Luc, Brussels, Belgium); Prof C Viscoli (Infectious Diseases Clinic, University of Genoa, Italy); Prof A De Maria (Department of Internal Medicine, University of Genoa, Italy); Prof G Bentivoglio, Dr S Ferrero, Dr C Gotta (Department of Obstetrics and Gynecology-Neonatology Unit, University of Genoa, Italy); Prof A Mûr, Dr A Payà, Dr MA López-Vilchez, Dr R Carreras (Hospital del Mar, Universidad Autonoma, Barcelona, Spain); Dr N H Valerius, Dr V Rosenfeldt (Hvidovre Hospital, Denmark); Dr O Coll, Dr A Suy and Dr J M Perez ( Hospital Clínic, Barcelona, Spain); Dr C Fortuny, Dr J Boguña (Hospital Sant Joan de Deu, Barcelona, Spain); Dr V Savasi, Dr S Fiore, Dr M Crivelli, Prof E Ferrazzi (Ospedale L. Sacco, Milan, Italy); Dr A Viganò, Dr V Giacomet, Dr C Cerini, Dr C Raimondi and Prof G Zuccotti (Department of Pediatrics, L. Sacco Hospital, University of Milan); Dr S.Alberico, Dr M Rabusin, M Bernardon (IRCCS Burlo Garofolo, Trieste, Italy); Dr G P Taylor, Dr EGH Lyall (St Mary’s Hospital, London); Ms Z Penn (Chelsea and Westminster Hospital, London); Drssa W. Buffolano, Dr R Tiseo, (Pediatric Dept, Federico II University, Naples), Prof P Martinelli, Drssa M Sansone, Dr G Maruotti, Dr A Agangi (Obstetric Dept, Federico II University, Naples, Italy); Dr C Tibaldi, Dr S Marini, Dr G Masuelli, Prof C Benedetto (University di Torino, Italy); Dr T Niemieç (National Research Institute of Mother & Child, Warsaw, Poland), Prof M Marczynska, Dr S Dobosz, Dr J Popielska, Dr A Oldakowska (Medical University of Warsaw, Infectious Diseases Hospital, Warsaw, Poland); Dr R Malyuta, Dr I Semenenko, T Pilipenko (ECS Ukraine coordinating centre).
References
- (1).Thorne C, Newell ML. Antenatal and neonatal antiretroviral therapy in HIV-infected women and their infants: a review of safety issues. Med Wieku Rozwoj. 2003;7:425–36. [PubMed] [Google Scholar]
- (2).Egger M, May M, Chene G, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360:119–29. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- (3).Sterne JA, Hernan MA, Ledergerber B, et al. Long-term effectiveness of potent antiretroviral therapy in preventing AIDS and death: a prospective cohort study. Lancet. 2005;366:378–84. doi: 10.1016/S0140-6736(05)67022-5. [DOI] [PubMed] [Google Scholar]
- (4).European Collaborative Study Mother-to-child transmission of HIV infection in the era of highly active antiretroviral therapy. Clin Infect Dis. 2005;40:458–65. doi: 10.1086/427287. [DOI] [PubMed] [Google Scholar]
- (5).Jasseron C, Mandelbrot L, Tubiana R, et al. Prevention of mother-to-child HIV transmission: similar access for sub-Sahara African immigrants and for French women? AIDS. 2008;22:1503–11. doi: 10.1097/QAD.0b013e3283065b8c. [DOI] [PubMed] [Google Scholar]
- (6).Townsend CL, Cortina-Borja M, Peckham CS, de RA, Lyall H, Tookey PA. Low rates of mother-to-child transmission of HIV following effective pregnancy interventions in the United Kingdom and Ireland, 2000-2006. AIDS. 2008;22:973–81. doi: 10.1097/QAD.0b013e3282f9b67a. [DOI] [PubMed] [Google Scholar]
- (7).Palella FJ, Jr., Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- (8).Heard I, Sitta R, Lert F. Reproductive choice in men and women living with HIV: evidence from a large representative sample of outpatients attending French hospitals (ANRS-EN12-VESPA Study) AIDS. 2007;21(Suppl 1):S77–S82. doi: 10.1097/01.aids.0000255089.44297.6f. [DOI] [PubMed] [Google Scholar]
- (9).Agangi A, Thorne C, Newell ML. Increasing likelihood of further live births in HIV-infected women in recent years. BJOG. 2005;112:881–8. doi: 10.1111/j.1471-0528.2005.00569.x. [DOI] [PubMed] [Google Scholar]
