Abstract
Background
Multiple micronutrient deficiencies are common among women in low-income countries and may adversely affect pregnancy outcomes Objective To conduct a meta-analysis of the effects on stillbirths, early and late neonatal mortality of multiple micronutrient (MMN) compared with iron and folic acid (Fe + FA)supplementation during pregnancy in recent randomised controlled trials.
Methods
Twelve randomised controlled trials were included in the analysis ( Bangladesh, Burkina Faso, China, Guinea Bissau, Indonesia, Mexico, Nepal, Niger, Pakistan and Zimbabwe), all providing approximately 1 Recommended Dietary Allowance (RDA) of MMN or Fe + FA to presumed HIV-negative women.
Results
MMN supplementation providing approximately 1 RDA of MMN did not decrease the risk of stillbirths (OR 1.01, 95% CI 0.88-1.16), early neonatal mortality (OR 1.23, 95% CI 0.95-1.59), late neonatal mortality (OR 0.94, 95% CI 0.73-1.23), or perinatal mortality (OR 1.11, 95% CI 0.93-1.33)
Conclusion
Our meta-analysis provides consistent evidence that MMN supplementation providing approximately 1 RDA of MMN during pregnancy does not result in any reduction in stillbirths, early or late neonatal deaths compared to FE + FA alone.
Introduction
The World Health Organization recommends universal distribution of Fe + FA supplements to all pregnant women in developing countries [1]. Because many women face additional micronutrient deficits, particularly in pregnancy, repletion of pregnant women with minerals and vitamins has been suggested as a way to further improve birth outcomes in countries with a high burden of under-nutrition [2]. Daily multiple micronutrients (MMN) during pregnancy have been shown to reduce the number of low birth weight and small-for-gestational age babies, and maternal anaemia [3]. A recent meta-analysis of MMN supplementation during pregnancy reported a small statistically significant increase in birth weight when compared with Fe + FA supplementation, but there was no effect on the incidence of pre-term birth [4]. Another meta-analysis showed reductions in anaemia and Fe deficiency and several other micronutrient deficiencies with the use of MMN [5].
If MMN affect fetal growth, particularly small-for-gestational-age births [6], a direct beneficial effect on perinatal or neonatal survival can be hypothesised. A systematic review of nine trials did not find a significant difference in perinatal mortality when comparing MMN supplementation with two or less micronutrients, Fe FA supplementation, no supplementation or placebo [3]. However, two trials in Nepal reported non-significant increases in perinatal and neonatal death associated with MMN supplementation compared to women who took only Fe and FA in pregnancy [7]. Although these results have been called into question [8.9], the possibility of adverse outcomes in relation to MMN supplementation certainly warrants further investigation.
At the time of the systematic review conducted by Haider and Bhutta [3], a number of trials of MMN supplementation were still ongoing. The present study updates the review and assesses the effect of supplementing pregnant women with a daily MMN tablet compared with Fe + FA on stillbirths, early and late neonatal mortality, and perinatal mortality.
Methods
Search protocol and study review
Details of the search strategy and study review are reported elsewhere [10]. In brief, findings are reported from randomised controlled trials supplementing pregnant women with daily MMN. For studies with more than one intervention or control arm only one comparison group was selected [10]. Table 1 lists the 12 trials included in this review. In brief, nine studies [10-13,15-19] used the UNICEF/UNU/WHO recommended daily composition of the multi-micronutrient tablet UNIMMAP (United Nations International Multiple Micronutrient Preparation), while the studies in Mexico [22], Nepal-Sarlahi [15] and Zimbabwe [21] used slightly different preparations. The UNIMMAP contains 30 mg Fe, 15 mg zinc, 2 mg copper, 65 μg selenium, 150 μg iodine, 800 ug RE vitamin A, 1.4 mg vitamin B1, 1.4 mg vitamin B2, 400 μg folic acid, 18 mg niacin, 1.9 mg vitamin B6, 2.6 μg vitamin B12, 70 mg vitamin C, 200 IU vitamin D and 10 mg vitamin E. The studies in Nepal-Sarlahi and Mexico used MMN containing 60 mg of Fe and in Zimbabwe Fe + FA were not included in the MMN but were supplied separately as part of routine antenatal care
Table 1.
