Abstract
Background
There is little data on the burden or causes of epilepsy in developing countries, particularly in children living in sub-Saharan Africa.
Methods
We conducted two surveys to estimate the prevalence, incidence and risk factors of epilepsy in children in a rural district of Kenya. All children born between 1991 and 1995 were screened with a questionnaire in 2001 and 2003, and those with a positive response were then assessed for epilepsy by a clinician. Active epilepsy was defined as two or more unprovoked seizures with one in the last year.
Results
In the first survey, 10,218 children were identified from a census, of whom 110 had epilepsy. The adjusted prevalence estimates of lifetime and active epilepsy were 41/1000 (95% CI=31-51) and 11/1000 (95% CI =5-15) respectively. Overall two thirds of children had either generalized tonic-clonic and/or secondary generalized seizures. A positive history of febrile seizures (OR=2.75; 95% CI: 1.240-6.09) and family history of epilepsy (OR=4.12; 95% CI= 2.00-8.49) were important risk factors for active epilepsy. After the second survey, 39 children from the same birth cohort with previously undiagnosed epilepsy were identified, thus the incidence rate of active epilepsy is 187 per 100,000 per year (95% CI: 133-256) in children aged 6-12 years.
Conclusions
There is a considerable burden of epilepsy in older children living in this area of rural Kenya, with a family history of seizures and a history of febrile seizures identified as risk factors for developing epilepsy.
Keywords: Prevalence, Incidence, Epilepsy, Risk factors
Introduction
Epilepsy is the most common neurological disorder in resource-poor countries (RPCs) (WHO, 2001). The World Bank identified epilepsy as a health priority for school age children because of its’ high psychosocial morbidity and the potential for control using low cost interventions (World Bank, 1993). The World Health Organization estimates that there are over 50 million people with epilepsy, of whom two thirds are children living in RPCs and one fifth in sub-Saharan Africa; but there is little data on which to base this estimate (WHO, 2001; Preux & Druet-Cabanac, 2005). Furthermore, there is even less data on incidence and risk factors associated with epilepsy in children, on which to plan public health interventions.
We have conducted two studies on children to determine the prevalence, incidence and risk factors for the development of epilepsy in older children living in a rural area of sub-Saharan Africa.
Methods
Study Setting
This study was conducted in a demarcated area in Kilifi District on the coast of Kenya. The area was mapped in 2000 and is described elsewhere (Mung’ala-Odera et al, 2004). The maps were used to locate each household during a census in October 2000 in which the cohort population was defined. The population in this area has been undergoing active surveillance since 2000.
Population
The study population consisted of children born from 01/06/1991 to 31/12/1995, who were identified from the population database of 108,896 people, resident in the study area for at least 6 months preceding the first survey. The population consists of the Mijikenda ethnic group, in which the Giriama sub-group predominates.
Study Design and Methods
The first survey was conducted from June 2001 to April 2002 as part of a survey of neurological impairment and disability (Mung’ala-Odera et al, 2006), in which parents or guardians of all the children born from 1991 to 1995 and residing in the study area were interviewed by fieldworkers using the ten questions questionnaire (TQQ) (Durkin et al, 1994). This questionnaire includes a single question enquiring about convulsions: “Does the child sometimes have fits, become rigid, or lose consciousness?” This question was found to have a sensitivity rate of 100% and specificity rate of 93% for moderate or severe lifetime and active convulsive epilepsy (ACE), defined as convulsions occurring at least once a month (Mung’ala-Odera et al, 2004). Children aged over 6 years were selected for this survey due to the difficulty in differentiating between febrile and unprovoked seizures in younger children and because by this age, most postnatal insults (particularly central nervous system infections) will have occurred.
The second survey was conducted from September 2003 to January 2004, as part of a larger survey of epilepsy in people of all ages (Edwards et al., in prep). In this survey, a three-phase approach was utilized to identify individuals with convulsive epilepsy. In phase one, a responsible respondent in each homestead was used to identify possible epilepsy cases with the question: Is there anybody in the household who has fits or has been told that they have fits? In phase two, individuals who were identified as positive in the first stage or their parents/guardians had a detailed epilepsy symptoms questionnaire administered to them, to determine those with possible ACE to be invited for detailed assessment.
