Abstract
Aim
Several studies have suggested a beneficial effect of infant breast-feeding on childhood cognitive function. Our main objective was to examine whether duration of breast-feeding and age at introduction of complementary foods are related to cognitive performance in 9-10 year old school going children in South-India.
Methods
We examined 514 children from the Mysore Parthenon birth cohort for whom breast-feeding duration (6 categories from <3 to ≥18 months) and age at introduction of complementary foods (4 categories from <4 to ≥6 months) were collected at the 1st, 2nd and 3rd year annual follow-up visits. Their cognitive function was assessed at a mean age of 9.7 years using 3 core tests from the Kaufman Assessment Battery for children and additional tests measuring long-term retrieval/storage, attention and concentration, visuo-spatial and verbal abilities.
Results
All the children were initially breast-fed. The mode for duration of breast-feeding was 12-17 months (45.7%) and for age at introduction of complementary foods 4 months (37.1%). There were no associations between longer duration of breast-feeding, or age of introduction of complementary foods, and cognitive function at 9-10 years, either unadjusted or after adjustment for age, sex, gestation, birth size, maternal age, parity, socio-economic status, parents’ attained schooling, and rural/urban residence.
Conclusions
Within this cohort, in which prolonged breast-feeding was the norm (90% breast-fed ≥6 months and 65% breast-fed for ≥12 months), there was no evidence suggesting a beneficial effect of longer duration of breast-feeding on later cognitive ability.
Keywords: Breast-feeding, Complementary foods, Children, Cognitive performance, India
Introduction
Several studies, summarised in three systematic reviews and a meta-analysis have concluded that children who were breast-fed rather than formula-fed in infancy have a small but significant advantage in cognitive ability, ranging from 2-8 developmental quotient points.[1-4] Some studies also reported a ‘dose-response’ effect of duration of breast-feeding on cognitive ability.[5-8] These effects have been attributed to breast milk constituents (long-chain polyunsaturated fatty acids required for brain development), and/or environmental factors (better mother-baby bonding and sensory stimulation in breast-fed infants.[1,9-10]
Evidence for a beneficial effect of breast-feeding on cognitive development, comes mainly from observational studies in children,[1-9,10-12] including those born small-for-gestational-age (SGA) and/or very-low-birthweight),[ 5,10] adults,[13] and elderly individuals.[14] Recently, a randomised trial of a population-based breast-feeding promotion programme reported a significant benefit of the intervention on childhood cognitive ability.[15] However, some observational studies, and another randomized controlled trial among preterm infants, found no associations between breast-feeding and later cognitive ability.[16-23]
Parents’ socio-economic status (SES) and intelligence/education are strongly related to childhood cognitive performance. In high-income countries these factors are also related to initiation and duration of breast-feeding.[24] and may confound the association of breast-feeding with cognitive function through post-natal nutrition, stimulation, growth and development. Therefore, adjustment for these potential confounders is important to assess the role of breast-feeding on cognitive performance. Data from developing countries are very few,[6,8] but may be helpful in addressing the confounding effects, since, in these populations, breast-feeding may be unrelated to, or inversely related to, maternal SES and education. In India, a recent survey reported that early termination of breast-feeding was associated with higher maternal SES and education.[25] Apart from breast-feeding, data examining associations between the age at introduction of complementary foods in infancy and later cognitive ability are scarce.
We have used data from the Mysore Parthenon Study[26,27] to examine whether duration of breast-feeding and age at introduction of complementary foods are associated with cognitive ability, independent of socio-demographic factors, among 9-10 year old South Indian children.
Methods
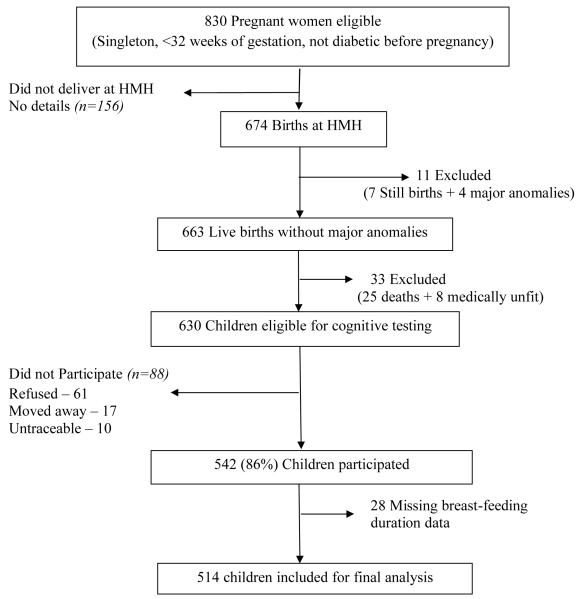
The Mysore Parthenon study, a prospective birth cohort study, was initiated in 1997-1998 mainly to examine the incidence and determinants of gestational diabetes in India and its short and long-term effects in the offspring.[26,27] Eight hundred and thirty women attending the antenatal clinic at the Holdsworth Memorial Hospital (HMH), Mysore, South-India participated in the study. Of them, 674 (81%) delivered their babies at HMH. Excluding 7 still born babies, and 4 with major congenital anomalies, newborn anthropometry was performed on 663 babies as reported previously.[26,27] Excluding 25 deaths and 8 with major medical problems 630 children were followed-up, with repeat anthropometry, annually till the age of 5 and every 6 months thereafter.
At one, two and three years of follow-up, information on infant feeding was obtained by asking mothers the same set of questions: How was the baby fed from birth (breast, bottle, breast+bottle or other)?; If breast-fed, was the baby still being breast-fed?; If no longer breast-fed, what was the age (months) at which breast-feeding stopped? In addition, at one year follow-up, mothers were asked the age (months) at which their baby started taking solid foods regularly. We did not collect data on duration of exclusive breast-feeding, nor on complementary foods other than solid foods. Breast-feeding and age at starting complementary foods data were available for 568 and 482 children respectively.
At 9-10 years of age (September 2007-May 2008) children were invited for assessment of their cognitive function. Of the 630 children, excluding 88 children (61-unwilling, 17-moved away from Mysore and 10-untraceable; of the 88 only 54 had breast-feeding data) 542 (86%) underwent cognitive testing. The current analysis is restricted to 514 children (249-boys and 265-girls) with complete breast-feeding data and cognitive outcomes (Figure 1).