Characteristics of study populations included in the meta-analyses for stillbirths, early and late neonatal mortality and perinatal mortality
| Country | Multiple micronutri ent regimen |
Control regimen |
Cluster / individual randomisation |
Number of pregnanciesa |
Gestation at supplement startb (median; inter quartile range) |
First pregnancies (%) |
Maternal height (cm) (Mean; SD) |
Maternal baseline body mass index (BMI) (kg/m2) < 18.5 (%) |
|---|---|---|---|---|---|---|---|---|
| Bangladesh11 | UNIMAPP | Fe 30mg, FA 400μg |
Individual | 2412 | 101 (95-108) | 33.7 | 149.8 (5.5) | 28.5 |
| China12 | UNIMAPP | Fe 60mg, FA 400μg |
Cluster | 3037 | 94 (66-126) | 64.2 | 158.8 (5.2) | 10.7 |
|
Indonesia (Indramayu)13 |
UNIMAPP | Fe 60mg, FA 400μg |
Cluster | 1598 | 101 (91-111) | 40.8 | 151.4 (5.1) | 11.4 |
|
Indonesia (Lombok)14 |
UNIMAPP | Fe 30mg, FA 400μg |
Cluster | 28643 | 142 (91-111) | 38.8 | 149.9 (4.9) | 8.3 |
|
Nepal (Sarlahi)15 |
Other | Fe 60mg, FA 400μg, Vitamin A |
Cluster | 1699 | 70 (56-91) | 26.2 | 150.1 (5.4) | 30.6 |
|
Nepal (Janakpur)16 |
UNIMAPP | Fe 60mg, FA 400μg |
Individual | 1139 | 114 (102-128) | 47.6 | 150.8 (5.7) | 27.9 |
| Pakistan17 | UNIMAPP | Fe 60mg, FA 400μg |
Cluster | 1711 | 98 (81-117) | 19.3 | 152.9 (5.9) | 20.4 |
| Burkina Faso18 | UNIMAPP | Fe 60mg, FA 400μg |
Individual | 1211 | 126 (79-166) | 21.5 | 162.2 (5.9) | 11.0 |
| Guinea Bissau19 | UNIMAPP | Fe 60mg, FA 400μg |
Individual | 1091 | 157 (124-190) | 31.4 | 160.5 (5.8) | 5.5 |
| Niger20 | UNIMAPP | Fe 60mg, FA 400μg |
Cluster | 2896 | 77 (56-84) | 19.5 | 158.0 (6.0) | 16.9 |
| Zimbabwe21 | Other | Routine antenatal Fe and FA supplements |
Individual | 715 | 204 (187-223) | 47.4 | 161.5 (5.7) | 3.4 |
| Mexico22 | Other | Fe 60mg, FA 400μg |
Individual | 611 | 65 (54-79) | 64.2 | 148.7 (4.9) | 6.3 |
excluding women known to be HIV positive, women known to have multiple pregnancies, women with fetal loss and women with unknown live/stillborn status at birth. Only one pregnancy was included for each woman. The number of pregnancies in this table are lower than those reported by Margetts et al 9 because we excluded women with fetal loss
days from last menstrual period
The control regimen consisted of 60 mg Fe and 400μg FA except for the studies in Indonesia-Lombok and Bangladesh which used 30 mg of Fe. Women in Zimbabwe received the routine antenatal prescription of Fe + FA. Six trials were cluster randomised and six used individual randomisation.
Statistical methods
From the selected studies, data were extracted on the number of live and still births and on early and late neonatal mortality. The Coding of data for fetal loss, still and live births was accepted from that reported in the data for each study. A stillbirth is defined as the death of a fetus after 28 weeks’ gestation but before delivery of the baby’s head, which is consistent with the definition generally used in developing countries. Early neonatal deaths was defined as death of a live born infant within seven completed days after birth. Late neonatal death refer to death of an infant between 8 and 28 completed days after birth. Perinatal mortality was also reported, pooling stillbirths and early neonatal deaths, because early neonatal deaths may be wrongly registered as stillbirths [23]. Denominators for stillbirths, early and late neonatal mortality and perinatal mortality are all births (live and stillbirths), all live births, all neonates surviving after the first week and all births respectively. Women were excluded if they were known to be HIV positive, to have multiple pregnancies, foetal loss or unknown live/stillborn status at birth. Only one pregnancy was included for each woman.