Individuals identified as having epilepsy in phase two of the second survey had a detailed assessment performed by a clinician to determine if they had seizures. Epilepsy was diagnosed from the history. A thirty-minute electroencephalogram (EEG), with hyperventilation and photostimulation was performed to help classify the type of epilepsy. Seizures were classified according to the International League against Epilepsy (ILAE, 1993). Active convulsive epilepsy was defined as more than one unprovoked convulsion, with at least one having occurred within the preceding 12 months, since this is the criteria for the administration of anti-epileptic drugs (AED) in Kenya (Dekker, 1994; Ministry of Health, 1994).
All the children seen in phase 2 of the first survey (2001 to 2002) also had assessments of cognition, vision, motor and hearing to determine impairments associated with epilepsy. Only those with moderate or severe impairments were included in the report (Mung’ala-Odera et al, 2006). Motor impairment was evaluated through physical examination, while cognitive assessment involved a seven-item battery testing verbal and non-verbal skills (Mung’ala-Odera et al, 2006). The Sonksen-Silver Acuity system was used to measure visual acuity, hearing was measured using the Kamplex screening audiometer (Mung’ala-Odera et al, 2006). All the assessments were performed within one week of identification. In addition, information was also collected on the previous and current use of AEDs for individuals with ACE. This information was used to estimate the treatment gap, defined as the difference between the number of people with ACE and those who reported not receiving AED in a given population at a given point in time, expressed as percentage. No blood or saliva testing for AEDs was included in the study.
Risk factor analysis
The study on risk factors was performed in phase two of the first survey. This involved collecting information on numerous potential risk factors for epilepsy. These risk factors were compared to a group of 816 children within the same age range, who tested negative on the TQQ and were randomly selected from the demographic database as part of the neurological impairment study (Mung’ala-Odera et al, 2006). This allowed 80% power to detect an odds ratio (OR) >3 for a variable that has a prevalence of 5% in the community with 95% confidence. For the analysis, 25 variables were selected as potential risk factors for epilepsy. We have categorized these into socio-demographic factors and factors operating primarily during one of the three stages of fetal and child development: prenatal, perinatal, and postnatal. After the initial univariate analysis, 12 variables were identified as potential risk factors for the development of epilepsy. For the purposes of this study, neonatal insult was defined by a history of features suggestive of birth asphyxia, neonatal tetanus, jaundice or sepsis.
The National Ethics Committee of Kenya granted permission for the study, and consent for the children to be involved in the study was obtained from the parents.
Data Storage and Analysis
The data was double entered into Fox-pro (version 4) and verified. For prevalence, ‘lifetime’ prevalence was used, when active and inactive epilepsy were considered together (Last et al, 1995) and prevalence of ACE was used in those who had one unprovoked convulsion occurring within the last year. Since only a sample of those children who screened negative were clinically evaluated, adjusted estimates of prevalence and their variance were computed. The adjustments followed methods suggested for the epidemiological study of rare disorders in populations (Shrout and Newman 1989). In this approach, individuals not evaluated in the second phase of the study are also considered in the computation of both the prevalence and its variance. For the calculation of incidence, only cases of active convulsive epilepsy were considered. The incidence was calculated from the number of new cases developing between the surveys, with the denominator as the number of children in whom information was obtained in the second survey, divided by the time period between the surveys. .
Unadjusted univariate ORs and their corresponding 95% confidence intervals (CI) were computed using logistic regression to evaluate associations between epilepsy and the potential risk factors. Factors with p values less or equal to 0.25 in the univariate analysis were used in the multiple regression model to determine the independent risk factors. Only 4 factors were retained in the final multiple logistic regression to evaluate the independent association of each factor with epilepsy.
Results
A total of 10,218 children were screened in the first survey, 125 (1.2%) of whom were reported to have convulsive epilepsy. The age and gender distribution of these children are similar within the population screened. Out of those positive on the epilepsy question in the TQQ, 113 (90%) were assessed in the second phase of the study, 12 children were not assessed because of lack of parental consent.
Prevalence
The total number of children with confirmed epilepsy was 110 of whom 35 (31.8%) had Active epilepsy. The unadjusted prevalences of active and lifetime epilepsy in the first survey were 3.4 per 1000 (95% CI: 2.4 - 4.8) and 10.7 per 1000 (95% CI: 8.9 - 13.0). Following adjustment, the prevalences were 11 (95% CI: 5.0-15.0) and 41 per 1000 (95% CI: 31.0-51.0) (Table 1). There were no significant differences in prevalence between age groups or gender.
