Abstract
Objectives
To examine temporal and geographical patterns of mode of delivery in the European Collaborative Study (ECS), identify factors associated with elective caesarean section (CS) delivery in the HAART era and explore associations between mode of delivery and mother-to-child transmission (MTCT).
Methods
The ECS is a cohort study in which HIV-infected pregnant women were enrolled and their infants prospectively followed. Data on 5238 mother-child pairs (MCPs) enrolled in Western European ECS sites between 1985 and 2007 were analyzed.
Results
The elective CS rate increased from 16% in 1985-1993 to 67% in 1999-2001, declining to 51% by 2005-2007. In 2002-2004, 10% of infants were delivered vaginally, increasing to 34% by 2005-2007. During the HAART era, women in Belgium, UK and the Netherlands were less likely to deliver by elective CS than those in Italy and Spain (Adjusted odds ratio (AOR) 0.07 [95%CI 0.04-0.12]). The MTCT rate in 2005-2007 was 1%. Among MCPs with maternal HIV RNA <400 copies/mL (n=960), elective CS was associated with 80% decreased MTCT risk (AOR 0.20 [0.05-0.65]) adjusting for HAART and prematurity. Two infants of 559 women with viral loads <50 copies/mL were infected, one of whom was delivered by elective CS (MTCT rate 0.4% [95%CI 0.04-1.29]).
Conclusions
Our findings suggest that elective CS prevents MTCT even at low maternal viral loads, but we were insufficiently powered to conclude whether this applies for levels <50 copies/ml. Diverging mode of delivery patterns in Europe reflect uncertainties regarding the risk-benefit balance of elective CS for women on successful HAART.
Introduction
Prevention of mother-to-child-transmission (PMTCT) of HIV-1 (HIV) has become increasingly effective in the past decade, with mother-to-child transmission (MTCT) rates declining from around 20-25% to less than 1-2% in developed country settings [1-4]. The effectiveness of elective caesarean section (CS) in reducing MTCT was first suggested by observational studies in the early 1990s, with an approximate halving of risk [5,6]. In 1998, the French Perinatal Cohort reported that among HIV-infected women on zidovudine prophylaxis, elective CS was associated with an 80% reduced risk of MTCT [7]. In 1999 the results of the only randomized controlled trial of vaginal delivery versus elective CS demonstrated an 80% efficacy for planned elective CS [8], while a large international individual patient data meta-analysis reported a 50% decreased MTCT risk associated with elective CS [9].
Use of antiretroviral drugs in pregnancy, initially zidovudine monotherapy [10, 11] and subsequently highly active antiretroviral therapy (HAART), has been a key factor behind declining MTCT rates [3,4,12]. Although no clinical trials have investigated the efficacy of HAART for PMTCT in developed country settings, combination drug regimens are now considered standard of care for PMTCT as well as for treatment of maternal HIV disease: guidelines in Western Europe and the United States generally advocate the application of HAART instead of zidovudine monotherapy to prevent MTCT in all HIV-infected pregnant women [13-17], or in those with HIV RNA loads above specific thresholds [18, 19]. In pregnant women who require therapy for their own health HAART is always advised.
There remains a lack of consensus regarding optimum obstetric management of pregnant HIV infected women in the HAART era. As a result of very low MTCT rates under effective HAART [1-4], the additional value of an elective CS for PMTCT has been questioned in cases where the HIV-RNA load is below detection (usually <40-50 copies/mL). Concerns relate to the risk-benefit balance of elective CS in such circumstances, particularly as HIV-infected women may be more likely to experience post-natal complications than uninfected women, and that women delivering by elective CS are more likely to have complications than those delivering vaginally [20, 21]. Some guidelines still recommend an elective CS for women on HAART with undetectable HIV-RNA loads [15, 16], whereas other guidelines no longer do so [13,14,17,19,22]. In the case of a measurable pre-labour HIV-RNA load an elective CS is generally recommended.
Our objectives were to examine temporal and geographical patterns of mode of delivery in the Western European centres of the European Collaborative Study (ECS), to identify factors associated with likelihood of elective CS delivery in the HAART era and to explore the associations between mode of delivery and MTCT.
Methods
The ECS is a birth cohort study, established in 1986, in which HIV-infected women are enrolled during pregnancy and their infants prospectively followed according to standard protocols [2,23]. The analyses presented here are limited to mother-child pairs (MCPs) enrolled from the eight participating Western European countries up to the end of 2007. All pregnant women are offered antenatal HIV testing, and those infected invited to participate; pregnant women already known to be HIV-infected due to earlier testing are also invited to take part. Informed consent is obtained before enrolment, according to local guidelines, and local ethics approval has been granted.
Information collected at enrolment and during pregnancy includes current antiretroviral treatment (ART), maternal immunological and virological status and mode of acquisition. Maternal CD4 counts were routinely collected since 1992 and HIV RNA measurements from 1998. Laboratory tests were performed locally; all laboratories were based in tertiary care hospitals. Maternal CD4 count and HIV RNA level nearest to delivery were used in the analyses. CD4 counts were categorized as <200, 200–499, and ≥500 cells/mm3.
Definitions
A CS performed before onset of contractions and rupture of membranes (ROM) was classified an elective CS and a CS performed after contractions had commenced or after ROM as an emergency CS; thus some CS for urgent medical reasons were included in elective CS category. Some analyses use an additional classification: “prophylactic CS”, which was defined as those CS deliveries where “HIV” or “randomized trial” were stated as the indication for the intervention (some women enrolled in the ECS concurrently participated in the European mode of delivery trial [8]); deliveries defined as “started vaginally” included all vaginal deliveries and those deliveries which started vaginally but finished as an emergency CS for the following reasons: abruptio placentae, fetal distress, lower genital tract infection, cervical dystocia, dyskinesia or small pelvis (i.e. intrapartum complications leading to switch from intended vaginal delivery to emergency CS); elective or emergency CS for maternal indication and for PROM were excluded.
Children with a positive virological marker of infection and/or children aged >18 months with persistence of antibody were defined as infected [2]. If a child was HIV antibody-negative and no virus or antigen had ever been detected, they were classified as uninfected. In case of a negative PCR test at >12 weeks postnatally the child was recorded as provisionally uninfected. In the analyses provisionally uninfected children were regarded as uninfected [2].MCPs were classified into one of three sub-regional groups: Italy/Spain, Belgium/Netherlands/UK and Germany/Denmark/Sweden. The following time periods were applied: 1985-1993 (pre-ACTG076 trial) [10], and 1994-1997 (pre-HAART era), with the HAART era divided into three groups (1998-2001, 2002-2004 and 2005-2007). Premature delivery was defined as delivery before 37 completed gestational weeks.