Statistical analyses and meta-analyses were performed using Stata 10. The overall effect of MMN supplementation on the risk of stillbirths, early neonatal deaths, perinatal deaths and late neonatal deaths was assessed. Crude mortality rates in intervention and control arms, with 95% CI adjusted for clustering where appropriate were also reported. Odds Ratios (OR) with 95% CI were used as a measure of effect of MMN supplementation on risk of mortality. Random effects models were used to calculate the pooled OR for MMN supplementation compared to controls, adjusting for cluster design where appropriate. Results were presented based on analyses using random-effects models because of the different levels of mortality, intervention and control regimens, intervention durations and dietary intake of the study populations. Heterogeneity between studies was measured using the I-square statistic, and was tested for significance using a chi-square test on the Q statistic. Because the review was heavily weighted towards the large Indonesia-Lombok study [14], an priori sensitivity analysis was undertaken by removing this study from the meta-analysis.
Results
Levels of mortality
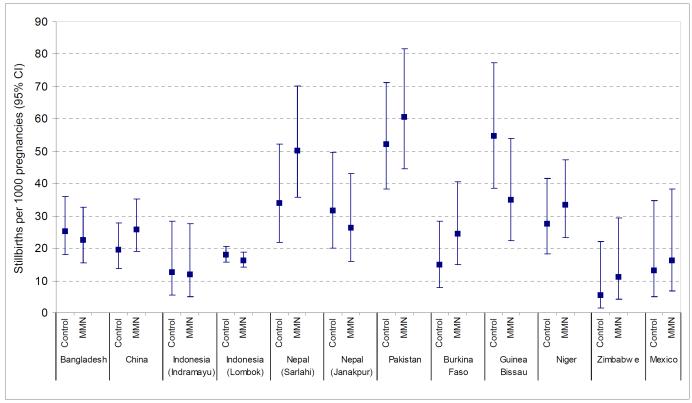
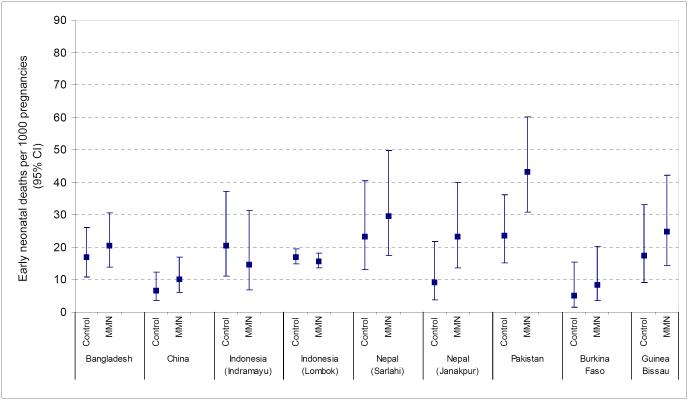
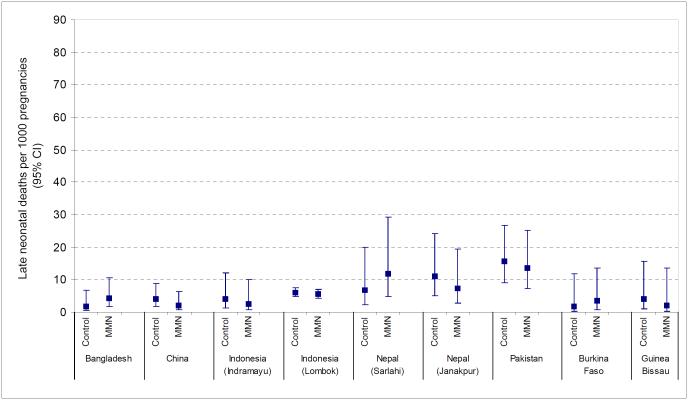
There were marked differences in the rate of stillbirths, early neonatal and late neonatal mortality across studies (Table 2, Figures 1-3). Stillbirth rates in the control arm ranged from 5.6 per 1000 births in Zimbabwe to over 50 per 1000 births in Pakistan and Guinea Bissau. Early neonatal mortality was low in Burkina Faso (5.0 per 1000 live births) but reached a level of 23.5 per 1000 live births in Pakistan. Similarly, for late neonatal mortality the range was between 1.7 and 15.7 deaths per 1000 children surviving the first week in Burkina Faso and Pakistan respectively.
Table 2.