Figure 1.
Flow chart of the study participants.
Cognitive tests
The cognitive measures consisted of a series of neuropsychological tests applicable for use in school-aged children related to specific cognitive domains (memory, attention, fluid reasoning) consistent with the Carroll model.[28] They included 3 core tests from the Kaufman Assessment Battery for children-second edition, 2004 (KABC-II)[29] and additional tests[30-32] that underwent an extensive adaptation process to ensure their applicability in the local cultural context.[33] These tests are described in Table 1 and covered the domains of short-term memory, long-term memory and retrieval ability, visuo-spatial ability and language production. These tests were administered at HMH research unit in separate rooms free from distraction by 2 trained masters’ level child psychologists (unaware of the children’s breast-feeding status) in the local Kannada language, to each child in a single session of 60-90 minutes. Intra class correlation coefficients for agreement between the 2 psychologists were 0.89 (intra-rater agreement) and 0.90 (inter-rater agreement).
Table 1.
Description of the cognitive tests used in the study
|
Tests from KABC-II [29]
| ||
|---|---|---|
| Name of the test | Description | Cognitive abilities |
| 1. Atlantis | The child is taught nonsense names for fish, plants and shells and is asked to point to the named object among an array of pictures |
Learning ability/long-term storage and retrieval, associative memory |
| 2. Word order | The child points to a series of silhouettes of common objects in the same order as mentioned by the examiner; an interference task (colour naming) is added between the stimulus and the response for the more difficult items |
Memory span, short term memory, working memory |
| 3. Pattern Reasoning | The child completes a pattern by selecting the correct image from a set of 4 to 6 options shown; most stimuli are abstract, geometric shapes and the difficulty of the task increases as the test progresses. |
Reasoning abilities such as induction and deduction and fluid reasoning |
|
| ||
|
Additional tests
| ||
|
4. Verbal fluency[30]
a) Animals b) First names |
The child is asked to name as many animals as possible in 1 minute and then asked to name as many first names as possible in 1 minute. |
Broad retrieval ability; speed and flexibility of verbal thought process; neuropsychological test of language production |
|
5. Kohs block
design[31] |
A psychometric test in which the child arranges groups of 4, 9, or 16 multi-coloured blocks to copy picture designs presented on test cards. |
Visuo-spatial problem solving, visual perception and organization |
| 6. Coding-WISC-II[32] | The child has to substitute specific symbols for numbers presented in boxes, and complete as many items as possible in 2 minutes. |
Visual-motor processing speed and coordination, short term memory, visual perception, visual scanning, cognitive flexibility, attention |
Covariates and confounders
We considered the following as potential covariates and confounders: ‘maternal factors’ (maternal age, parity, BMI and height in pregnancy); ‘infant factors’(gender, gestational age at birth and birthweight); ‘child factors’(current age, BMI and height) and ‘parental factors’(parents’ school attainment, rural/urban residence and current SES, assessed using the Standard of Living Index (SLI) designed by National Family Health Survey-2 which derives a score based on type and size of the house, household sanitary facilities, source of water and power supply, cooking fuel used, ownership of house/property, land, livestock and household assets. In Indian terms, SLI scores of 0–14 indicate a ‘low’ standard of living; scores of 15–24 indicate a ‘medium’ standard; and scores of 25–67 indicate a ‘high’ standard. [34]. Most of the families in our study would be described in India as ‘middle class’ or ‘lower middle class’. 95% of our families had a private water supply direct to the house and 5% obtain water from a public tap/pump/well; 99% of families used electricity as the main source of household lighting; 59% owned a bicycle; 12% owned a car; 83% owned a colour television and 12% a black and white television; and 40% owned a refrigerator. None of the mothers had ever smoked or consumed alcohol.
The HMH Research Ethics Committee approved the study and informed verbal consent was obtained from parents and children.
Statistical Methods
Skewed distributions were examined based on histograms and skewness statistics. For maternal BMI and the child’s Kohs block design score, log transformation was the most appropriate, while for the child’s pattern reasoning score square root transformation provided a better approximation of a normal distribution. All cognitive tests scores were then converted to z-scores which represent the difference from the mean score for each individual and are expressed in units of standard deviations. All the children were initially breast-fed and the main breast-feeding exposure was total duration of breast-feeding in months. Categories for duration of breast-feeding were chosen based on their meaningfulness from a public health perspective, (eg, having a bin for <3 months) and also from a statistical perspective (i.e. having enough subjects in each bin), and to reduce the effect of outliers and maintain ordering, breast-feeding duration was split into 6 categories (<3, 3-5, 6-8, 9-11, 12-17 and 18+ months) and considered as a quantitative variable.. Age at starting complementary foods was split into 4 categories (<4, 4, 5, ≥6 months). Initially associations of breast-feeding duration (6 categories) and age at starting complementary foods (4 categories) with potential confounders and outcomes were analysed using univariable linear regression model. Tests of a departure from linearity were performed using likelihood ratio (LR) tests, and where a departure from linearity was found (indicated by a LR test [p<0.05]) we reported F tests of general association. The associations between breast-feeding duration or age at starting complimentary foods with cognitive outcomes were then analysed using a series of multivariable regression models, to examine whether any association between exposures and cognitive outcomes could be acting through socio-economic and parental factors, infant factors and/or the child’s current size. Stata v10 was used for all analyses.
Results
All the children were initially breast-fed, and very few stopped breast-feeding before the age of 3 months. The mode for duration of breast-feeding was 12-17 months and for age at starting complementary foods 4 months, similar in boys and girls (Table 2). Duration of breast-feeding was similar among children who underwent cognitive testing and children who did not take part in cognitive testing (data not shown). Girls scored better than boys in tests of word order (p=0.03), pattern reasoning (p=0.003), verbal fluency-names (p<0.001) and coding (p<0.001). One percent of mothers were illiterate, approximately 35% had received primary school education; 51% secondary school education, and 13% were graduates or postgraduates/ professionals. Corresponding figures for fathers were 3%, 35%, 40% and 22% respectively. Approximately 74% of the families were from urban areas and 26% from rural areas.
Table 2.