Statistical analyses
Univariable comparisons for categorized variables were tested with the χ2 test. Logistic regression analyses were used to obtain unadjusted and adjusted odds ratios (OR and AOR) and 95% confidence intervals (CI); the analyses investigating factors associated with likelihood of elective CS delivery included geographical region, maternal ART, CD4 count and viral load and prematurity. Analyses were carried out for 1998-2002 and 2003-2007. We performed two logistic regression analyses to explore the association between MTCT risk and mode of delivery, adjusting for confounding factors, in infants born at term and in those born prematurely. A sub-analysis was carried out among all MCPs with maternal viral load <400 copies/ml, adjusting for antenatal HAART and prematurity. Analyses used SAS statistical software (v8.02, SAS Institute, Cary, North Carolina, USA) and STATA (version 10; College Station, Texas, USA).
Results
A total of 5238 MCPs were enrolled by December 2007. Maternal and delivery characteristics are presented in Table 1. Enrolled women were predominantly white in the earlier years of the study, and black in the latter years (mainly black African), with substantial increases in maternal age at delivery and knowledge of positive HIV-status before pregnancy (Table 1). The most common mode of HIV acquisition shifted over time from injecting drug use (IDU) to heterosexual acquisition. The proportion of severely immunosuppressed women (CD4 counts <200 cells/mm3) at delivery more than halved over time (χ2trend=5.7, p=0.017, df 8), while the proportion with HIV-RNA load above versus below 1000 copies/mL decreased significantly (χ2trend=145.3, p<0.02 df 4) (Table 1).
Table 1. Characteristics of mother-child pairs, stratified by time period (n=5238*).
| 1985-1993 | 1994-1997 | 1998-2001 | 2002-2004 | 2005-2007 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| median (n) |
IQR | median (n) |
IQR | median (n) |
IQR | median (n) |
IQR | median (n) |
IQR | |
| Age (years) | 26 (1685) | 23-29 | 29 (810) | 26-32 | 31 (1053) | 27-34 | 32 (952) | 27-36 | 31 (397) | 27-36 |
| Gestational age (weeks) | 39 (1743) |
38-40 | 38 (880) | 37-40 | 38 (1151) |
37-38 | 37 (964) | 37-38 | 38 (395) | 37-39 |
| Birth weight (grams) | 2950 (1726) |
2560- 3310 |
2970 (848) |
2650- 3320 |
2900 (1074) |
2550- 3190 |
2920 (913) |
2580- 3250 |
3030 (388) |
2658- 3350 |
| CD4 count (cells/mm3)# | 470 (426) | 330- 690 |
430 (293) |
240- 580 |
435 (600) |
300- 632 |
425 (577) | 291- 627 |
455 (172) | 339- 614 |
| HIV-RNA load (copies/mL) # | 400 (173) | 200- 4830 |
1480 (69) | 400- 8750 |
200 (546) |
50- 1100 |
50 (563) | 50- 200 |
50 (137) | 50-50 |
| N | % | N | % | N | % | N | % | N | % | |
| Ethnicity | ||||||||||
| White | 1545 | 88 | 673 | 77 | 626 | 59 | 471 | 49 | 109 | 28 |
| Black | 149 | 9 | 172 | 20 | 380 | 36 | 447 | 46 | 260 | 66 |
| Other | 53 | 3 | 31 | 4 | 60 | 6 | 46 | 5 | 24 | 6 |
| Mode of HIV acquisition | ||||||||||
| Injecting drug use | 1195 | 69 | 401 | 46 | 293 | 26 | 141 | 15 | 26 | 8 |
| Heterosexual | 473 | 27 | 410 | 47 | 772 | 68 | 773 | 82 | 288 | 88 |
| Other | 57 | 3 | 65 | 7 | 78 | 7 | 23 | 2 | 12 | 4 |
| Timing of first positive HIV test | ||||||||||
| Before pregnancy | 838 | 49 | 528 | 63 | 759 | 67 | 656 | 70 | 287 | 75 |
| Antenatal | 633 | 37 | 246 | 29 | 338 | 30 | 269 | 29 | 91 | 24 |
| Labour | 247 | 14 | 62 | 7 | 29 | 3 | 16 | 2 | 3 | 1 |
| CD4 count (cells/mm3) # | ||||||||||
| <200 | 56 | 13 | 50 | 17 | 62 | 10 | 69 | 12 | 11 | 6 |
| 200-499 | 173 | 41 | 133 | 45 | 293 | 49 | 284 | 49 | 87 | 51 |
| ≥500 | 197 | 46 | 110 | 38 | 245 | 41 | 224 | 39 | 74 | 43 |
| HIV-RNA load (copies/mL) # | ||||||||||
| 50 or less | 1 | 1 | 178 | 33 | 331 | 59 | 106 | 77 | ||
| 51 - 500 | 88 | 51 | 26 | 38 | 189 | 35 | 146 | 26 | 15 | 11 |
| 501 - 1000 | 6 | 3 | 4 | 6 | 37 | 7 | 20 | 4 | 9 | 7 |
| > 1000 | 79 | 46 | 38 | 55 | 142 | 26 | 66 | 12 | 7 | 5 |
| Mode of delivery | ||||||||||