Numbers of pregnancies, live births, stillbirths, early neonatal deaths and late neonatal deaths in Multiple Micronutrient (MMN) and control groups in each study
| Country | Stillbirths | Early neonatal deaths | Late neonatal deaths | |||
|---|---|---|---|---|---|---|
|
Control N pregnancies (n stillbirths) |
MMN N pregnancies (n stillbirths) |
Control N live births (n deaths) |
MMN N live births (n deaths) |
Control N neonates surviving after one week (n deaths) |
MMN N children surviving after one week (n deaths) |
|
| Bangladesh11 | 1218 (31) | 1194 (27) | 1187 (20) | 1167 (24) | 1167 (2) | 1143 (5) |
| China12 | 1529 (30) | 1508 (39) | 1499 (10) | 1469 (15) | 1489 (6) | 1454 (3) |
|
Indonesia
(Indramayu)13 |
792 (13) | 806 (13) | 779 (18) | 793 (14) | 761 (3) | 779 (2) |
|
Indonesia (Lombok)14 |
14170 (259) | 14473 (239) | 13911 (241) | 14234 (228) | 13670 (86) | 14006 (81) |
| Nepal (Sarlahi)15 | 792 (28) | 907 (47) | 764 (20) | 860 (29) | 744 (8) | 831 (15) |
|
Nepal (Janakpur)16 |
568 (18) | 571 (15) | 550 (5) | 556 (13) | 545 (6) | 543 (4) |
| Pakistan17 | 898 (48) | 813 (50) | 850 (20) | 763 (33) | 830 (13) | 730 (10) |
| Burkina Faso18 | 604 (9) | 607 (15) | 595 (3) | 592 (5) | 592 (1) | 587 (2) |
| Guinea Bissau19 | 547 (30) | 544 (19) | 517 (9) | 525 (13) | 508 (2) | 512 (1) |
| Niger20 | 1381 (45) | 1515 (57) | - | - | - | - |
| Zimbabwe21 | 358 (2) | 357 (4) | - | - | - | - |
| Mexico22 | 302 (4) | 309 (5) | - | - | - | - |
Figure 1.
Stillbirths per 1000 births (95% confidence intervals) in Multiple Micronutrient (MMN) and control groups in each study
Figure 2.
Early neonatal deaths per 1000 live births (95% confidence intervals) in Multiple Micronutrient (MMN) and control groups in each study
Figure 3.
Late neonatal deaths per 1000 children surviving the first week of life (95% confidence intervals) in Multiple Micronutrient (MMN) and control groups in each study
Effect of MMN supplementation on stillbirths, early neonatal and perinatal mortality
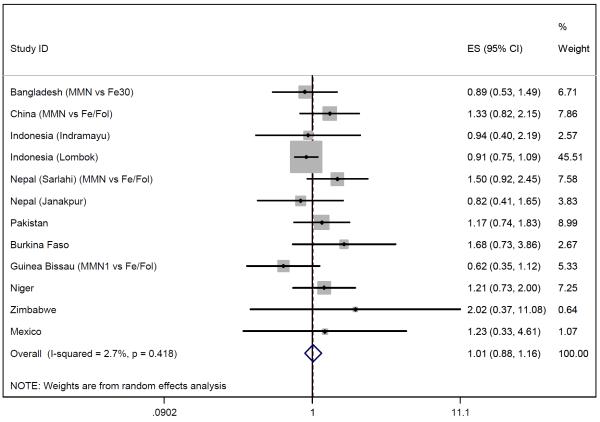
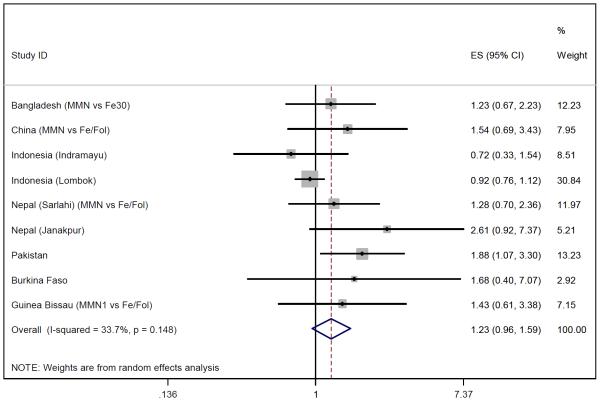
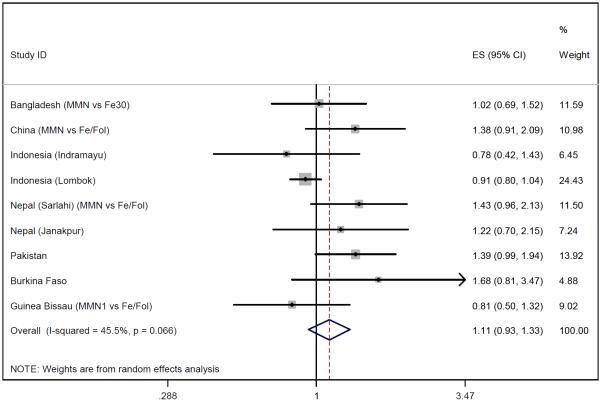
Of the 12 eligible studies, all provided data on stillbirths and nine on early neonatal deaths (Niger, Zimbabwe and Mexico did not). Figures 4-6 show the meta-analysis for stillbirths and early neonatal and perinatal mortality. With a random effects model, MMN supplementation was not associated with stillbirths (OR 1.01, 95% CI 0.88-1.16), but there was a non-significant 23% increase in early neonatal mortality (OR 1.23, 95% CI 0.96-1.59) and a non-significant 11% increase in perinatal mortality (OR 1.11, 95% CI 0.93-1.33). Between-study heterogeneity was moderate for perinatal mortality (I2=46%, p=0.07) and early neonatal mortality (I2=34%, p=0.15) but low for stillbirths (I2=3%, p=0.42). Excluding the large Indonesia-Lombok study increased all relative risk estimates. The subsequent OR of stillbirths, early neonatal and perinatal mortality were 1.12 (95% CI 0.93-1.34), 1.40 (95% CI 1.08-1.82) and 1.20 (95% CI 1.01-1.42) respectively.