Characteristics of the study cohort
| Boys (n=249) | Girls (n=265) | |
|---|---|---|
| Breast-feeding Categories: No (%) | ||
| < 3months | 6 (2.4) | 6 (2.3) |
| 3-5 months | 23 (9.2) | 17 (6.4) |
| 6-8 months | 27 (10.8) | 22 (8.3) |
| 9-11 months | 32 (12.9) | 45 (17.0) |
| 12-17 months | 107 (43.0) | 128 (48.3) |
| 18+ months | 54 (21.7) | 47 (17.7) |
| Age at starting regular solids: No (%) | ||
| < 4 months | 44 (18.7) | 51 (20.7) |
| 4 months | 82 (34.9) | 97 (39.3) |
| 5 months | 73 (31.1) | 57 (23.1) |
| ≥ 6 months | 36 (15.3) | 42 (17.0) |
| Tests of cognitive function: Mean(SD) | ||
| Atlantis (score) | 67.5 (17.8) | 67.5 (16.8) |
| Word order (score) | 16.1 (2.6) | 16.6 (2.6) |
| Pattern reasoning (score)* | 9 (4, 13) | 11 (6, 14) |
| Verbal fluency (score) – test1 – animals | 11.9 (3.2) | 12.1 (3.4) |
| – test2 – first names | 14.7 (4.0) | 17.5 (5.3) |
| Kohs Block Design (score)* | 77.4 (63.7, 88.2) | 76.6 (63.1, 88.5) |
| Coding-WISC-III (score) | 30.1 (7.6) | 34.9 (7.8) |
| Maternal characteristics at recruitment in pregnancy: Mean(SD) | ||
| Age (years) | 23.9 (4.2) | 23.7 (4.2) |
| Parity (No (%)) – primipara | 121 (48.6) | 141 (53.2) |
| – multipara | 128 (51.4) | 124 (46.8) |
| Height (cm) | 154.0 (5.5) | 154.6 (5.2) |
| BMI (kg/m2)* | 22.9 (21.0, 25.7) | 23.3 (20.9, 26.5) |
| Birth measurements: Mean(SD) | ||
| Birthweight (kg) | 2.910 (0.471) | 2.837 (0.412) |
| Gestational age (weeks) | 39.1 (1.7) | 39.4 (1.5) |
| Preterm (No (%)) | 21 (8.4) | 16 (6.0) |
| Measurements at the time of cognitive testing: Mean(SD) | ||
| Age (years) | 9.7 (0.3) | 9.7 (0.3) |
| Height (cm) | 131.2 (5.5) | 130.3 (5.8) |
| BMI (kg/m2) | 14.6 (1.7) | 14.7 (1.9) |
| Parent’s current socio-economic status | ||
| Standard of living index (score): Mean (SD) | 36.3 (7.8) | 36.4 (8.6) |
| Maternal education (No (%)) | ||
| a) <10 years of education | 102 (41.1) | 83 (31.3) |
| b) 10 years of education | 72 (29) | 87 (32.8) |
| c) >10 years of education | 74 (29.8) | 95 (35.9) |
| Paternal education (No (%)) | ||
| a) <10 years of education | 101 (40.7) | 93 (35.1) |
| b) 10 years of education | 88 (35.5) | 116 (43.8) |
| c) >10 years of education | 59 (23.8) | 56 (21.1) |
| Residence (No (%)) a) Rural | 72 (28.9) | 64 (24.1) |
| b) Urban | 177 (71.1) | 201 (75.9) |
transformed variable; values are median and inter quartile range
Associations of breast-feeding duration with covariates and cognitive outcomes
There were significant non-linear associations between duration of breast-feeding and maternal age at pregnancy, birthweight, the child’s current BMI and urban/rural dwelling (Table 3). Both short and long duration of breast-feeding were associated with higher maternal age, birthweight and the child’s current BMI. Rural mothers tended to breast-feed for longer duration than urban mothers. There were no significant associations between duration of breast-feeding and parental attained schooling or SLI score (Table 3). However, we noted that SLI score rose across the first 4 categories of duration of breast-feeding, and this was statistically significant (p=0.044).
Table 3.
Associations of duration of breast-feeding with covariates and cognitive function scores. Values are Mean (SD) unless otherwise stated.
| Covariates | Breast-feeding duration categories |
||||||
|---|---|---|---|---|---|---|---|
| <3 months n=12 |
3-5 months n=40 |
6-8 months n=49 |
9-11 months n=77 |
12-17 months n=235 |
18+ months n=101 |
p | |
| Maternal factors at pregnancy | |||||||
| Age (years) | 24.7 (5.9) | 24.1 (4.4) | 23.9 (4.0) | 23.2 (4.4) | 23.4 (3.9) | 25.0 (4.6) | 0.04 † |
| Parity: Primipara (No (%)) : Multipara (No (%)) |
5 (1.9) 7 (2.8) |
22 (8.4) 18 (7.1) |
28 (10.7) 21 (8.3) |
42 (16.0) 35 (13.9) |
122 (46.6) 113 (44.8) |
43 (16.4) 58 (23.0) |
0.2 |
| Height (cm) | 152.5 (6.2) | 153.4 (5.8) | 155.4 (4.7) | 154.0 (5.6) | 154.5 (5.2) | 154.2 (5.4) | 0.5 |
| Body mass index (kg/cm2)* | 23.8 (20.2, 26.1) | 22.8 (21.2, 26.8) | 22.5 (21.0, 25.9) | 22.4 (21.0, 25.8) | 23.2 (20.7, 26.2) | 23.9 (21.4, 26.5) | 0.4 |
| Infant factors at birth | |||||||
| Birthweight (kg) | 2.762 (0.294) | 2.966 (0.439) | 2.787 (0.476) | 2.817 (0.379) | 2.845 (0.447) | 2.998 (0.454) | 0.01† |
| Gestational age (weeks) | 38.8 (1.7) | 39.4 (1.3) | 38.9 (2.2) | 39.2 (1.6) | 39.4 (1.6) | 39.3 (1.4) | 0.2 |
| Preterm: Yes (No (%)) : No (No (%)) |
2 (5.41) 10 (2.1) |
1 (2.7) 39 (8.2) |
5 (13.5) 44 (9.2) |
5 (13.5) 72 (15.1) |
17 (46.0) 218 (45.7) |
7 (18.9) 94 (19.7) |
0.8 |
| Children’s factors at the time of cognitive testing | |||||||
| Height (cm) | 128.2 (5.2) | 131.5 (6.2) | 132.2 (5.8) | 129.9 (5.6) | 130.5 (5.4) | 131.3 (6.0) | 0.067 † |