- (10).Floridia M, Tamburrini E, Ravizza M, et al. Antiretroviral therapy at conception in pregnant women with HIV in Italy: wide range of variability and frequent exposure to contraindicated drugs. Antivir Ther. 2006;11:941–6. [PubMed] [Google Scholar]
- (11).Garcia PM, Kalish LA, Pitt J, et al. Women and Infants Transmission Study Group Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. N Engl J Med. 1999;341:394–402. doi: 10.1056/NEJM199908053410602. [DOI] [PubMed] [Google Scholar]
- (12).US Public Health Service Task Force . Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. [Accessed 28 Apr 2009]. Updated July 8 2008. Available from http://aidsinfo.nih.gov/contentfiles/PerinatalGL.pdf. [Google Scholar]
- (13).Panel on Antiretroviral Guidelines for Adults and Adolescents . Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; USA: [Accessed 28 Apr 2009]. Updated Nov 3 2008. Available from http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf. [Google Scholar]
- (14).British HIV Association BHIVA guidelines for the treatment of HIV-1 infected adults with antiretroviral therapy 2008. HIV Medicine. 2008;9:563–608. doi: 10.1111/j.1468-1293.2008.00636.x. [DOI] [PubMed] [Google Scholar]
- (15).European Collaborative Study Time to undetectable viral load after highly active antiretroviral therapy initiation among HIV-infected pregnant women. Clin Infect Dis. 2007;44:1647–56. doi: 10.1086/518284. [DOI] [PubMed] [Google Scholar]
- (16).European Collaborative Study The mother-to-child HIV transmission epidemic in Europe: evolving in the East and established in the West. AIDS. 2006;20:1419–27. doi: 10.1097/01.aids.0000233576.33973.b3. [DOI] [PubMed] [Google Scholar]
- (17).Thiebaut R, Jacqmin-Gadda H. Mixed models for longitudinal left-censored repeated measures. Comput Methods Programs Biomed. 2004;74:255–60. doi: 10.1016/j.cmpb.2003.08.004. [DOI] [PubMed] [Google Scholar]
- (18).Hughes JP. Mixed Effects Models with Censored Data with Application to HIV RNA Levels. Biometrics. 1999;55:625–9. doi: 10.1111/j.0006-341x.1999.00625.x. [DOI] [PubMed] [Google Scholar]
- (19).European Collaborative Study Levels and patterns of HIV RNA viral load in untreated pregnant women. International Journal of Infectious Diseases. 2009;13:266–73. doi: 10.1016/j.ijid.2008.07.004. [DOI] [PubMed] [Google Scholar]
- (20).Phillips AN, Staszewski S, Weber R, et al. HIV viral load response to antiretroviral therapy according to the baseline CD4 cell count and viral load. JAMA. 2001;286:2560–7. doi: 10.1001/jama.286.20.2560. [DOI] [PubMed] [Google Scholar]
- (21).British HIV Association British HIV Association and Children’s HIV Association guidelines for the management of HIV infection in pregnant women 2008. HIV Medicine. 2008;9:452–502. doi: 10.1111/j.1468-1293.2008.00619.x. [DOI] [PubMed] [Google Scholar]
- (22).Thorne C, Newell ML. Safety of agents used to prevent mother-to-child transmission of HIV: is there any cause for concern? Drug Saf. 2007;30:203–13. doi: 10.2165/00002018-200730030-00004. [DOI] [PubMed] [Google Scholar]
- (23).Fiore S, Heard I, Thorne C, et al. Reproductive experience of HIV-infected women living in Europe. Hum Reprod. 2008;23:2140–4. doi: 10.1093/humrep/den232. [DOI] [PubMed] [Google Scholar]
- (24).Coll O, Lopez M, Hernandez S. Fertility choices and management for HIV-positive women. Current Opinion in HIV and AIDS. 2008;3:186–92. doi: 10.1097/COH.0b013e3282f51219. [DOI] [PubMed] [Google Scholar]
- (25).Massad LS, Evans CT, Wilson TE, et al. Contraceptive use among U.S. women with HIV. J Womens Health. 2007;16:657–66. doi: 10.1089/jwh.2006.0204. [DOI] [PubMed] [Google Scholar]