Figure 4.
Random effects model forest plots for effects of MMN supplementation on stillbirths
Figure 5.
Random effects model forest plots for effects of MMN supplementation on early neonatal deaths
Figure 6.
Random effects model forest plots for effects of MMN supplementation on perinatal mortality
Late neonatal mortality
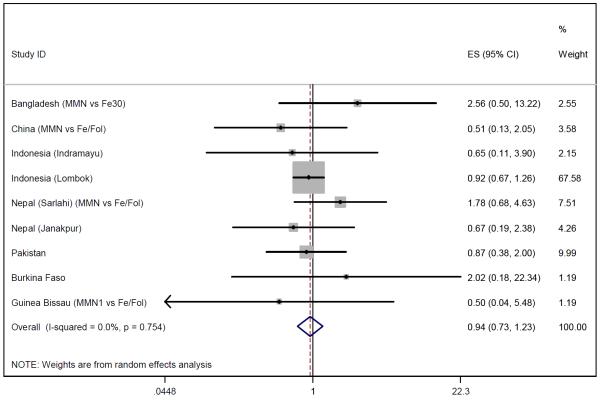
Data on late neonatal mortality were available for eight studies which included 22,396 children whose mothers received multiple micronutrient supplementation and 22,003 children in the control arm. The pooled data showed a non-significant 6% reduction in late neonatal mortality (OR 0.94, 95% CI 0.73-1.23) (Figure 7). Heterogeneity was low (I2=0%, p=0.75) and sensitivity analysis altered the odds ratio slightly (OR=1.01, 95% CI 0.64-1.60).
Figure 7.
Random effects model forest plots for effects of MMN supplementation on late neonatal mortality
Discussion
This meta-analysis provides coherent evidence that MMN supplementation does not result in any reduction in stillbirths or early and late neonatal deaths. However, after removing the large Indonesian study from the analysis the data were consistent with an increased risk in early neonatal and perinatal mortality in women taking MMN supplements during pregnancy compared to Fe + FA.
The lack of an effect of MMN supplements on perinatal or neonatal survival – and the possibility of increased early neonatal and perinatal mortality in women taking MMN supplements in some settings - needs to be scrutinised very carefully. This finding is surprising given that MMN supplementation does result in small increases in birthweight [4].
It was expected that there would be a direct beneficial effect on early neonatal and perinatal survival. Increased survival of infants who would otherwise have died in utero is unlikely since MMN was not associated with preterm birth [4] or stillbirth. It could be argued that newborns in the control arm had enhanced survival because most mothers in the control arm received higher amounts of Fe than the MMN group. There is no evidence however that routine Fe supplementation in pregnancy improves health outcomes for babies [24]. Huffman and colleagues [8] suggested that the earlier Nepal findings might be due to a misclassification of stillbirths into neonatal deaths. It was for that reason that perinatal mortality (pooled stillbirths and early neonatal deaths) was assessed, rather than reporting only on neonatal mortality. Increased asphyxia of children born at the upper end of the birth weight distribution [7] may partly explain the adverse effects of MMN supplementation on perinatal mortality. In Nepal, children whose mothers had received MMN were reported to have a 60% higher risk of birth asphyxia [32].