| Body mass index (kg/cm2) | 14.3 (1.5) | 15.2 (2.7) | 14.8 (1.7) | 14.2 (1.9) | 14.5 (1.7) | 14.9 (1.7) | 0.02 † |
| Parents socio-economic status | |||||||
| Standard of living index (score) | 33.3 (7.7) | 35.9 (8.3) | 36.7 (7.7) | 38.1 (9.1) | 36.3 (7.9) | 35.5 (8.6) | 0.8 |
| Maternal education (years) | 8.2 (2.2) | 9.9 (3.5) | 10.5 (3.0) | 9.7 (3.6) | 9.7 (3.3) | 10.0 (3.7) | 0.8 |
| Paternal education (years) | 8.0 (3.3) | 9.8 (4.6) | 9.5 (4.1) | 10.1 (4.4) | 10.1 (4.3) | 9.6 (4.9) | 0.5 |
| Residence: Rural (No (%)) : Urban (No (%)) |
5 (3.7) 7 (1.9) |
3 (2.2) 37 (9.8) |
8 (5.9) 41 (10.9) |
32 (23.5) 45 (11.9) |
69 (50.7) 166 (43.9) |
19 (14.0) 82 (21.7) |
<0.001† |
| Tests of cognitive function | |||||||
| Atlantis (score) | 62.5 (18.1) | 67.8 (21.5) | 68.3 (13.2) | 67.8 (15.3) | 66.9 (18.2) | 68.7 (16.5) | 0.6 |
| Word order (score) | 15.7 (2.9) | 16.4 (2.5) | 16.9 (2.4) | 16.2 (2.5) | 16.2 (2.7) | 16.6 (2.4) | 0.9 |
| Pattern reasoning (score)* | 7.5 (1.5, 10.0) | 9.0 (3.0, 14.0) | 11.0 (7.0, 14.0) | 11.0 (6.0, 14.0) | 10.0 (4.0, 14.0) | 10.0 (5.0, 14.0) | 0.9 |
| Verbal fluency (score) – test1 – animals – test2 – first names |
10.6 (2.6) 14.3 (4.1) |
12.3 (3.2) 16.1 (4.5) |
12.2 (2.9) 15.3 (3.9) |
12.7 (3.7) 16.8 (6.2) |
12.0 (3.2) 16.4 (4.8) |
11.5 (3.3) 15.5 (4.9) |
0.4
0.7 |
| Kohs Block Design (score)* | 71.1 (61.2, 77.6) | 84.7 (68.4, 94.4) | 81.8 (65.7, 89.3) | 80.3 (65.7, 86.8) | 75.0 (63.0, 88.5) | 74.3 (60.3, 87.0) | 0.1 |
| Coding-WISC-III (score) | 29.9 (10.8) | 30.8 (8.7) | 32.8 (6.9) | 31.9 (7.9) | 33.2 (7.8) | 32.6 (8.5) | 0.09 |
Transformed variable; values are median and inter quartile range. P value (assuming a linear trend across breast-feeding duration categories) derived by univariable (unadjusted) linear regression;
If there was significant departure from a linear association (indicated by a likelihood ratio test [p<0.05]) the p value (†) is an F-test of general association.
There were no significant linear or non-linear associations, for any of the cognitive outcomes, with duration of breast-feeding (Table 3). This remained true after adjusting, in a series of models, for all the covariates (Table 4). We also re-checked our results using breast-feeding duration as a continuous rather than categorised variable; the findings were unchanged. We noted that, as for SLI score, cognitive test scores tended to rise across the first four categories of duration of breast-feeding (Table 3); however none of these trends was statistically significant. Since some studies reported stronger associations of breast-feeding duration with later cognitive performance among SGA children (gestation and sex specific birthweight<10th percentile)[5] or low-birthweight children (full-term birthweight<2500 g),[6] we repeated the above analyses limiting the sample to SGA children (gestation and sex specific birthweight<10th percentile)[27], and (separately) to low-birthweight children (full-term (>37weeks) birthweight<2500 g). Once again, there were no significant associations between duration of breast-feeding and cognitive measures in this group.
Table 4.
Associations between duration of breast-feeding, age at introduction of complementary foods and tests of cognitive function: Multiple linear regression analysis
| Cognitive Tests | |||||||
|---|---|---|---|---|---|---|---|
| Atlantis (SD) | Word Order (SD) | Pattern Reasoning (SD) |
Verbal Fluency Animals (SD) |
Verbal Fluency First Names (SD) |
Koh’s block design (SD) |
Coding-WISC-III (SD) |
|
|
|
|||||||
| β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | |
| Breast-feeding duration (n=514) | |||||||
| Model 1 | 0.02 (−0.05, 0.09) | −0.002 (−0.07, 0.07) | −0.01(−0.08, 0.06) | −0.04 (−0.10, 0.03) | 0.004 (−0.06, 0.07) | −0.06 (−0.12, 0.01) | 0.04 (−0.02, 0.10) |
| Model 2 | 0.02 (−0.05, 0.08) | −0.002 (−0.07, 0.06) | −0.01(−0.08, 0.05) | −0.03 (−0.10, 0.03) | 0.004 (−0.06, 0.07) | −0.06 (−0.12, 0.01) | 0.04 (−0.02, 0.10) |
| Model 3 | 0.02 (−0.05, 0.09) | −0.001 (−0.07, 0.07) | −0.01(−0.07, 0.06) | −0.03 (−0.10, 0.03) | 0.005(−0.06, 0.07) | −0.06 (−0.12, 0.01) | 0.04 (−0.02, 0.10) |
| Model 4 | 0.02 (−0.05, 0.08) | −0.001 (−0.07, 0.07) | −0.01(−0.07, 0.05) | −0.03 (−0.10, 0.03) | 0.01 (−0.06, 0.07) | −0.06 (−0.13, 0.01) | 0.04 (−0.02, 0.10) |
| Model 5 | 0.02 (−0.04, 0.09) | 0.0005 (−0.07, 0.07) | −0.01(−0.07, 0.06) | −0.03 (−0.09, 0.04) | 0.01 (−0.06, 0.07) | −0.06 (−0.13, 0.006) | 0.04 (−0.02, 0.10) |
| Age at introduction of complementary foods (n=482) | |||||||
| Model 1 | −0.09 (−0.18, 0.01) | −0.005 (−0.10, 0.09) | −0.04 (−0.13, 0.05) | −0.05 (−0.14, 0.04) | −0.03 (−0.12, 0.06) | −0.08 (−0.18, 0.01) | 0.01 (−0.08, 0.09) |