Table 1. Prevalence of epilepsy for lifetime and active epilepsy in children during 2001/2 survey by sex and age, per 1000, with 95% confidence intervals (CI) in parentheses.
| Age (Years) |
Number resident in study area |
Number with epilepsy |
Number with active epilepsy |
Adjusted Prevalence Lifetime Epilepsy* |
Adjusted Prevalence Active Epilepsy |
|---|---|---|---|---|---|
| 6 | 2940 | 33 | 10 | 35.8 (19.5-55.3) |
8.7 (1.1-16.3) |
| 7 | 2493 | 24 | 9 | 31.7 (14.4-49.0) |
12.6 (2.0-23.2) |
| 8 | 2391 | 28 | 8 | 42.0 (22.4-61.6) |
11.9 (1.8-22.0) |
| 9 | 2394 | 25 | 8 | 43.5 (24.0-63.0) |
8.0 (0.0-16.0) |
| Gender | |||||
| Male | 5160 | 54 | 17 | 37.0 (24.0-49.0) |
7.5 (2.3-12.7) |
| Female | 5058 | 56 | 18 | 45.0 (30.0-59.0) |
12.2 (5.1-19.3) |
| Total | 10218 | 110 | 35 | 41.0 (31.0-51.0) |
11.0 (5.0-15.0) |
Includes both active and inactive epilepsy
Incidence of Active Convulsive Epilepsy
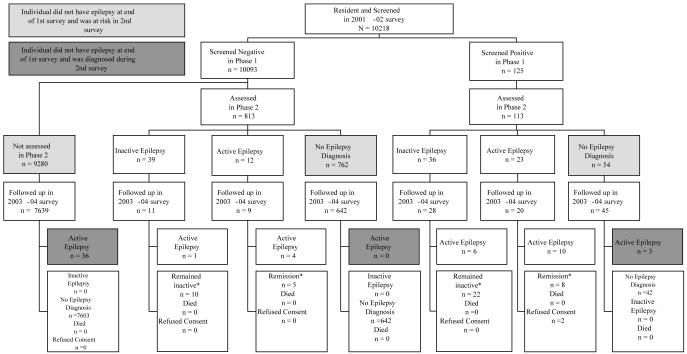
Over a period of 2 years and 6 months, 39 children with previously undiagnosed epilepsy were identified in 8,326 children who could be traced and re-interviewed from the original cohort of 10,093 who screened negative during the first survey (figure 1). This corresponds to annual minimum incidence of 187 per 100,000 (95%CI: 133 - 256) per year. During the second survey in 2003, thirteen of the original active cases were found to be in remission (no seizures in the last 12 months) with 2 still taking AEDs at the time of second survey, while six had moved out of the study area. None of the children identified with epilepsy in the first survey had died.
Figure 1. Active Epilepsy Incidence 2001-2004.
Types of Seizures and age at onset
All the children had convulsive seizures, in whom about a third of children had generalized tonic-clonic and a further third had secondary generalized seizures as their dominant type of seizures (Table 2). Other identified seizure types were complex partial, tonic attacks and absences. . In 24 (69%) of the active cases and 56 (75%) of the inactive cases, the onsets of seizures were reported to be within the first two years of life.
Table 2. Dominant seizure types in the Lifetime and Active Epilepsy groups of the children diagnosed in the 2001-2002 survey.
| Seizure Type | Lifetime Epilepsy* n (%) |
Active Epilepsy n (%) |
|---|---|---|
| Generalized Tonic-Clonic | 37(33.6) | 16(45.7) |
| Secondary-Generalized | 35(31.8) | 8(22.9) |
| Complex Partial | 8(7.3) | 3(8.6) |
| Tonic Attacks | 10(9.1) | 2(5.7) |
| Generalized Absence | 4(3.6) | 4(11.4) |
| Others | 14(12.7) | 0(0) |
| Unclassifiable | 2(1.8) | 2(5.7) |
| Total | 110(100) | 35(100) |
Includes both active and inactive epilepsy
EEG characteristics of seizures
Electroencephalograms could be performed on 80 children of whom 16 (20%) had abnormal tracings, and six were found to have interictal epileptiform activity. Two children with general tonic-clonic seizures had evidence of focal features on their EEG.