Meta-analyses have inherent limitations. First, the sample size of the trials included in this analysis varied, and the results were more likely to be affected by the trials with larger sample sizes. However, the only study powered to examine effects on mortality – the Indonesia Lombok study - also failed to find a statistically significant effect of MMN supplementation on perinatal or neonatal survival [14]. Second, there was significant heterogeneity among studies for perinatal mortality, though the source of this heterogeneity is difficult to ascertain. The study populations differed substantially in terms of nutritional status, reproductive history and timing and duration of MMN, all of which may affect the effect of MMN on perinatal mortality. Effects of supplements that include one RDA have been found to vary in HIV infected and uninfected pregnant women in Tanzania [25], suggesting that the burden of infectious diseases may affect nutritional requirements and the size of effect of MMN.
Intervention trials have now expanded the range of endpoints to include neonatal morbidity [32], mortality after the first month of life [14.26], infant growth and development and health outcomes later in childhood [25]. In Lombok, Indonesia, early infant mortality after the first month of life was 30% lower in the MMN group compared to the Fe + FA group [14]. In Nepal, children whose mothers had taken MMN during pregnancy were heavier and of greater body size at the age of 2.5 years than those born to mothers who had only received Fe + FA [27]. In another study in Nepal, MMN supplementation did not improve symptoms of neonatal morbidity, and reported birth asphyxia was higher in the MMN group [32]. In the present analysis, data were not available on survival beyond one month and it was not possible to expand the meta-analysis beyond the neonatal period.
It has been suggested that higher doses of MMN may be required in malnourished populations [25]. However, the one study involving a supplement containing twice the RDA of vitamin E and 6 to 10 times the RDAs for several B vitamins and vitamin C in HIV-uninfected pregnant women in Tanzania, and which was powered for effects on fetal death, also had no effect on stillbirths or perinatal mortality [25]. It may be that MMN supplementation before conception rather than during pregnancy is the way forward. Periconceptional folic acid supplementation, for example, reduces the incidence of neural tube defects [27], and there is growing evidence that maternal undernutrition in the periconceptional period increases the risk of pre-term delivery [29,30], and reduces fetal growth even if nutrition improves later in pregnancy. Future research should include studies of maternal nutrient repletion before pregnancy.
What are the implications of our findings for public health? Any suggestion of harm must be carefully weighed against evidence of potential gain [9]. Improving micronutrient intake during pregnancy contributes to reduced micronutrient deficiencies in mothers and small increases in birth weight, and there is some evidence that infant survival after the first month may be improved. However, MMN supplementation in pregnancy does not lead to a gain in perinatal survival in the pooled results of 11 studies of women receiving MMN supplementation, and MMN may increase the risk of birth asphyxia. Three quarters of neonatal deaths happen in the first week of life, and the burden of mortality in the early neonatal period is much greater than that in later infancy. There has also been least progress in reducing deaths during the perinatal period [31]. Given the limited resources available for public health in poor countries, attention should focus on interventions that are known to positively reduce the burden of stillbirths and early neonatal mortality. On current evidence, MMN supplementation started in mid-pregnancy is not one of them. Success in reducing perinatal deaths is possible through outreach and community care, including health education to improve home-care practices, to create demand for skilled care and to improve care seeking. Expansion of skilled care for babies and mothers is essential to achieve the reduction in neonatal deaths needed to meet the Millennium Development Goal for child survival.
Acknowledgments
We thank Sarah Thomas and Shabbar Jaffar for helpful advice on the meta-analysis
Support for this paper came from the United Nations Children’s Fund (UNICEF) and the United Nations System Standing Committee on Nutrition (SCN).