| Model 2 | −0.06 (−0.15, 0.02) | 0.03 (−0.06, 0.11) | −0.004 (−0.09, 0.08) | −0.03 (−0.12, 0.06) | −0.01 (−0.09, 0.08) | −0.05 (−0.14, 0.04) | 0.03 (−0.06, 0.11) |
| Model 3 | −0.04 (−0.13, 0.04) | 0.04 (−0.05, 0.13) | 0.01 (−0.07, 0.10) | −0.02 (−0.11, 0.07) | −0.005 (−0.09, 0.08) | −0.04 (−0.13, 0.05) | 0.03 (−0.06, 0.11) |
| Model 4 | −0.04 (−0.13, 0.05) | 0.05 (−0.04, 0.13) | 0.02 (−0.07, 0.11) | −0.02 (−0.11, 0.07) | −0.006 (−0.09, 0.08) | −0.03 (−0.12, 0.06) | 0.03 (−0.06, 0.11) |
| Model 5 | −0.04 (−0.12, 0.05) | 0.05 (−0.04, 0.13) | 0.02 (−0.06, 0.11) | −0.02 (−0.11, 0.07) | −0.002 (−0.09, 0.09) | −0.03 (−0.12, 0.06) | 0.03 (−0.06, 0.11) |
Regression co-efficient (β) is the effect size (SD) per category increase in breast feeding (<3, 3-5, 6-8., 9-11, 12-17 and 18+ months), and age at introduction of complementary foods (<4, 4, 5, 6+ months); Model1 adjusted for sex, gestation and child’s age at the time of study; Model 2 adjusted for model 1 parameters + SLI, parent’s education; Model 3 adjusted for model 2 parameters + parity, maternal age at pregnancy, rural/urban residence; Model 4 adjusted for model 3 parameters + child’s birthweight; Model 5 adjusted for model 4 parameters + child’s current body mass index and height.
Associations of age at introduction of complementary foods with covariates and cognitive outcomes
Breast-feeding duration weakly correlated with age at starting complementary foods (Spearman r=0.11; p=0.02). Earlier introduction of complementary foods was associated with higher children’s current BMI, family’s SES and maternal education. Primiparous and urban mothers introduced complementary foods to their infants earlier compared to multiparous and rural mothers (Table 5).
Table 5.
Associations of age at introduction of complementary foods with covariates and cognitive function scores. Values are Mean (SD) unless otherwise stated.
| Covariates | Age at introduction of solid food categories |
||||
|---|---|---|---|---|---|
| <4 months n=95 |
4 months n=179 |
5 months n=130 |
6+ months n=78 |
P | |
| Maternal factors at pregnancy | |||||
| Age (years) | 24.0 (4.8) | 24.2 (4.2) | 23.4 (4.0) | 23.8 (4.3) | 0.3 |
| Parity: Primipara (No (%)) : Multipara (No (%)) |
56 (23.1) 39 (16.3) |
93 (38.3) 86 (36.0) |
61 (25.1) 69 (28.9) |
33 (13.6) 45 (18.8) |
0.02 |
| Height (cm) | 154.2 (5.4) | 154.6 (5.3) | 153.6 (5.2) | 154.5 (5.2) | 0.7 |
| Body mass index (kg/cm2)* | 23.0 (21.0, 26.1) | 23.4 (21.0, 26.5) | 22.0 (20.6, 24.7) | 23.6 (21.5, 26.2) | 0.9 |
| Infant factors at birth | |||||
| Birthweight (kg) | 2.929 (0.394) | 2.901 (0.441) | 2.808 (0.441) | 2.858 (0.492) | 0.09 |
| Gestational age (weeks) | 39.3 (1.5) | 39.3 (1.6) | 39.4 (1.7) | 39.1 (1.7) | 0.7 |
| Preterm: Yes (No (%)) : No (No (%)) |
4 (11.3) 91 (20.4) |
13 (37.1) 166 (37.1) |
12 (34.3) 118 (26.4) |
6 (17.1) 72 (16.1) |
0.3 |
| Children’s factors at the time of cognitive testing | |||||
| Height (cm) | 130.7 (5.7) | 131.1 (5.4) | 129.9 (6.1) | 131.0 (5.2) | 0.7 |
| Body mass index (kg/cm2) | 14.9 (2.3) | 14.7 (1.6) | 14.3 (1.7) | 14.5 (1.7) | 0.050 |
| Parents socio-economic status | |||||
| Standard of living index (score) | 37.3 (7.6) | 37.0 (8.1) | 35.9 (8.4) | 35.4 (8.5) | 0.06 |
| Maternal education (years) | 10.0 (3.7) | 10.4 (3.4) | 9.4 (3.3) | 9.2 (3.3) | 0.02 |
| Paternal education (years) | 9.9 (4.8) | 10.2 (4.1) | 9.8 (4.4) | 9.1 (4.6) | 0.2 |
| Residence: Rural (No (%)) : Urban (No (%)) |
24 (20.5) 71 (19.5) |
32 (27.4) 147 (40.3) |
36 (30.8) 94 (25.8) |
25 (21.4) 53 (14.5) |
0.059 † |
| Tests of cognitive function | |||||
| Atlantis (score) | 67.8(19.6) | 69.8 (15.9) | 66.3 (17.2) | 64.6 (16.3) | 0.07 |
| Word order (score) | 16.0 (3.0) | 16.6 (2.5) | 16.5 (2.7) | 16.0 (1.9) | 0.9 |
| Pattern reasoning (score)* | 10.0 (4.0, 14.0) | 10.0 (6.0, 15.0) | 9.0 (5.0, 13.0) | 9.0 (4.0, 13.0) | 0.3 |
| Verbal fluency (score): test1 – animals : test 2 – first names |
12.5 (3.7) 17.0 (5.0) |
12.0 (3.5) 15.8 (5.2) |
11.9 (2.9) 16.0 (4.7) |
11.9 (3.1) 16.1 (4.3) |
0.3
0.4 |
| Kohs Block Design (score)* | 75.8 (66.3, 89.0) | 82.0 (62.5, 93.8) | 74.0 (61.9, 84.2) | 75.3 (63.7, 86.7) | 0.08 |
| Coding-WISC-III (score) | 31.9 (8.0) | 33.5 (8.1) | 31.6 (7.6) | 32.9 (8.2) | 0.9 |
Transformed variable; values are median and inter quartile range. P value (assuming a linear trend across age of introduction of complementary foods categories) derived by univariable (unadjusted) linear regression;
If there was significant departure from a linear association (indicated by a likelihood ratio test [p<0.05]) the p value (†) is an F-test of general association.