| vaginal | 1330 | 75 | 485 | 55 | 188 | 16 | 188 | 10 | 135 | 34 |
| elective CS | 287 | 16 | 292 | 33 | 770 | 67 | 628 | 65 | 203 | 51 |
| emergency CS | 148 | 8 | 108 | 12 | 198 | 17 | 158 | 16 | 58 | 15 |
| Infant infection status | ||||||||||
| uninfected | 1372 | 84.5 | 705 | 90.4 | 962 | 97.1 | 752 | 97.9 | 190 | 99.0 |
| infected | 251 | 15.5 | 75 | 9.6 | 29 | 2.9 | 16 | 2.1 | 2 | 1.0 |
| Sub-region | ||||||||||
| Italy/Spain | 1376 | 78 | 606 | 67 | 635 | 55 | 432 | 45 | 91 | 23 |
| UK,Belgium,Netherlands | 239 | 13 | 162 | 18 | 294 | 25 | 314 | 33 | 235 | 60 |
| Germany,Denmark,Sweden | 156 | 9 | 130 | 14 | 236 | 20 | 217 | 23 | 68 | 17 |
| Total | 1771 | 898 | 1165 | 963 | 394 | |||||
missing data are left out of the table; totals for each time period are given in the bottom row; for the numerical data they are provided separately;
measured 30 days pre - 1day post-labour
Mode of delivery: temporal and geographical trends
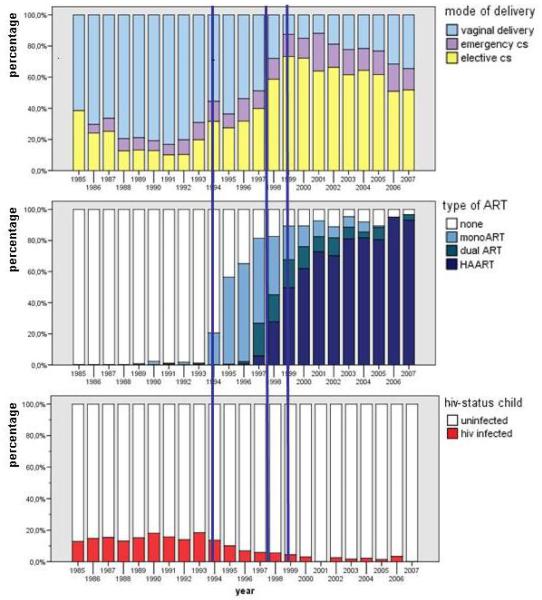
The changing pattern of mode of delivery, together with trends in antenatal ART use and MTCT rates between 1985 and 2007 are shown in Figure 1. The proportion of vaginal deliveries decreased significantly over the study period as a whole (χ2trend=989.4, p<0.001), but reached its lowest level (10%) in 2002-04, increasing in the most recent time period to 34%. The elective CS rate declined since 2000 (Figure 1). Overall, 1.7% (39/2326) vaginal deliveries were instrumental, all but two of whiche occurred in the earliest time period. The emergency CS rate increased in the HAART era, but peaked in 1998-2001, decreasing in 2005-2007.
Figure 1. Trends in use of (HA)ART, mode of delivery and MTCT*.
* in MCPs with known child’s infection; the three vertical blue lines mark from left to right the publication year of the ACTG 076 trial [10 ], the start of the HAART era and the publication of the European Mode of Delivery trial [8]
Among women delivering before 1994, three-quarters delivered vaginally and 99% received no ART (Table 1, Figure 1). Figure 1 shows the rapid implementation of use of zidovudine monotherapy during the four years following the ACTG076 trial results in 1994, and the subsequent uptake of HAART. In the HAART era, 119 (10%) women did not receive (HA)ART, of whom 34% delivered vaginally, 23% by emergency CS and 43% by elective CS; among the 2526 women on HAART, 511 (20%) delivered vaginally, 414 (16%) by emergency CS and 1601 (63%) by elective CS.
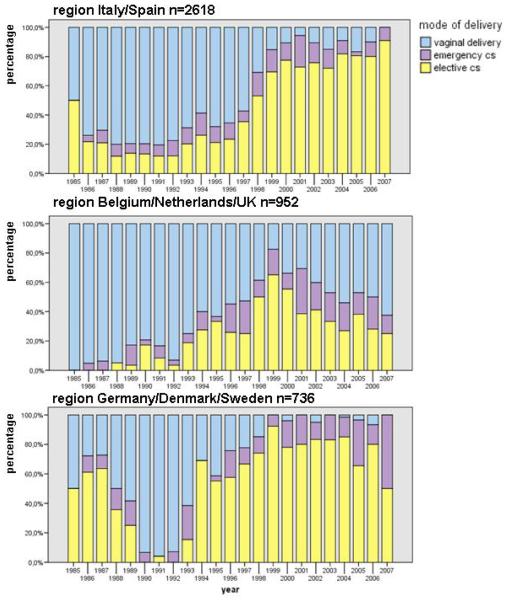
There was a distinct pattern in mode of delivery across different geographic regions, with a relatively rapid decline in elective CS rates in Belgium/Netherlands/UK since 1999 but virtually no drop until 2006 in the two other European regions (Figure 2). In univariable analysis of factors associated with elective CS delivery (Table 2), geographic area, ART type, prematurity and viral load were all significantly associated with likelihood of delivering by elective CS in one or both periods. The multivariable results demonstrated a significantly reduced likelihood of elective CS delivery in Belgium/Netherlands/UK versus Italy/Spain, with the most pronounced difference seen in 2003-2007 with a 93% decreased risk. Women delivering in Germany/Denmark/ Sweden were more likely to have an elective CS than women from Italy/Spain, but this increase was only significant in 1998-2002. Use of antenatal mono- or dual therapy was associated with an independent 1.6 times increased likelihood of elective CS in 1998-2002 and a nearly 3 times increase in 2003-07 compared to HAART (Table 2). Women with undetectable HIV RNA levels (<50 copies/ml) were significantly less likely to have an elective CS in the group delivering between 1998 and 2002.
Figure 2. Mode of delivery in three Western European regions*.
* for 4309 MCPs with known child’s infection status
Table 2. Factors associated with likelihood of an elective caesarean section delivery in the HAART era.