Risk factors
The risk factors for lifetime and active epilepsy were compared to 816 children who tested negative on phase two evaluations. The univariate logistic regression analysis (Table 3) identified a history of febrile seizures, family history of seizures, birth difficulties and neonatal insults to be significantly associated with the development of lifetime epilepsy. For active epilepsy, a history of febrile seizures and family history of seizures were identified as risk factors. Out of twenty-six children with epilepsy and who were reported to have been admitted to hospital, only ten were found in the Kilifi District Hospital database. Malaria with convulsions (n=4) and febrile convulsions (n=1) were the only central nervous system infections identified.
Table 3. Univariate odds ratios for selected risk factors of epilepsy in children.
| Characteristics | Proportion of Children with Characteristics, n (%) |
Lifetime Epilepsy* | Active Epilepsy | ||||
|---|---|---|---|---|---|---|---|
| No Epilepsy |
Lifetime Epilepsy* |
Active Epilepsy |
Odds Ratios† |
95% CI | Odds Ratios† | 95% CI | |
| Maternal age at first birth >30 years |
189 (27.5) | 24 (22.6.0) | 7 (22.6) | 0.76 | 0.47-1.24 | 0.78 | 0.33-1.85 |
| Lack of maternal education |
423 (61.8) | 56 (52.8) | 18 (58.1) | 0.69 | 0.45-1.04 | 0.89 | 0.43-1.89 |
| Child's age > 7 years | 215 (29.1) | 33 (30.0) | 9 (26.5) | 1.04 | 0.67-1.62 | 0.87 | 0.39-1.88 |
| Male children | 385 (52.1) | 54 (49.1) | 17 (50.0) | 0.88 | 0.59-1.32 | 0.93 | 0.46-1.87 |
| Family history of seizures | 87 (11.8) | 23 (20.9) | 10 (29.4) | 1.97 | 1.18-3.29 | 2.96 | 1.37-6.38 |
| Antenatal problems | |||||||
| Lack of prenatal care | 16 (2.2) | 2 (1.8) | 2 (6.1) | 0.82 | 0.18-3.631 | 3.15 | 0.69-14.3 |
| Perinatal problems | |||||||
| Home birth | 626 (85.9) | 84 (76.4) | 25 (73.5) | 0.53 | 0.33-0.86 | 0.49 | 0.22-1.06 |
| Untrained birth attendant | 572 (79.3) | 77 (70.6) | 24 (72.7) | 0.76 | 0.59-0.96 | 0.73 | 0.49-1.10 |
| Birth difficulty | 36 (4.9) | 11 (10.2) | 4 (12.1) | 2.16 | 1.06-4.38 | 2.41 | 0.81-7.19 |
| Postnatal problems | |||||||
| Neonatal insults | 51 (7.0) | 18 (16.5) | 4 (12.1) | 1.61 | 1.20-2.17 | 1.24 | 0.73-2.14 |
| Child not immunized | 70 (9.8) | 8 (7.5) | 4 (11.7) | 0.74 | 0.34-1.58 | 1.28 | 0.43-3.73 |
| History of febrile seizures |
130 (17.6) | 78 (70.9) | 19 (55.8) | 11.4 | 7.25-17.96 | 4.19 | 2.09-8.42 |
| Total | 816 | 110 | 35 | ||||
Includes both active and inactive epilepsy
Odds ratios calculated using logistic regression
After adjustment for a history of febrile seizures, other individual risk factors for lifetime epilepsy still suggested an increased risk of epilepsy but the adjusted ORs were not statistically significant (Table 4). For active epilepsy, after taking into account, history of febrile seizures and family history of seizures, other independent risk factors were no longer statistically significant (Table 4).
Table 4. Multiple logistic regression odds ratios for the risk factors of epilepsy.
| Risk factors | Lifetime epilepsy* | Active epilepsy | ||
|---|---|---|---|---|
| Odds Ratios | 95% CI | Odds Ratios | 95% CI | |
|
Family history of
seizures |
1.67 | 0.93-2.99 | 2.75 | 1.24-6.09 |
| Birth difficulty | 1.69 | 0.74-3.84 | 1.57 | 0.53-4.70 |
|
History of febrile
seizures |
10.75 | 6.77-17.09 | 4.12 | 2.00-8.49 |
| Neonatal insults | 1.23 | 0.88-1.72 | 0.92 | 0.52-1.63 |
Includes both active and inactive epilepsy
Odds ratios calculated using logistic regression
Associated impairments
In children with lifetime epilepsy, 34 (31%) of them had moderate or severe neurological impairments. This included 22 (65%) with cognitive impairment, 5 (15%) with motor impairment, 1 (3%) with vision impairment and 6 (18%) with hearing difficulties. In those with active epilepsy, 17 (49%) of them had other neurological problems: 8 (23%) with cognitive impairment, 2(6%) with motor impairment, 3 (9%) with hearing difficulties and 4 (11%) with speech difficulties.