There were no significant associations between the age at starting complementary foods and any of the cognitive measures either unadjusted (Table 5) or adjusted, in a series of models, for all covariates (Table 4).
Discussion
The results of this study from India support the null hypothesis, that there was no association between either duration of breast-feeding, or age at introduction of complementary foods, and cognitive abilities in 9-10 year old South Indian children.
Strengths of the study were that in a large sample of children, we had a battery of cognitive function tests specifically adapted for, and validated in, a South Indian population and collected data on a variety of potential confounders including birthweight, gestational age, maternal age, parity, height and BMI at pregnancy, SES, parents’ education and rural/urban residence. Breast-feeding duration was obtained by maternal recall at one year for 94% of children (of whom more than 60% of the children were still breast-fed making our data reasonably accurate) and for the remaining at two and three years of follow-up. Limitations are that the dataset was relatively homogeneous in terms of breast-feeding (majority of the children were breast-fed for a year or more) and we were unable to differentiate between exclusive and partial breast-feeding, and we did not have information on frequency of breast-feeding, the nutritional quality of breast-milk, or the type and nutritional quality of complementary foods.
To our knowledge, only two studies from developing countries have examined cognitive performance in relation to breast-feeding. One study in the Philippines reported that longer duration (12 months+) of breast-feeding was associated with higher cognitive performance among low-birthweight children aged 8 and 11 years.[6] Another study (in Chile) found a non-linear association, with higher cognitive abilities in 5½ year old children exclusively breast-fed for 2-8 months compared to those breast-fed for <2 months or >8 months.[9] Many observational studies in high-income countries have shown higher cognitive performance among children[4,7,10-12,16-22] and adults[13] who were breast-fed compared to those not breast-fed, and/or breast-fed for a longer duration. Adjusting for SES, parental education, intelligence and other confounders these associations lost their significance in some studies,[16-22] while in some other studies tended to diminish although they remained significant.[7,10-12] It is therefore unclear whether there is a genuine biological effect of breast-feeding upon cognitive development, or whether the association results entirely from confounding, and remains in the latter studies only because of an inability to measure and adjust for all the relevant confounding factors. One possible explanation for the negative findings in our study is that SES was only weakly related to breast-feeding duration in Mysore and confounding was not an issue, thus revealing a genuine lack of effect of breast-feeding on cognitive ability. An alternative explanation is that we failed to detect an effect because of a lack of heterogeneity in breast-feeding duration in our population. A striking difference between the studies in high-income countries and ours was that most infants in the former stopped breast-feeding <6 months,[7,11,12] and ‘longer duration’ could mean anything from 2+ to 8+ months; few of our children were breast-fed for as short a duration as this and 65% were breast-fed for 12 months or more. If the first 6 months of life is a critical period in which breast-feeding can influence cognition, we may have had inadequate power to detect this because almost all our children were breast-fed during that time. It is possible that the nutritional quality of breast-milk is important in this context. We have no data on the docosahexanoic (DHA) and arachidonic acid (AA) (fatty acids important for infant brain development [1]) concentrations in our study. Studies in India reporting fatty acid composition in breast-milk are scarce and only one recent study reported that milk DHA levels in Indian women (consuming predominantly vegetarian diet) were similar to milk DHA levels reported from Western and European women. Levels of plasma DHA and AA in Indian women were lower compared to the levels in American and European women. Maternal plasma omega-3 and omega-6 fatty acids levels were positively associated with their respective levels in milk though there was no direct association between maternal plasma and milk DHA or AA levels.[35] The study suggest that levels of LCPUFA vary between different populations and may be dependent on their dietary intakes. Low levels of these or other nutrients in breast-milk could be another explanation for our negative findings.
Randomized intervention studies are a way of overcoming confounding, but it is impractical to randomise healthy babies to be breast-fed or formula-fed. In a large randomised trial of a breast-feeding promotion programme in Belarus, which led to significant differences in breast-feeding initiation and continuation between intervention and control groups, children in the experimental group had higher test scores of intelligence and teachers’ academic ratings compared to the control group at 6.5 years.[15] However, this study was criticised[36] because the paediatricians who administered the cognitive tests were not blind to the intervention status of the children, although the teacher ratings and results based on audit data were blinded. In another trial among preterm infants, 8 year old children who were breast-fed during infancy had higher test scores for IQ than formula-fed children.[37] However, this trial involved non-randomized comparisons between breast milk fed and formula-fed infants, which might be biased by the socio-biological differences between these groups. The same authors, in a subsequent randomized multicentre study of preterm infants, reported no differences in psychomotor and developmental indices at 18 months between those receiving donor’s breast milk and those fed on nutrient enriched preterm formula.[23] They concluded that considering the lower nutrient value of donor’s breast milk their data add significant support to the opinion that breast milk promotes neurodevelopment.
We found no association between the age at introduction of complementary foods and cognitive performance. Two studies have investigated associations between cognitive function and diet quality in infancy based on dietary history collected from/or after 4 months.[38,39] One study reported that meat consumption from 4-12 and 4-16 months was positively associated with children’s psychomotor but not mental developmental indices up to 24 months of age.[38] The other study reported that 4 year old children who consumed more fruits, vegetables and home-prepared foods at infancy (6 and 12 months) had higher full-scale and verbal intelligence scores, independent of confounding variables.[39] Infancy is a period of rapid brain growth, and it seems likely that nutritive quality of complementary foods, including their timely introduction, is important for cognitive development. More research is required in this area.