References
- 1.Mother-baby package: implementing Safe Motherhood in Countries. World Health Organization; WHO/FHE/MSM/94.11, 1994. [Google Scholar]
- 2.Bhutta ZA, Ahmed T, Black RE, Cousens S, Dewey K, Giugliani E, Haider BA, Kirkwood B, Morris SS, Sachdev HPS, Shekar M, for the Maternal and Child Undernutrition Study Group Lancet. 2008;371:417–440. doi: 10.1016/S0140-6736(07)61693-6. [DOI] [PubMed] [Google Scholar]
- 3.Haider BA, Bhutta ZA. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database of Systematic Reviews. 2006;4 doi: 10.1002/14651858.CD004905.pub5. CD004905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fall C, Fisher D, Osmond C, Margetts B, the Maternal Micronutrient Supplementation Study Group Multiple micronutrient supplementation during pregnancy in low-income countries; a meta-analysis of effects on birth size and length of gestation. Food Nutr Bull. doi: 10.1177/15648265090304S408. (this volume) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen L, Peerson JM. Impact of multiple micronutrient versus iron-folic acid supplements on maternal anaemia and micronutrient status in pregnancy. Food Nutr Bull. doi: 10.1177/15648265090304S407. (this volume) [DOI] [PubMed] [Google Scholar]
- 6.Osrin D, Viadya A, Shrestha Y, et al. Effects of antenatal multiple micronutrient supplementation on birthweight and gestational duration in Nepal: double-blind, randomized controlled trial. Lancet. 2005;365:955–962. doi: 10.1016/S0140-6736(05)71084-9. [DOI] [PubMed] [Google Scholar]
- 7.Christian P, Osrin D, Manandhar DS, Khatry SK, Costello AM, West KP. Antenatal micronutrient supplements in Nepal. Lancet. 2005;366:711. doi: 10.1016/S0140-6736(05)67166-8. [DOI] [PubMed] [Google Scholar]
- 8.Huffman SL, Habicht JP, Scrimshaw N. Micronutrient supplementation in pregnancy. Lancet. 2005;366:2001. doi: 10.1016/S0140-6736(05)67807-5. [DOI] [PubMed] [Google Scholar]
- 9.Shrimpton R, Dalmiya N, Danton-Hill I, Gross R. Micronutrient supplementation in pregnancy. Lancet. 2005;366:2001–2. doi: 10.1016/S0140-6736(05)67808-7. [DOI] [PubMed] [Google Scholar]
- 10.Margetts B, Fall CHD, Ronsmans C, Allen L, the Maternal Micronutrient Supplementation Study Group Multiple Micronutrient supplementation during pregnancy in low-income countries: review of methods and study characteristics for studies included in the meta-analyses. Food Nutr Bull. doi: 10.1177/15648265090304S406. (this volume) [DOI] [PubMed] [Google Scholar]
- 11.Tofail F, Persson LA, El Arifeen S, et al. Effects of prenatal food and micronutrient supplementation on infant development: a randomized trial from the Maternal and Infant Nutrition Interventions, Matlab (MINIMat) study. Am J Clin Nutr. 2008;87:704–11. doi: 10.1093/ajcn/87.3.704. [DOI] [PubMed] [Google Scholar]
- 12.Zeng L, Dibley M, Cheng Y, et al. Impact of micronutrient supplementation during pregnancy on birth weight, duration of gestation and perinatal mortality in rural western China: double-blind cluster randomised controlled. BMJ. 2008 doi: 10.1136/bmj.a2001. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sunawang, Budi Utomo, Adi Hidayat, Kusharisupeni, Subarkah Preventing low birth weight through maternal multiple micronutrient supplementation: A cluster-randomized controlled trial in Indramayu, West Java. Food Nutr Bull. doi: 10.1177/15648265090304S403. (this volume) [DOI] [PubMed] [Google Scholar]
- 14.Shankar A, Multiple Micronutrients Intervention Trial (SUMMIT) Study Group Effect of maternal multiple micronutrient supplementation on fetal loss and infant death in Indonesia: a double-blind cluster-randomised trial. Lancet. 2008;371:215–27. doi: 10.1016/S0140-6736(08)60133-6. [DOI] [PubMed] [Google Scholar]
- 15.Christian P, West K, Khatry SK, et al. Effects of maternal micronutrient supplementation on fetal loss and infant mortality:a cluster-randomized trial in Nepal. Am J Clin Nutr. 2003;78:1194–1202. doi: 10.1093/ajcn/78.6.1194. [DOI] [PubMed] [Google Scholar]
- 16.Osrin D, Vaidya A, Shrestha Y, et al. Effects of antenatal multiple micronutrient supplementation on birthweight and gestational duration in Nepal: double-blind, randomised controlled trial. Lancet. 2005;365:955–62. doi: 10.1016/S0140-6736(05)71084-9. [DOI] [PubMed] [Google Scholar]