| 1998-2002 | 2003-2007 | |||
|---|---|---|---|---|
| OR (95%CI), p value |
AOR (95% CI), p value |
OR (95%CI), p value |
AOR (95% CI), p value |
|
| Region | ||||
| Italy/Spain | 1.00 | 1.00 | 1.00 | 1.00 |
| UK/Netherlands/Belgium | 0.38 (0.30-0.49), p<0.001 |
0.31 (0.21-0.46), p<0.001 |
0.12 (0.08-0.16), p<0.001 |
0.07 (0.04-0.12), p<0.001 |
| Germany/Denmark/Sweden | 1.73 (1.25-2.39), p<0.001 |
3.71 (2.15-6.41), p<0.001 |
0.85 (0.57-1.28), p=0.44 |
1.49 (0.79-3.06), p=0.20 |
| Antenatal ART type | ||||
| HAART | 1.00 | 1.00 | 1.00 | 1.00 |
| Mono or dual therapy | 1.73 (1.34-2.24), p<0.001 |
1.58 (1.04-2.38), p=0.030 |
3.45 (2.06-5.79), p<0.001 |
2.85 (1.22-6.60), p=0.02 |
| None | 0.42 (0.29-0.59), p<0.001 |
N/A | 1.46 (0.86-2.47), p=0.16 |
N/A |
| Start of antenatal ART | ||||
| Pre conception | 1.00 | 1.00 | 1.00 | 1.00 |
| 1st or 2nd trimester | 0.88 (0.66-1.18), p=0.41 |
0.68 (0.46-1.01), p=0.057 |
0.94 (0.68-1.29), p=0.69 |
0.77 (0.47-1.25), p=0.29 |
| 3rd trimester | 0.82 (0.60-1.13), p=0.24 |
0.70 (0.44-1.12), p=0.14 |
0.91 (0.64-1.31), p=0.62 |
0.94 (0.55-1.58), p=0.81 |
| At/after delivery | 0.77 (0.51-1.15), p=0.20 |
0.76 (0.41-1.40), p=0.37 |
2.04 (1.24-3.35), p=0.005 |
0.80 (0.32-2.38), p=0.80 |
| Premature delivery | ||||
| No | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 3.38 (2.65-4.30), p<0.001 |
8.27 (3.47-19.69), p<0.001 |
1.55 (1.13-2.12), p<0.001 |
6.03 (3.36-10.8), p<0.001 |
| CD4 count (cells/mm3) | ||||
| ≥500 | 1.00 | 1.00 | 1.00 | 1.00 |
| 200-499 | 0.95 (0.64-1.43), p=0.823 |
1.12 (0.66-1.91) p=0.664 |
0.80 (0.49-1.30), p=0.376 |
0.88 (0.45-1.69), p=0.70 |
| <200 | 1.12 (0.73-1.70), p=0.60 |
1.00 (0.58-1.73), p=0.99 |
0.63 (0.39-1.03), p=0.068 |
0.47 (0.23-0.94), p=0.032 |
| HIV RNA load (copies/ml) | ||||
| <50 | 1.00 | 1.00 | 1.00 | 1.00 |
| 51-1000 | 1.62 (1.20-2.18), p=0.002 |
1.66 (1.15-2.40), p=0.007 |
1.75 (1.18-2.60), p=0.005 |
1.51 (0.91-2.51), p=0.11 |
| >1000 | 1.91 (1.36-2.66), p<0.001 |
1.63 (1.04-2.55), p=0.03 |
1.22 (0.78-1.89), p=0.38 |
0.64 (0.34-1.21), p=0.17 |
MTCT risk and mode of delivery
The MTCT rate decreased substantially after 1994, reaching 1% in 2005-2007 (Table 1). Among premature infants, the MTCT rates for those delivered by elective CS, by emergency CS and vaginally were 2.8% (9/319), 6.2% (14/226) and 21.6% (58/268) respectively; 79% (251/319) of those delivered by elective CS were born at 35-36 weeks and for 96% maternal HIV infection was stated as the CS indication. Elective CS and emergency CS delivery were both univariably associated with a statistically significant reduction in MTCT risk overall versus vaginal delivery (respective ORs 0.06 [95%CI 0.02-0.16] and 0.19 [95%CI 0.09-0.42]). In multivariable analysis adjusting for maternal CD4 count and receipt of antenatal ART (classified as none, mono/dual therapy and HAART) including 496 premature infants, elective CS was associated with an 89% decreased risk of MTCT (AOR 0.11 [0.03-0.32] p<0.001) and emergency CS with a 63% reduced risk (AOR 0.37 [0.16-0.87] p=0.02). Repeating this analysis for the 2081 MCPs with term delivery, elective CS was associated with a halving of MTCT risk (AOR 0.49 [0.30-0.80] p=0.004), but the association with emergency CS was not significant (AOR 0.74 [0.38-1.43] p=0.37). Results from a sub-analysis among all MCPs with maternal viral load <400 copies/ml, (n=960) are presented in Table 3. Elective CS and emergency CS were associated with a reduced MTCT risk versus vaginal delivery, but the emergency CS association was only of borderline significance. We were unable to repeat this analysis restricted to the 559 MCPs with maternal viral load <50 copies/ml, as there were only two cases of vertical transmissions (overall MTCT rate 0.4% [95%CI 0.04-1.29]): one infected infant was born vaginally at <34 weeks and the other by elective CS at 37 weeks; both mothers were receiving HAART in pregnancy, the former from before pregnancy and the latter for 2 months prior to delivery.
Table 3. Risk factors associated with mother-to-child transmission among women with viral loads <400 copies/ml (n=960).
| Unadjusted MTCT rate |
Odds ratio (95%CI) p value |
Adjusted odds ratio (95%CI), p value |
|
|---|---|---|---|
| Mode of delivery | |||
| Vaginal | 4.6% (11/242) | 1.00 | 1.00 |
| Emergency CS | 1.4% (2/147) | 0.29 (0.06-1.33) p=0.11 | 0.19 (0.03-1.02) p=0.05 |
| Elective CS | 0.7% (4/571) | 0.15 (0.05-0.47) p=0.001 | 0.20 (0.05-0.65) p=0.008 |
| Antenatal HAART | |||
| No | 5.3% (12/227) | 1.00 | 1.00 |
| Yes | 0.7% (5/733) | 0.12 (0.04-0.35) p<0.001 | 0.15 (0.05-0.45) p<0.001 |
| Gestational age | |||
| Term (≥37 weeks) | 1.2% (9/730) | 1.00 | 1.00 |
| 34-36 weeks | 2.2% (4/179) | 1.83 (0.56-6.02) p=0.32 | 2.21 (0.64-7.59) p=0.21 |
| <34 weeks | 7.8% (4/51) | 6.82 (2.03-23.0) p=0.002 | 8.47 (1.99-36.1) p=0.004 |
95%CI – 95% confidence interval
A further analysis was performed to explore the value of a policy of an elective CS (prophylactic CS) to prevent MTCT versus a policy of vaginal delivery (including vaginal deliveries converted to an emergency CS) in women on HAART. Among 1132 women on HAART with viral load measurements available 30 days before delivery or one day post-partum, the MTCT rate was 0.65% (2/310) among women who started their labour vaginally and 1.3% (11/822) among those who had a prophylactic CS (p=0.64). There were no transmissions among the women who started their labour vaginally, with a viral load <1000 copies/mL (0/155 [one-sided 97.5% CI 2.35%]), and three among women who had prophylactic CS (0.7%, 3/424 [95%CI 0.15-2.05]; the MTCT rate among women undergoing prophylactic CS with HIV RNA levels <50 copies/mL was 0.4% (1/238) (p=0.48).