To conclude, within this cohort, most of whom were breast-fed for >12 months, we found no evidence that longer duration of breast-feeding promotes cognitive development. We highlight the fact that prolonged breast-feeding was the norm among these children, and since brain development is most rapid in the first 6 post-natal months, it may be that breast-feeding at this time is beneficial but that we were unable to detect an effect since almost all children were breast-fed throughout that period. In support of this, cognitive scores tended to rise across the first 3 categories of duration of breast-feeding (although none of the trends was significant) suggesting that longer breast-feeding up to the age of 8 months may benefit cognitive development. There was no evidence of an association between the age at introduction of complementary foods and cognitive performance. Our study adds to a very small literature on this topic from developing countries. Despite the negative findings in relation to cognitive function, we strongly support WHO guidelines on infant feeding practices (exclusive breast-feeding for 6 months, introduction of nutritious complementary foods from 6 months, and continued breast-feeding up to 2 years), which have been clearly shown to reduce infant infections and mortality, and prevent stunting, in developing country populations.[40]
What is known already on this topic?
In high-income countries, children who were breast-fed rather than formula-fed and breast-fed for a longer duration, tend to score higher on tests of cognitive function.
It is controversial whether this is causal or due to confounding, as higher parental education or socio-economic status influence breast-feeding, and also predict childhood cognitive ability
Data examining associations between the age at introduction of complementary foods and later cognitive ability are scarce.
What this study adds?
This study adds to a very small literature on this topic from low-income countries.
Unlike high-income countries, duration of breast-feeding in this south Indian population was unrelated to parental school attainment and only weakly related to socio-economic status.
In this cohort, in which prolonged breast-feeding was the norm, we found no evidence that longer duration of breast-feeding promotes childhood cognitive development
Acknowledgements
We are grateful to the families who participated in the study and to Dr BDR Paul (former Medical Director). We acknowledge the substantial contribution made to the study by Annamma, Baby, Lalitha, Lalithakala, Savitha, Prathibha, Asha, Jayakumar, Saroja, Geetha, Chachyamma, Stephen, Kiran, Rumana, Jane Pearce and Patsy Coakley.
Funding: Parthenon Trust, Switzerland, Wellcome Trust, UK, Medical Research Council, UK.
Footnotes
Competing Interests: None to declare
Authors consent to publication (copyright) The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its licencees, to permit this article (if accepted) to be published in ADC and any other BMJ Group products and to exploit all subsidiary rights, as set out in our licence (http://adc.bmjjournals.com//ifora/licence.pdf).
References
- 1.Michaelsen KF, Lauritzen L, Mortensen EL. Effects of breast-feeding on cognitive function. Adv Exp Med Biol. 2009;639:199–215. doi: 10.1007/978-1-4020-8749-3_15. [DOI] [PubMed] [Google Scholar]
- 2.Jain A, Concato J, Leventhal JM. How good is the evidence linking breastfeeding and intelligence? Pediatrics. 2002;109:1044–1053. doi: 10.1542/peds.109.6.1044. [DOI] [PubMed] [Google Scholar]
- 3.Drane DL, Logemann JA. A critical evaluation of the evidence on the association between type of infant feeding and cognitive development. Paediatr Perinat Epidemiol. 2000;14:349–356. doi: 10.1046/j.1365-3016.2000.00301.x. [DOI] [PubMed] [Google Scholar]
- 4.Anderson JW, Johnstone BM, Remley DT. Breast-feeding and cognitive development: a meta-analysis. Am J Clin Nutr. 1999;70:525–535. doi: 10.1093/ajcn/70.4.525. [DOI] [PubMed] [Google Scholar]
- 5.Slykerman RF, Thompson JMD, Becroft DMO, et al. Breastfeeding and intelligence of preschool children. Acta Pædiatrica. 2005;94:832–837. doi: 10.1111/j.1651-2227.2005.tb01997.x. [DOI] [PubMed] [Google Scholar]
- 6.Daniels MC, Adair LS. Breast-Feeding Influences Cognitive Development in Filipino Children. J. Nutr. 2005;135:2589–2595. doi: 10.1093/jn/135.11.2589. [DOI] [PubMed] [Google Scholar]
- 7.Gomez-Sanchiz M, Canete R, Rodero I, et al. Influence of breast-feeding and parental intelligence on cognitive development in the 24-month-old child. Clin Pediatr (Phila) 2004;43:753–761. doi: 10.1177/000992280404300811. [DOI] [PubMed] [Google Scholar]
- 8.Clark KM, Castillo M, Calatroni A, et al. Breastfeeding and Mental and Motor Development at 5 ½ Years. Ambul Pediatr. 2006;6:65–71. doi: 10.1016/j.ambp.2005.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uauy R, Peirano P. Breast is best: human milk is the optimal food for brain development. Am J Clin Nutr. 1999;70:433–434. doi: 10.1093/ajcn/70.4.433. [DOI] [PubMed] [Google Scholar]
- 10.Horwood LJ, Darlow BA, Mogridge N. Breast milk feeding and cognitive ability at 7–8 years. Arch Child Fetal Neonatal Ed. 2001;84:23–27. doi: 10.1136/fn.84.1.F23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Angelsen NK, Vik T, Jacobsen G, et al. Breastfeeding and cognitive development at age one and five years. Arch Dis Child. 2001;85:183–188. doi: 10.1136/adc.85.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oddy WH, Kendall GE, Blair E, De Klerk NH, Stanley FJ, Landau LI, Silburn S, Zubrick S. Breast feeding and cognitive development in childhood: a prospective birth cohort study. Paediatr Perinat Epidemiol. 2003;17:81–90. doi: 10.1046/j.1365-3016.2003.00464.x. [DOI] [PubMed] [Google Scholar]