- 17.Bhutta ZA, Rizvi A, Raza F, et al. A comparative evaluation of multiple micronutrient and iron-folate supplementation during pregnancy in Pakistan: impact on pregnancy outcomes. Food Nutr Bull. doi: 10.1177/15648265090304S404. (this volume) [DOI] [PubMed] [Google Scholar]
- 18.Roberfroid D, Huybregts L, Lanou H, et al. for the MISAME study group Effects of maternal multiple micronutrient supplementation on fetal growth: a double-blind, randomised controlled trial in rural Burkina Faso. Am J Clin Nutr. 2008;88:1330–40. doi: 10.3945/ajcn.2008.26296. [DOI] [PubMed] [Google Scholar]
- 19.Kæstel P, Michaelsen KF, Aaby P, Friis H. Effects of prenatal micronutrient supplements on birth weight and perinatal mortality: a randomised, controlled trial in Guinea-Bissau. Eur J Clin Nutr. 2005;59:1081–9. doi: 10.1038/sj.ejcn.1602215. [DOI] [PubMed] [Google Scholar]
- 20.Zagre NM, Desplats G, Adou P, Mamadoultaibou A, Aguayo VM. Prenatal multiple micronutrient supplementation has greater impact on birthweight than supplementation with iron and folic acid: a cluster randomised, double-blind, controlled programmatic study in rural Niger. Food Nutr Bull. 2007;28:317–327. doi: 10.1177/156482650702800308. [DOI] [PubMed] [Google Scholar]
- 21.Friis H, Gomo E, Nyazema N, et al. Effect of multimicronutrient supplementation on gestational length and birth size: a randomized, placebo-controlled, double-blind effectiveness trial in Zimbabwe. Am J Clin Nutr. 2004;80:178–84. doi: 10.1093/ajcn/80.1.178. [DOI] [PubMed] [Google Scholar]
- 22.Ramakrishnan U, Gonzalez-Cossio T, Neufeld LM, Rivera J, Martorell R. Multiple micronutrient supplementation during pregnancy does not lead to greater infant birth size than does iron-only supplementation: a randomized controlled trial in a semirural community in Mexico. Am J Clin Nutr. 2003;77:720–5. doi: 10.1093/ajcn/77.3.720. [DOI] [PubMed] [Google Scholar]
- 23.Kramer MS, Liu S. Analysis of perinatal mortality and its components: time for a change? Am J Epidemiol. 2000;156:493–7. doi: 10.1093/aje/kwf077. [DOI] [PubMed] [Google Scholar]
- 24.Pena-Rosas JP, Viteri FE. Effects of routine oral iron supplementation with or without folic acid for women during pregnancy. Cochrane Database of Systematic Reviews. 2006;(Issue 3) doi: 10.1002/14651858.CD004736.pub2. [DOI] [PubMed] [Google Scholar]
- 25.Fawzi FW, Msamanga GI, Urassa W, Hertzmark E, Petraro P, Willett WC, Spiegelman D. Vitamins and Perinatal Outcomes among HIV-Negative Women in Tanzania. New Engl J Med. 2007;356:1423–1431. doi: 10.1056/NEJMoa064868. [DOI] [PubMed] [Google Scholar]
- 26.West KP, Christian P. Antenatal micronutrients in undernourished people. Lancet. 2008;371:452–453. doi: 10.1016/S0140-6736(08)60214-7. [DOI] [PubMed] [Google Scholar]
- 27.Vaidya A, Saville N, Shrestha BP, Costello AM, Manandhar DS, Osrin D. Effects of antenatal multiple micronutrient supplementation on children’s weight and size at 2 years of age in Nepal: follow-up of double-blind randomised controlled trial. Lancet. 2008;371:492–499. doi: 10.1016/S0140-6736(08)60172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lumley J, Watson L, Watson M, Bower C. Periconceptional supplementation with folate and/or multivitamins for preventing neural tube defects. Cochrane Database Syst Rev. 2001 doi: 10.1002/14651858.CD001056. CD001056. [DOI] [PubMed] [Google Scholar]
- 29.Oliver MH, Jaqueiry AL, Bloomfield FH, Harding JE. The effects of maternal nutrition around the time of conception on the health of the offspring. Soc Reprod Fertil Suppl. 2007;64:397–410. doi: 10.5661/rdr-vi-397. [DOI] [PubMed] [Google Scholar]
- 30.Rayco-Solon P, Fulford AJ, Prentice AM. Maternal preconceptional weight and gestation length. Am J Obstet Gynecol. 2005;192:1133–6. doi: 10.1016/j.ajog.2004.10.636. [DOI] [PubMed] [Google Scholar]
- 31.Lawn JE, Cousens S, Zupan J, for the Lancet Neonatal Survival Steering Team Lancet. 2005;365:891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- 32.Christian P, Darmstadt GL, Wu L, et al. The effect of maternal micronutrient supplementation on early neonatal morbidity in rural Nepal: a randomised, controlled, community trial. Arch Dis Childhood. 2008;93:660–664. doi: 10.1136/adc.2006.114009. [DOI] [PubMed] [Google Scholar]