Discussion
In this large, long-running study of HIV-infected pregnant women, we have documented fluctuations in vaginal delivery and CS delivery rates over time. During 2005-2007,, a third of women delivered vaginally, half by elective CS and the remainder by emergency CS. In contrast, at the start of the HAART era, two-thirds of women delivered by elective CS. We document geographical variation in mode of delivery in the HAART era, with an increasing proportion of vaginal deliveries, mainly in the UK, Belgium and the Netherlands. In multivariable analysis of MTCT risk among MCPs with maternal HIV RNA <400 copies/mL, elective CS was associated with an 80% decreased MTCT risk. However, among women with viral loads <50 copies/mL there were only two transmissions overall.
Although clinical trials are the gold standard for clinical care, observational studies often provide the initial evidence for trial inception and design. Use of elective CS as a PMTCT intervention is a case in point: the ECS first published results showing an association between reduced MTCT risk and elective CS in 1994 [5], with subsequent confirmation from a large meta-analysis [9]. Our finding here, that the highest implementation of an elective CS policy had already been reached in 1999, when the mode of delivery trial was published [8], is probably largely explained by participating clinicians changing their practices before the trial results were released as a result of the observational evidence that they helped to provide; furthermore, a number of women were concomitantly enrolled in both the trial and the ECS. The somewhat paradoxical finding of a declining elective CS rate in the years immediately following the trial publication may be partly explained by the concurrent implementation of antenatal HAART instead of mono- or dual therapy for PMTCT, when the first studies suggesting the benefit of HAART for decreasing MTCT risk were published [24-27] and guidelines started to change. In the Netherlands, for instance the national guideline in 2000 only mentioned an elective CS as a rescue therapy in case of HAART failure or refusal [28].
Other European studies have also documented declining elective CS rates in the HAART era. In an analysis from the French Perinatal Study involving over 5000 pregnant women receiving antenatal ART and delivering between 1997 and 2004, the elective CS rate declined from 56% in 2000 to 41% in 2004 [4]. In the UK and Ireland National Study of HIV in Pregnancy and Childhood (NSHPC), the elective CS rate peaked in 1999 at 66%, declining to around 50% in 2006. The emergency CS rate we report here was relatively stable but high and ranged from 15-17% in the HAART era; the French Perinatal Study also reported stable emergency CS rates between 1997 and 2004, but higher at around 29% [4]. In contrast, the NSHPC documented an increasing emergency CS rate, from 17% in 1999 to 23% in 2006 in the UK and Ireland, concurrent with updated guidelines recommending a vaginal delivery for women on HAART with viral suppression [29]; most emergency CS deliveries were in women delivering at term, consistent with the likelihood that these women opted for a vaginal delivery but delivered by CS due to intrapartum complications [29]. The lower emergency CS rate in our centres in Italy and Spain compared to that in Belgium, the Netherlands and the UK may be largely explained by the greater proportion of women opting for vaginal deliveries in the latter.
A prominent factor associated with likelihood of an elective CS was geographic location. In our adjusted analysis, women delivering in Belgium, the Netherlands or UK were 93% less likely to have an elective CS compared with women living in Italy or Spain by 2003-2007. Geographic differences may be explained by differences in national guidelines [13-17,19,52] and may also reflect variation in the elective CS rate in the general population. The association between antenatal ART and mode of delivery strengthened over time: in 1998-2002, women on mono- or dual therapy were 1.6 times more likely to deliver by elective CS than women on HAART, increasing to 2.5 times by 2003-2007. Although women with a last HIV RNA viral load in pregnancy <50 copies/ml were significantly less likely to have an elective CS in the group delivering between 1998 and 2003, this was not the case in the more recent time period. This might be due to the fact that the policy to perform an elective CS was very region bound and that more CS that were intentionally prophylactic became an emergency CS because of changed guidelines with respect to the week of the planned CS (37-37+6 weeks instead of 36-36+6 weeks) [15].
Prematurity is a well-defined risk factor for MTCT [2,4,30,31] and some studies have suggested that infants born to HIV-infected mothers may be particularly susceptible to intrapartum acquisition of infection [32]. In our analysis among MCPs with viral loads <400 copies/mL, infants born before 34 weeks had an eight-fold increased risk of HIV infection compared with term infants. Among premature infants, both elective CS and emergency CS were effective in reducing MTCT risk (independent of maternal CD4 count and ART), whilst in term infants, emergency CS was not associated with a significantly lower MTCT risk. Associations between prematurity and HAART use have been reported in several studies, mainly in Europe, with prematurity rates in cohorts of HIV-infected women reported up to 34% [33-37]. A recent risk-benefit analysis using UK data indicated that the risk-benefit ratio associated with exclusive HAART (vs zidovudine monotherapy) was an estimated 0.59 premature infants for each infection averted [38]. It is clear that the relationships between preterm delivery, HAART use and MTCT are complex, and the role that mode of delivery may play in these requires further research.
We found that elective CS was an effective PMTCT intervention among nearly 1000 women with viral load <400 copies/mL, with an 80% decreased risk, independent of HAART use and gestational age. This extends our previous finding, whereby elective CS was associated with a 93% decreased MTCT risk in 560 women with undetectable viral loads (around half of whom were tested with less sensitive assays than those currently used) [12]. A decision regarding mode of delivery has to be made before labour starts on the basis of the instituted antiretroviral treatment and the last measured HIV-RNA viral load. Emergency CS can be the result of a woman with a planned elective CS starting labour earlier than the planned date or the consequence of a complication during a planned vaginal delivery. Here, emergency CS was associated with an 80% decreased risk of MTCT among infants born to women with viral loads <400 copies/mL, although only with borderline statistical significance. We also described MTCT rates by mode of delivery, reclassified as prophylactic CS and an attempted vaginal delivery to reflect intended delivery. The possibility exists that some conditions potentially favourable for MTCT like placental abruption, IUGR and infection of the lower genital tract were also included in the “started vaginally” group. Most HAART-using women here with a known HIV-RNA load in the last month of pregnancy had undetectable levels (<50 copies/mL) and virtually all had levels <500 copies/mL. In our analysis comparing MTCT rates among women delivering by prophylactic CS and those starting vaginally, we carried out a sub-analysis restricted to women with undetectable viral loads because it could be that a prophylactic CS is mainly performed in cases where there is a perceived high risk of MTCT (i.e. confounding by indication): there were no transmissions among women with viral loads <50 copies/mL starting labour vaginally at ≥34 weeks and a MTCT rate of 0.4% for those having a prophylactic CS.