Anti-epileptic drugs utilization
In 2001, only 4 (11%) children were reported to be on AEDs treatment. Two children were taking two AEDs (one on phenobarbitone and phenytoin and the other on phenobarbitone and carbamazepine). The other two were each on a single drug therapy, one on phenobarbitone, while the other was using carbamazepine. Only one child (previously on phenobarbitone) had stopped taking AEDs at the time of the second survey.
There was no difference in the nutritional status between the children with epilepsy and those without epilepsy. In the children with ACE, significantly less attended school than controls (14 (40%) vs 878 (60%), χ2 = 4.98, p=0.026), but there was no difference in school attendance between those with lifetime epilepsy (55%) and those without.
Discussion
This study identifies a significant burden of epilepsy in this rural area of Africa, with around 4% of children aged 6-9 years having developed epilepsy by this age, a third of whom had an unprovoked convulsion during the year prior to the survey. In this Kenyan population, nearly 0.2% of children aged between 6-12 years develop epilepsy each year. A history of febrile seizures and a family history of seizures were identified as the most important risk factors. Most children (89%) were not receiving AED. Nearly half of the children with active epilepsy had other impairments, in particular cognitive impairment, and were less likely to attend school.
Prevalence
The estimate for active epilepsy in this area is higher than that established earlier in this same community and in which a different protocol was utilized (Snow et al. 1994) and also higher than that reported by a study done in the Rift Valley of Kenya (reported rates were 3.6/1000 when the key informants approach was used and 18.2/1000 when a randomized cluster sampling was utilized) (Kaamugisha and Feksi 1988). Compared to studies that utilized a protocol similar to ours, the prevalence of active epilepsy was higher than that measured in Bangladesh, Jamaica (Durkin et al, 1992) and South Africa (Christianson et al, 2000) (Table 5). The prevalences reported in this study are minimum estimates, since the tools used to identify cases detected convulsive seizures and not other type of seizures e.g. the absences. However the high prevalence of lifetime epilepsy may have been confounded by febrile seizures, since the recall as to whether the seizures were associated with febrile illness may be inaccurate.
Table 5. Prevalence estimates for lifetime and active in resource poor counties (per 1000).
| Author | Year of study | Location | Age-group studied (years) |
Lifetime Epilepsy* (95% CI) |
Active Epilepsy (95% CI) |
|---|---|---|---|---|---|
| Durkin et al, 1992 | 1987-1988 | Clarendon, Jamaica |
2-9 | 5.8 (0.0-11.7) |
5.2 (0.0-11.1) |
| Durkin et al, 1992 | 1987-1988 | Karachi, Pakistan | 2-9 | 15.5 (9.6-21.4) |
12.4 (6.6-18.2) |
| Durkin et al, 1992 | 1987-1988 | Bangladesh | 2-9 | 6.5 (2.2-10.6) |
5.8 (1.6-10.1) |
| Christianson et al, 2000 | 2001 | South Africa | 6-9 | 93 ** | 8.3** |
| Mung’ala-Odera et al. | 2001-2002 | Kilifi, Kenya | 6-9 | 41 (31.0-51.0) |
11 (5.0-15.0) |
Includes both active and inactive epilepsy
Confidence limits not presented
Incidence of epilepsy
There are few studies that have attempted to estimate the incidence of epilepsy in Africa. A study of a small population in Tanzania over a 10 year period estimated the incidence of epilepsy in children aged 0-9 years to be 94/100 000 per year (Rwiza et al, 1992). A community based study in Ethiopia, also in children aged 0-9 years, estimated the incidence to be 68/100 000 per year (Tekle-Haimanot et al, 1997) over a period of 3.5 years. In our study, incidence among children aged 6-9 years was 187 (95% CI: 133-256) per 100,000 per year, considerably higher than in these studies. The other studies used different methodologies; in particular there was reliance on the recall of seizures over a longer period of time, which may have under reported the occurrence of seizures and use different approaches to identify individuals with epilepsy at different time points. Furthermore it is unclear how these studies differentiated between epilepsy and febrile seizures, since children under six years were also included in the study. Although our study may have also been affected by the different methods of detecting epilepsy used in the two surveys, the incidence figure is likely to be a minimum estimate, suggesting that epilepsy is a considerable burden in these older children, not only from the seizure perspective but also because of additional impairments.