- 13.Richards M, Hardy R, Wadsworth MEJ. Long-term effects of breast-feeding in a national birth cohort: Educational attainment and midlife cognitive function. Public Health Nutr. 2002;5:631–635. doi: 10.1079/PHN2002338. [DOI] [PubMed] [Google Scholar]
- 14.Elwood PC, Pickering J, Gallacher JEJ, et al. Long term effect of breast feeding: cognitive function in the Caerphilly cohort. J Epidemiol Community Health. 2005;59:130–133. doi: 10.1136/jech.2004.022913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kramer MS, Aboud F, Mironova E, et al. Breastfeeding and child cognitive development: new evidence from a large randomized trial. Arch Gen Psychiatry. 2008;65:578–584. doi: 10.1001/archpsyc.65.5.578. [DOI] [PubMed] [Google Scholar]
- 16.Wigg NR, Tong S, McMichael AJ, et al. Does breastfeeding at six months predict cognitive development? Aust N Z J Public Health. 1998;22:232–236. doi: 10.1111/j.1467-842x.1998.tb01179.x. [DOI] [PubMed] [Google Scholar]
- 17.Jacobson SW, Chiodo LM, Jacobson JL. Breastfeeding effects on intelligence quotient in 4- and 11-year old children. Pediatrics. 1999;103(5):e71. doi: 10.1542/peds.103.5.e71. [DOI] [PubMed] [Google Scholar]
- 18.Zhou SJ, Baghurst P, Gibson RA, et al. Home environment, not duration of breast-feeding, predicts intelligence quotient of children at four years. Nutrition. 2007;23:236–241. doi: 10.1016/j.nut.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 19.Malloy MH, Berendes H. Does breast-feeding influence intelligence quotients at 9 and 10 years of age? Early Hum Dev. 1998;50:209–217. doi: 10.1016/s0378-3732(97)00044-1. [DOI] [PubMed] [Google Scholar]
- 20.Der G, Batty D, Deary IJ. Effect of breast feeding on intelligence in children: prospective study, sibling pairs analysis, and meta-analysis. BMJ. 2006;333:945. doi: 10.1136/bmj.38978.699583.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibson-Davis CM, Brooks-Gunn J. Breastfeeding and verbal ability of 3-year-olds in a multicity sample. Pediatrics. 2006;118:e1444–e1451. doi: 10.1542/peds.2006-0072. [DOI] [PubMed] [Google Scholar]
- 22.Richards M, Wadsworth M, Rahimi-Foroushani A, et al. Infant nutrition and cognitive development in the first offspring of a national UK birth cohort. Dev Med Child Neurol. 1998;40:163–167. [PubMed] [Google Scholar]
- 23.Lucas A, Morley R, Cole TJ, et al. A randomised multicentre study of human milk versus formula and later development in preterm infants. Arch Dis Child Fetal Neonatal Ed. 1994;70:F141–146. doi: 10.1136/fn.70.2.f141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bolling K, Grant C, Hamlyn B. Infant Feeding Survey 2005. The Information Centre; London: 2007. [Google Scholar]
- 25.Malhotra R, Noheria A, Amir O, et al. Determinants of termination of breastfeeding within the first 2 years of life in India: evidence from the National Family Health Survey-2. Matern Child Nutr. 2008;4:181–193. doi: 10.1111/j.1740-8709.2007.00130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill JC, Krishnaveni GV, Annamma I, et al. Glucose tolerance in pregnancy in South India: Relationships to neonatal anthropometry. Acta Obstet Gynecol Scand. 2005;84:159–165. doi: 10.1111/j.0001-6349.2005.00670.x. [DOI] [PubMed] [Google Scholar]
- 27.Krishnaveni GV, Hill JC, Veena SR, et al. Truncal adiposity is present at birth and in early childhood in South Indian children. Indian Pediatrics. 2005;42:527–538. [PubMed] [Google Scholar]
- 28.Carroll JB. The nature of intelligence and the principles of cognition. Spearman C Contemp Psychol. 1991;36:557–559. [Google Scholar]
- 29.Kaufman AS, Kaufman LN. Kaufman Assessment Battery for Children, Second Edition: Manual. AGS Publishing; Circle Pines, MN: 2004. [Google Scholar]
- 30.Korkman M, Kemp SL, Kirk U. Effects of age on neurocognitive measures of children ages 5 to 12: A cross-sectional study on 800 children from the United States. Dev Neuropsychol. 2001;20:331–354. doi: 10.1207/S15326942DN2001_2. [DOI] [PubMed] [Google Scholar]
- 31.Kohs SC. Intelligence measurement: a psychological and statistical study based upon the Block-design test. Macmillan; New York: 1923. [Google Scholar]
- 32.Wechsler D. Manual for the Wechsler Intelligence Scale for Children. 3rd ed Psychological Corporation; San Antonio, TX: 1991. [Google Scholar]
- 33.Malda M, van de Vijver FJR, Srinivasan K. Adapting a cognitive test for a different cultures: An illustration of qualitative procedures. Psychol Sci Quarterly. 2008;50:451–468. at al. [Google Scholar]
- 34.International Institute for Population Sciences (IIPS) Operations Research Centre (ORC) Macro . National Family Health Survey (NFHS-2), India 1998-1999. IIPS; Maharashtra, Mumbai: 2001. [Google Scholar]
- 35.Kilari AS, Mehendale SS, Dangat KD, et al. Long chain polyunsaturated fatty acids in mothers and term babies. J Perinat Med. 2009 Jun 3; doi: 10.1515/JPM.2009.096. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 36.Der G, Batty GD, Deary IJ. Results from the PROBIT breastfeeding trial may have been overinterpreted. Arch Gen Psychiatry. 2008;65:1456–1457. doi: 10.1001/archpsyc.65.12.1456-b. [DOI] [PubMed] [Google Scholar]
- 37.Lucas A, Morley R, Cole TJ, et al. Breast milk and subsequent intelligence quotient in children born preterm. Lancet. 1992;339:261–264. doi: 10.1016/0140-6736(92)91329-7. [DOI] [PubMed] [Google Scholar]
- 38.Morgan J, Taylor A, Fewtrell M. Meat consumption is positively associated with psychomotor outcome in children up to 24 months of age. J Pediatr Gastroenterol Nutr. 2004;39:493–498. doi: 10.1097/00005176-200411000-00009. [DOI] [PubMed] [Google Scholar]
- 39.Gale CR, Martyn CN, Marriott LD, et al. Dietary patterns in infancy and cognitive and neuropsychological function in childhood. J Child Psychol Psychiatry. 2009 Jan 5; doi: 10.1111/j.1469-7610.2008.02029.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.World Health Organization . Global strategy for infant and young child feeding. WHO; Geneva: 2000. [Google Scholar]