Our findings suggest a protective effect of elective CS even at low maternal viral loads, but when the HIV-RNA load is <50 copies/mL we were insufficiently powered to draw any conclusions about the benefit of intended elective CS or the risk of intended vaginal delivery in this group of patients, who can achieve MTCT rates below 0.5% [1, 3, 4]. The effectiveness of elective CS in PMTCT is just one of the factors requiring consideration in decision-making around mode of delivery; the potential risks of CS also need consideration as CS, particularly in HIV-infected women, may cause maternal morbidity in the short-term [20,21,39] and in subsequent pregnancies [40]. A further factor to consider is that delivery may not take place as planned: recent studies have shown that between 38% and 55% of women opting for a vaginal delivery have actually delivered by CS, for a variety of reasons [1,22].
A limitation of our study is the lack of data on what the planned mode of delivery was. We thus could not address the issue of … We were not able to investigate the likely timing of transmission in infected infants ..
In conclusion, we show that implementation of obstetric interventions for PMTCT are not only influenced by evidence-based medicine but also by “opinion-based” medicine. Our data highlight the effectiveness of antenatal HAART in PMTCT, which has resulted in a very small number of infections in recent years and has contributed to a declining elective CS rate overall. The numbers needed to treat (i.e. the number of elective CS deliveries) to prevent a single transmission will be high taking into account the results of the present and other studies [1,3,4]. Cohort collaborations and risk benefit analyses are needed to address the important question of whether elective CS has any additional benefit for women reaching the end of their pregnancy with undetectable viral loads, and to explore the role and feasibility of CS after labour and/or rupture of membranes in preventing MTCT among specific groups, including premature infants.
Acknowledgements
We thank Prof L Chieco Bianchi, Prof F Zacchello, Dr E Ruga, Dr AM Laverda, Dr R D’Elia, Mrs S Oletto (Padua); Dr T Schmitz, Dr R Weigel, Dr S Casteleyn (Berlin); Dr S Burns, Dr N Hallam, Dr PL Yap, Dr J Whitelaw (Edinburgh); A van der Plas, E.M. Lepoole (Amsterdam); Dr E Belfrage, Dr A Kaldma, Dr AC Lindholm (Sweden); Dr A Ferrazin, Dr R Rosso, G Mantero, Prof S Trasino, Dr J Nicoletti (Genoa); Dr E Mur (Barcelona); Dr G Zucotti (Milan); Prof PA Tovo, Dr C Gabiano (Turino); Dr T Bruno (Naples), The Regional Health Office and RePuNaRC (Naples); G Mantero, Dr A Nicoletti, Dr B Bruzzone, Dr R Rosso and Dr M Setti (Genoa); M Kaflik (Medical University of Warsaw, Poland). We would like to thank Dr C Townsend for her helpful comments on drafts of this paper.
The ECS is a coordination action of the European Commission (PENTA/ECS 018865). Claire Thorne is supported by a Wellcome Trust Research Career Development Fellowship. The centre at Universita degli Studi di Padova is supported by Progetto di Ricerca sull AIDS - Istituto Superiore di Sanità – 2006.
European Collaborative Study
Dr C Thorne, Prof ML Newell, S Mahdavi, K England (ECS coordinating Centre, UCL Institute of Child Health, UK), Dr C Giaquinto, Dr O Rampon, Dr A Mazza and Prof A De Rossi (Universita degli Studi di Padova, Italy); Prof I Grosch Wörner (Charite Virchow-Klinikum, Berlin, Germany); Dr J Mok (Royal Hospital for Sick Children, Edinburgh); Dr Ma I de José, Dra B Larrú Martínez, Dr J Ma Peña, Dr J Gonzalez Garcia, Dr JR Arribas Lopez and Dr MC Garcia Rodriguez (Hospital Infantil La Paz, Madrid); Prof F Asensi-Botet, Dr MC Otero, Dr D Pérez-Tamarit (Hospital La Fe, Valencia, Spain); Dr H J Scherpbier, M Kreyenbroek, Dr MH Godfried, Dr FJB Nellen and Dr K Boer (Academisch Medisch Centrum, Amsterdam, The Netherlands); Dr L Navér, Dr AB Bohlin, Dr S Lindgren, Dr B Anzén, Dr K Lidman, Prof A Ehrnst (Karolinska University Huspital, Huddinge and Solna, Sweden); Prof J Levy, Dr P Barlow, Dr Y Manigart, Dr M Hainaut and Dr T Goetghebuer (Hospital St Pierre, Brussels, Belgium); Prof B Brichard, J De Camps, N Thiry, G Deboone, H Waterloos (UCL Saint-Luc, Brussels, Belgium); Prof C Viscoli (Infectious Diseases Clinic, University of Genoa, Italy); Prof A De Maria (Department of Internal Medicine, University of Genoa and S.S.Infettivologia, Istituto Nazionale per la Ricerca sul Cancro, IST- Genova, Italy); Prof G Bentivoglio, Dr S Ferrero, Dr C Gotta (Department of Obstetrics and Gynecology-Neonatology Unit, University of Genoa, Italy); Prof A Mûr, Dr A Payà, Dr MA López-Vilchez, Dr R Carreras (Hospital del Mar, Universidad Autonoma, Barcelona, Spain); Dr N H Valerius, Dr V Rosenfeldt (Hvidovre Hospital, Denmark); Dr O Coll, Dr A Suy and Dr J M Perez (Hospital Clínic, Barcelona, Spain); Dr C Fortuny, Dr J Boguña (Hospital Sant Joan de Deu, Barcelona, Spain); Dr V Savasi, Dr S Fiore, Dr M Crivelli, (Ospedale L. Sacco, Milan, Italy); Dr A Viganò, Dr V Giacomet, Dr C Cerini, Dr C Raimondi and Prof G Zuccotti (Department of Pediatrics, L. Sacco Hospital, University of Milan); S. Alberico, M. Tropea, C. Businelli (IRCCS Burlo Garofolo, Trieste, Italy); Dr G P Taylor, Dr EGH Lyall (St Mary’s Hospital, London); Ms Z Penn (Chelsea and Westminster Hospital, London); Drssa W. Buffolano, Dr R Tiseo, (Pediatric Dept, Federico II University, Naples), Prof P Martinelli, Drssa M Sansone, Dr G Maruotti, Dr A Agangi (Obstetric Dept, Federico II University, Naples, Italy); Dr C Tibaldi, Dr S Marini, Dr G Masuelli, Prof C Benedetto (University di Torino, Italy); Dr T Niemieç (National Research Institute of Mother & Child, Warsaw, Poland), Prof M Marczynska, Dr S Dobosz, Dr J Popielska, Dr A Oldakowska (Medical University of Warsaw, Infectious Diseases Hospital, Warsaw, Poland); Dr R Malyuta, Dr I Semenenko, T Pilipenko (ECS Ukraine coordinating centre).