Seizure types and EEG findings
The high proportion of partial becoming secondary generalized seizures is similar to that reported from other studies in Africa (Rwiza et al, 1992;Tekle-Haimanot et al, 1997; Osuntokun, 1978), and suggests a focal aetiology. Most of these studies detected convulsive epilepsies and may have missed partial epilepsies. In this study, the finding of moderate to severe neurological impairments associated with epilepsy supports that there may be identifiable causes. A relatively low percentage (20%) of children with active epilepsy had abnormal EEG findings compared to studies in Tanzania (49%) (Matuja et al, 2001), Ethiopia (43%) (Tekle-Haimanot et al, 1990) and Libya (62%) (Sridharan et al, 1986). These later studies were hospital based and may have selected for more severe cases. However the EEG findings do not support a focal etiology, although further EEGs and neuroimaging may detect more abnormalities.
Risk factors
From the analysis of risk factors, the higher prevalence and incidence in this area could be caused by genetic causes or febrile illnesses. We were not able to investigate the role of antenatal or perinatal events, which have been noted in other studies (Matuja et al, 2001) as a result of relatively small numbers and poor recall (Mung’ala-Odera & Newton, 2001). A family history of seizures was an important risk factor identified in this study, which has been found in earlier studies in this community (Versteeg et al, 2003) and also in studies performed in Tanzania (Matuja et al, 2001) and Ethiopia (Tekle-Haimanot et al, 1990). This may be related to genetic factors, which predispose individuals in a family to epilepsy, although, environmental factors may also contribute. The finding that a seven and three-fold increased risk of lifetime and active epilepsy respectively following febrile seizures highlight the importance of infections as a possible cause of epilepsy. The relationship is however complex with damage following prolonged febrile seizures, acute CNS infections particularly malaria and illnesses provoked by other epilepsies tending to be lumped together in epidemiological studies. Incidence studies in this area have established rates of 190 and 202/100 000 child years for cerebral malaria (Snow et al 1993) and bacterial meningitis respectively (Mwangi et al 2002). Infections have also been reported in other studies of epilepsy from Africa (Annegers et al, 1979; Leviton & Cowan,1982; Ogunniyi et al, 1987; (Matuja et al, 2001), and we have documented an association between admission with severe falciparum malaria and the development of epilepsy (Carter et al, 2004).
Conclusion
As in many RPCs, most epilepsies in this community still remain untreated. In this population, 89% of the active epilepsy cases had not received any medical treatment. Probable reasons for this include lack of awareness about the availability of AEDs, affordability and individuals’ perception about the cause of epilepsy. In this community, the decision to seek western medical help depends upon whether the parents think the seizures are considered as an illness, or fall within the health sphere (El Sharkawy et al, 2006). Although children with epilepsy do not appear to be ostracized in this community (El Sharkawy et al, 2006), those with active epilepsy are less likely to attend school possibly due to the associated cognitive impairments, and focus group discussion have suggested that their opportunity of marrying or obtaining jobs is impaired (El Sharkawy et al, 2006).
This study has identified a considerable burden of epilepsy in older children in this part of rural Kenya. Genetic, neonatal insults and infections may be important causes, but further studies are required to assess the contribution of these factors. In addition, further examination of the treatment gap should also be performed to improve treatment of children with epilepsy in this area.
Acknowledgements
The Wellcome Trust-UK and Kenya Medical Research Institute supported this study. We thank the mapping, census, and epilepsy field teams who made this study possible. In particular we thank Eddie Chengo, Judy Dzombo, Mary Mwangoma, Francis Yaa, Douglas Konde, Mary Karisa, Francis Kanyetta, Silas Haro, Karen Konde and Janet Chea. We also thank Rachael Odhiambo for the data entry Neil Alexander for statistical advice during the analysis. Prof. Kevin Marsh and Dr. Norbert Peshu for their advice on the study design. This paper is published with the permission of the director of KEMRI. Prof. CRJC Newton holds a Wellcome Trust Career Post in Clinical Tropical Medicine (No. 070114).