- (26).Koenig LJ, Espinoza L, Hodge K, Ruffo N. Young, seropositive, and pregnant: epidemiologic and psychosocial perspectives on pregnant adolescents with human immunodeficiency virus infection. Am J Obstet Gynecol. 2007;197:S123–S131. doi: 10.1016/j.ajog.2007.03.004. [DOI] [PubMed] [Google Scholar]
- (27).Bardeguez AD, Lindsey JC, Shannon M, et al. Adherence to antiretrovirals among US women during and after pregnancy. J Acquir Immune Defic Syndr. 2008;48:408–17. doi: 10.1097/QAI.0b013e31817bbe80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Mellins CA, Chu C, Malee K, et al. Adherence to antiretroviral treatment among pregnant and postpartum HIV-infected women. AIDS Care. 2008;20:958–68. doi: 10.1080/09540120701767208. [DOI] [PubMed] [Google Scholar]
- (29).Vaz MJ, Barros SM, Palacios R, et al. HIV-infected pregnant women have greater adherence with antiretroviral drugs than non-pregnant women. Int J STD AIDS. 2007;18:28–32. doi: 10.1258/095646207779949808. [DOI] [PubMed] [Google Scholar]
- (30).Zorrilla CD, Santiago LE, Knubson D, et al. Greater adherence to highly active antiretroviral therapy (HAART) between pregnant versus non-pregnant women living with HIV. Cell Mol Biol. 2003;49:1187–92. [PubMed] [Google Scholar]
- (31).Hart E, Curtis H, Wilkins E, Johnson M. National review of first treatment change after starting highly active antiretroviral therapy in antiretroviral-naive patients. HIV Med. 2007;8:186–91. doi: 10.1111/j.1468-1293.2007.00451.x. [DOI] [PubMed] [Google Scholar]
- (32).Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- (33).Maggiolo F, Ravasio L, Ripamonti D, et al. Similar adherence rates favor different virologic outcomes for patients treated with nonnucleoside analogues or protease inhibitors. Clin Infect Dis. 2005;40:158–63. doi: 10.1086/426595. [DOI] [PubMed] [Google Scholar]
- (34).Bangsberg DR. Less than 95% adherence to nonnucleoside reverse-transcriptase inhibitor therapy can lead to viral suppression. Clin Infect Dis. 2006;43:939–41. doi: 10.1086/507526. [DOI] [PubMed] [Google Scholar]
- (35).Mirochnick M, Capparelli E. Pharmacokinetics of antiretrovirals in pregnant women. Clin Pharmacokinet. 2004;43:1071–87. doi: 10.2165/00003088-200443150-00002. [DOI] [PubMed] [Google Scholar]
- (36).Villani P, Floridia M, Pirillo MF, et al. Pharmacokinetics of nelfinavir in HIV-1-infected pregnant and nonpregnant women. Br J Clin Pharmacol. 2006;62:309–15. doi: 10.1111/j.1365-2125.2006.02669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Stek AM, Mirochnick M, Capparelli E, et al. Reduced lopinavir exposure during pregnancy. AIDS. 2006;20:1931–9. doi: 10.1097/01.aids.0000247114.43714.90. [DOI] [PubMed] [Google Scholar]
- (38).Roustit M, Jlaiel M, Leclercq P, Stanke-Labesque F. Pharmacokinetics and therapeutic drug monitoring of antiretrovirals in pregnant women. Br J Clin Pharmacol. 2008;66:179–95. doi: 10.1111/j.1365-2125.2008.03220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Galli L, Puliti D, Chiappini E, et al. Is interruption of antiretroviral treatment during pregnancy an additional major risk factor for mother-to-child transmission of HIV type 1? Clin Infect Dis. 2009;48:1310–1317. doi: 10.1086/597774. [DOI] [PubMed] [Google Scholar]
- (40).Schmitz T, Kleinkauf N, Klempa B, Ringe H, Varnholt V, Grosch-Worner I. Transmission of human immunodeficiency virus type 1 nevirapine resistance mutation K103N from a treatment-naive mother to her child. Pediatr Infect Dis J. 2006;25:275–6. doi: 10.1097/01.inf.0000202142.34502.5e. [DOI] [PubMed] [Google Scholar]
- (41).Karchava M, Pulver W, Smith L, et al. Prevalence of drug-resistance mutations and non-subtype B strains among HIV-infected infants from New York State. J Acquir Immune Defic Syndr. 2006;42:614–9. doi: 10.1097/01.qai.0000225871.87456.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]