Reference List
- 1.Boer K, Nellen JF, Patel D, Timmermans S, Tempelman C, Wibaut M, et al. The AmRo study: pregnancy outcome in HIV-1-infected women under effective highly active antiretroviral therapy and a policy of vaginal delivery. BJOG. 2007;114(2):148–155. doi: 10.1111/j.1471-0528.2006.01183.x. [DOI] [PubMed] [Google Scholar]
- 2.European Collaborative Study The mother-to-child HIV transmission epidemic in Europe: evolving in the East and established in the West. AIDS. 2006;20(10):1419–1427. doi: 10.1097/01.aids.0000233576.33973.b3. [DOI] [PubMed] [Google Scholar]
- 3.Townsend CL, Cortina-Borja M, Peckham CS, de RA, Lyall H, Tookey PA. Low rates of mother-to-child transmission of HIV following effective pregnancy interventions in the United Kingdom and Ireland, 2000-2006. AIDS. 2008;22(8):973–981. doi: 10.1097/QAD.0b013e3282f9b67a. [DOI] [PubMed] [Google Scholar]
- 4.Warszawski J, Tubiana R, Le CJ, Blanche S, Teglas JP, Dollfus C, et al. Mother-to-child HIV transmission despite antiretroviral therapy in the ANRS French Perinatal Cohort. AIDS. 2008;22(2):289–299. doi: 10.1097/QAD.0b013e3282f3d63c. [DOI] [PubMed] [Google Scholar]
- 5.European Collaborative Study Caesarean section and risk of vertical transmission of HIV-1 infection. Lancet. 1994;343(8911):1464–1467. [PubMed] [Google Scholar]
- 6.Villari P, Spino C, Chalmers TC, Lau J, Sacks HS. Cesarean section to reduce perinatal transmission of human immunodeficiency virus. A metaanalysis. Online J Curr Clin Trials. 1993 Doc No 74:5107. [PubMed] [Google Scholar]
- 7.Mandelbrot L, Le CJ, Berrebi A, Bongain A, Benifla JL, Delfraissy JF, et al. Perinatal HIV-1 transmission: interaction between zidovudine prophylaxis and mode of delivery in the French Perinatal Cohort. JAMA. 1998;280(1):55–60. doi: 10.1001/jama.280.1.55. [DOI] [PubMed] [Google Scholar]
- 8.European Mode of Delivery Collaboration Elective caesarean-section versus vaginal delivery in prevention of vertical HIV-1 transmission: a randomised clinical trial. Lancet. 1999;353(9158):1035–1039. doi: 10.1016/s0140-6736(98)08084-2. [DOI] [PubMed] [Google Scholar]
- 9.International Perinatal HIV Group The mode of delivery and the risk of vertical transmission of human immunodeficiency virus type 1--a meta-analysis of 15 prospective cohort studies. N Engl J Med. 1999;340(13):977–987. doi: 10.1056/NEJM199904013401301. [DOI] [PubMed] [Google Scholar]
- 10.Connor EM, Sperling RS, Gelber R, Kiselev P, Scott G, O’Sullivan MJ, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;331(18):1173–1180. doi: 10.1056/NEJM199411033311801. [DOI] [PubMed] [Google Scholar]
- 11.Sperling RS, Shapiro DE, Coombs RW, Todd JA, Herman SA, McSherry GD, et al. Maternal viral load, zidovudine treatment, and the risk of transmission of human immunodeficiency virus type 1 from mother to infant. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1996;335(22):1621–1629. doi: 10.1056/NEJM199611283352201. [DOI] [PubMed] [Google Scholar]
- 12.European Collaborative Study Mother-to-child transmission of HIV infection in the era of highly active antiretroviral therapy. Clin Infect Dis. 2005;40(3):458–465. doi: 10.1086/427287. [DOI] [PubMed] [Google Scholar]
- 13.Werkgroep Antiretrovirale Werkgroep antiretrovirale behandeling van de Nederlandse Vereniging van AIDS Behandelaren. Ned Tijdschr Geneeskd. 2005;149:2399–2405. Revised guidelines “Antiretroviral Treatment”. [PubMed] [Google Scholar]
- 14.Burdge DR, Money DM, Forbes JC, Walmsley SL, Smaill FM, Boucher M, et al. Canadian consensus guidelines for the management of pregnancy, labour and delivery and for postpartum care in HIV-positive pregnant women and their offspring (summary of 2002 guidelines) CMAJ. 2003;168(13):1671–1674. [PMC free article] [PubMed] [Google Scholar]
- 15.Buchholz B, Beichert M, Marcus U, Grubert T, Gingelmaier A, Haberl A, et al. German-Austrian recommendations for HIV-therapy in pregnancy and in HIV-exposed newborn - update 2005. Eur J Med Res. 2006;11(9):359–376. [PubMed] [Google Scholar]
- 16.Naver L, Bohlin AB, Albert J, Flamholc L, Gisslen M, Gyllensten K, et al. Prophylaxis and treatment of HIV-1 infection in pregnancy: Swedish Recommendations 2007. Scand J Infect Dis. 2008;40(6-7):451–461. doi: 10.1080/00365540801894787. [DOI] [PubMed] [Google Scholar]
- 17.US Public Health Service Task Force . Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. 2008. [Google Scholar]
- 18.Eramova I, Matic S, Munz M. HIV/AIDS treatment and care. Clinical protocols for the WHO European Region. World Health Organization Regional Office for Europe; Copenhagen: 2007. [Google Scholar]
- 19.de Ruiter A, Taylor G, Mercey D, Anderson J, Chakraborty R, Clayden P, et al. British HIV Association and Children’s HIV Association guidelines for the management of HIV infection in pregnant women 2008. BHIVA; London: 2008. [DOI] [PubMed] [Google Scholar]
- 20.Read JS, Newell MK. Efficacy and safety of cesarean delivery for prevention of mother-to-child transmission of HIV-1. Cochrane Database Syst Rev. 2005;(4):CD005479. doi: 10.1002/14651858.CD005479. [DOI] [PubMed] [Google Scholar]
- 21.European HIV Obstetrics Group Higher rates of post-partum complications in HIV-infected than in uninfected women irrespective of mode of delivery. AIDS. 2004;18(6):933–938. doi: 10.1097/00002030-200404090-00011. [DOI] [PubMed] [Google Scholar]