References
- Annegers J, Hauser WA, Elveback, Kurland LT. The risk of epilepsy following febrile convulsions. Neurology. 1979;29(3):297–303. doi: 10.1212/wnl.29.3.297. [DOI] [PubMed] [Google Scholar]
- Carter JA, Neville BGR, White S, Ross AJ, Otieno G, Mturi N, Musumba C, Newton CRJC. Increased prevalence of epilepsy associated with severe falciparum malaria in children. Epilepsia. 2004;45(8):978–981. doi: 10.1111/j.0013-9580.2004.65103.x. [DOI] [PubMed] [Google Scholar]
- Christianson A, Zwane ME, Manga P, Rosen E, Venter A, Kromberg JGR. Epilepsy in rural South African children-prevalence, associated disability and management. South African Medical Journal. 2000;90(3):262–266. [PubMed] [Google Scholar]
- Dekker P. A manual for medical and clinical officers in Kenya. . Leiden. SMD Educatieve Uitgevers; Foundation Epilepsy Care Developing Countries: 1994. [Google Scholar]
- Durkin MS, Davidson LL, Hasan ZM, Hauser WA, Khan N, Paul TJ, Shrout PE, Thorburn MJ, Zaman S. Estimates of the prevalence of childhood seizure disorders in communities where professional resources are scarce: results from Bangladesh, Jamaica and Pakistan. Paediatric and Perinatal Epidemiology. 1992;6:166–180. doi: 10.1111/j.1365-3016.1992.tb00758.x. [DOI] [PubMed] [Google Scholar]
- Durkin M, Davidson LL, Desai P, Hasan ZM, Khan N, Shrout PE, Thorburn MJ, Wang W, Zaman SS. Validity of the ten questions screened for childhood disability: results from population-based studies in Bangladesh, Jamaica, and Pakistan [see comments] Epidemiology. 1994;5(3):283–9. [PubMed] [Google Scholar]
- El Sharkawy G, Newton C, Hartley S. Attitudes and practices of families and health care personnel toward children with epilepsy in Kilifi, Kenya. Epilepsy & Behavior. 2006;8:201–212. doi: 10.1016/j.yebeh.2005.09.011. [DOI] [PubMed] [Google Scholar]
- ILAE Guidelines for Epidemiologic studies on Epilepsy. Epilepsia. 1993;34(4):592–596. doi: 10.1111/j.1528-1157.1993.tb00433.x. [DOI] [PubMed] [Google Scholar]
- Kaamugisha J, Feksi A. Determining the prevalence of epilepsy in the semi-urban population of Nakuru, Kenya, comparing two independent methods not apparently used before in epilepsy studies. Neuroepidemiology. 1988;7:115–121. doi: 10.1159/000110144. [DOI] [PubMed] [Google Scholar]
- Last J, Abramson J, editors. A Dictionary of Epidemiology. Oxford University Press; New York: 1995. [Google Scholar]
- Leviton A, Cowan L. Epidemiology of seizure disorders in children. Neuroepidemiology. 1982;1:40–83. [Google Scholar]
- Matuja W, Kilonzo G, Mbena P, Mwang’ombola RL, Wong P, Goodfellow P, Jillek-Aall L. Risk factors for epilepsy in a rural area in Tanzania. A community-based case-control study. Neuroepidemiology. 2001;20(4):242–7. doi: 10.1159/000054797. [DOI] [PubMed] [Google Scholar]
- Ministry of Health, G. o. K . Central Nervous System:Clinical Guidelines. Vol. 4. M.O.H Government Printers; 1994. pp. 41–45. [Google Scholar]
- Mung’ala-Odera V, Newton C. Recall of perinatal events by mothers living in rural Kenya. Epidemiology. 2001;12:366. doi: 10.1097/00001648-200105000-00021. [DOI] [PubMed] [Google Scholar]
- Mung’ala-Odera V, Meehan R, Njuguna P, Mturi N, Alcock K, Carter JA, Newton CRJC. Validity and reliability of the ‘Ten Questions’ questionnaire for detecting moderate to severe neurological impairment in children aged 6-9 years in rural Kenya. Neuroepidemiology. 2004;23(1-2):67–72. doi: 10.1159/000073977. [DOI] [PubMed] [Google Scholar]