- 22.Suy A, Hernandez S, Thorne C, Lonca M, Lopez M, Coll O. Current guidelines on management of HIV-infected pregnant women: Impact on mode of delivery. Eur J Obstet Gynecol Reprod Biol. 2008 doi: 10.1016/j.ejogrb.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 23.European Collaborative Study Time to undetectable viral load after highly active antiretroviral therapy initiation among HIV-infected pregnant women. Clin Infect Dis. 2007;44(12):1647–1656. doi: 10.1086/518284. [DOI] [PubMed] [Google Scholar]
- 24.Clarke SM, Mulcahy F, Healy CM, Condon S, Butler KM. The efficacy and tolerability of combination antiretroviral therapy in pregnancy: infant and maternal outcome. Int J STD AIDS. 2000;11(4):220–223. doi: 10.1258/0956462001915741. [DOI] [PubMed] [Google Scholar]
- 25.Morris AB, Cu-Uvin S, Harwell JI, Garb J, Zorrilla C, Vajaranant M, et al. Multicenter review of protease inhibitors in 89 pregnancies. J Acquir Immune Defic Syndr. 2000;25(4):306–311. doi: 10.1097/00042560-200012010-00003. [DOI] [PubMed] [Google Scholar]
- 26.McGowan JP, Crane M, Wiznia AA, Blum S. Combination antiretroviral therapy in human immunodeficiency virus-infected pregnant women. Obstet Gynecol. 1999;94(5 Pt 1):641–646. doi: 10.1016/s0029-7844(99)00526-8. [DOI] [PubMed] [Google Scholar]
- 27.Cooper ER, Charurat M, Burns DN, Blattner W, Hoff R. Trends in antiretroviral therapy and mother-infant transmission of HIV. The Women and Infants Transmission Study Group. J Acquir Immune Defic Syndr. 2000;24(1):45–47. doi: 10.1097/00126334-200005010-00007. [DOI] [PubMed] [Google Scholar]
- 28.Borleffs JCC, Danner SA, Lange JMA, van Everdingen JJE, Orichtlijn CB. Antiretrovirale behandeling in Nederland. Ned Tijdschr Geneeskd. 2001;145:1585–1589. [PubMed] [Google Scholar]
- 29.Townsend CL, Cortina-Borja M, Peckham CS, Tookey PA. Trends in management and outcome of pregnancies in HIV-infected women in the UK and Ireland, 1990-2006. BJOG. 2008;115(9):1078–1086. doi: 10.1111/j.1471-0528.2008.01706.x. [DOI] [PubMed] [Google Scholar]
- 30.Jourdain G, Mary JY, Coeur SL, Ngo-Giang-Huong N, Yuthavisuthi P, Limtrakul A, et al. Risk factors for in utero or intrapartum mother-to-child transmission of human immunodeficiency virus type 1 in Thailand. J Infect Dis. 2007;196(11):1629–1636. doi: 10.1086/522009. [DOI] [PubMed] [Google Scholar]
- 31.Thorne C, Newell ML. Mother-to-child transmission of HIV infection and its prevention. Curr HIV Res. 2003;1(4):447–462. doi: 10.2174/1570162033485140. [DOI] [PubMed] [Google Scholar]
- 32.Kuhn L, Steketee RW, Weedon J, Abrams EJ, Lambert G, Bamji M, et al. Perinatal AIDS Collaborative Transmission Study Distinct risk factors for intrauterine and intrapartum human immunodeficiency virus transmission and consequences for disease progression in infected children. J Infect Dis. 1999;179(1):52–58. doi: 10.1086/314551. [DOI] [PubMed] [Google Scholar]
- 33.Thorne C, Patel D, Newell ML. Increased risk of adverse pregnancy outcomes in HIV-infected women treated with highly active antiretroviral therapy in Europe. AIDS. 2004;18(17):2337–2339. doi: 10.1097/00002030-200411190-00019. [DOI] [PubMed] [Google Scholar]
- 34.Townsend CL, Cortina-Borja M, Peckham CS, Tookey PA. Antiretroviral therapy and premature delivery in diagnosed HIV-infected women in the United Kingdom and Ireland. AIDS. 2007;21(8):1019–1026. doi: 10.1097/QAD.0b013e328133884b. [DOI] [PubMed] [Google Scholar]
- 35.Cotter AM, Garcia AG, Duthely ML, Luke B, O’Sullivan MJ. Is antiretroviral therapy during pregnancy associated with an increased risk of preterm delivery, low birth weight, or stillbirth? J Infect Dis. 2006;193(9):1195–1201. doi: 10.1086/503045. [DOI] [PubMed] [Google Scholar]
- 36.Grosch-Woerner I, Puch K, Maier RF, Niehues T, Notheis G, Patel D, et al. Increased rate of prematurity associated with antenatal antiretroviral therapy in a German/Austrian cohort of HIV-1-infected women. HIV Med. 2008;9(1):6–13. doi: 10.1111/j.1468-1293.2008.00520.x. [DOI] [PubMed] [Google Scholar]
- 37.Schulte J, Dominguez K, Sukalac T, Bohannon B, Fowler MG. Declines in low birth weight and preterm birth among infants who were born to HIV-infected women during an era of increased use of maternal antiretroviral drugs: Pediatric Spectrum of HIV Disease, 1989-2004. Pediatrics. 2007;119(4):e900–e906. doi: 10.1542/peds.2006-1123. [DOI] [PubMed] [Google Scholar]
- 38.Townsend C, Cortina-Borja M, Tookey P. Premature delivery and mother to child HIV transmission: risk:benefit analysis of HAART in pregnancy; Montreal. February 8-11 2009; 2009. abstract 927. [Google Scholar]
- 39.Semprini AE, Castagna C, Ravizza M, Fiore S, Savasi V, Muggiasca ML, et al. The incidence of complications after caesarean section in 156 HIV-positive women. AIDS. 1995;9(8):913–917. doi: 10.1097/00002030-199508000-00013. [DOI] [PubMed] [Google Scholar]
- 40.Stamilio DM, DeFranco E, Pare E, Odibo AO, Peipert JF, Allsworth JE, et al. Short interpregnancy interval: risk of uterine rupture and complications of vaginal birth after cesarean delivery. Obstet Gynecol. 2007;110(5):1075–1082. doi: 10.1097/01.AOG.0000286759.49895.46. [DOI] [PubMed] [Google Scholar]