- Mung’ala-Odera V, Meehan R, Njuguna P, Mturi N, Alcock K, Newton CRJC. Prevalence and risk factors of neurological disability and impairment in children living in rural Kenya. International Journal of Epidemiology. 2006;(35):683–688. doi: 10.1093/ije/dyl023. [DOI] [PubMed] [Google Scholar]
- Mwangi I, Berkley J, Lowe B, Peshu N, Marsh K, Newton CRJC. Acute bacterial meningitis in children admitted to a rural Kenyan hospital: increasing antibiotic resistance and outcome. Pediatric Infectious Disease Journal. 2002;21(11):1042–1048. doi: 10.1097/00006454-200211000-00013. [DOI] [PubMed] [Google Scholar]
- Ogunniyi A, Osuntokun BO, Bademosi O, Adeuja AOG, Schonberg BS. Risk factors for Epilepsy: Case-control study in Nigeria. Epilepsia. 1987;28(3):280–85. doi: 10.1111/j.1528-1157.1987.tb04219.x. [DOI] [PubMed] [Google Scholar]
- Osuntokun BO. Epidemiology of Epilepsy in Developing Countries in Africa. Tropical Geographic Medicine. 1978;30(1):23–32. [PubMed] [Google Scholar]
- Preux P, Druet-Cabanac M. Epidemiology and aetiology of epilepsy in sub-Saharan Africa. Review. Lancet Neurology. 2005;4(1):21–31. doi: 10.1016/S1474-4422(04)00963-9. [DOI] [PubMed] [Google Scholar]
- Rwiza H, Kilonzo G, Haule J, Matuja WBP, Mteza I, Mbena P, Kilima PM, Mwaluku G, Mwang’ombola R, Mwaijande F, Rweyemamu G, Mwatowo A, Jillek-All LM. Prevalence and incidence of epilepsy in Ulanga, a Rural Tanzanian District: A community-based study. Epilepsia. 1992;33(6):1051–1056. doi: 10.1111/j.1528-1157.1992.tb01758.x. [DOI] [PubMed] [Google Scholar]
- Shrout EP, Newman SC. Design of two-phase prevalence surveys of rare disorders. Biometrics. 1989;45:549–555. [PubMed] [Google Scholar]
- Snow RW, Armstrong Schellenberg JRM, Peshu N, Forster D, Newton CRJC, Winstanley PA, Mwangi I, Waruiru C, Warn PA, Newbold C, Marsh K. Periodicity and space-time clustering of severe childhood malaria on the coast of Kenya. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1993;87:386–390. doi: 10.1016/0035-9203(93)90007-d. [DOI] [PubMed] [Google Scholar]
- Snow RW, Williams REM, Rogers JE, Mung’ala VO, Peshu N. The prevalence of epilepsy among a rural Kenyan population: Its association with premature mortality. Tropical and Geographical Medicine. 1994;46(3):175–179. [PubMed] [Google Scholar]
- Sridharan RK, Radhakrishnan K, Ashok PP, Mousa ME. Epidemiological and Clinical study of epilepsy in Benghazi, Libya. Epilepsia. 1986;27(1):60–5. doi: 10.1111/j.1528-1157.1986.tb03502.x. [DOI] [PubMed] [Google Scholar]
- Tekle-Haimanot R, Forsgren L, Abebe M, Gebre-Mariam A, Heijbel J, Holmgren G, Ekstedt J. Clinical and electroencephalographic characteristics of epilepsy in rural Ethiopia: a community-based study. Epilepsy Research. 1990;7(3):230–9. doi: 10.1016/0920-1211(90)90020-v. [DOI] [PubMed] [Google Scholar]
- Tekle-Haimanot R, Forsgren L, Ekstedt J. Incidence of Epilepsy in rural central Ethiopia. Epilepsia. 1997;38(5):541–546. doi: 10.1111/j.1528-1157.1997.tb01138.x. [DOI] [PubMed] [Google Scholar]
- Versteeg A, Carter J, Dzombo J, Neville BG, Newton CR. Seizure disorders among relatives of Kenyan children with severe falciparum malaria. Trop Med Int Health. 2003;8(8):12–6. doi: 10.1046/j.1365-3156.2003.00965.x. [DOI] [PubMed] [Google Scholar]
- WHO . The World Health Report: 2001: Mental health: new understanding, new hope. WHO; Geneva: 2001. [Google Scholar]
- World Bank . Investing in Health. World Development Indicators. World Bank; New York: 1993. [Google Scholar]