Abstract
Objective
Weight gain and growth in early life may influence adult pro-inflammatory and pro-thrombotic cardiovascular risk factors.
Methods
Follow-up of a birth cohort in New Delhi, India, whose weight and height were measured 6-monthly until age 21 years. BMI at birth, during infancy (2 years), childhood (11 years) and adulthood (26-32 years) and BMI gain between these ages were analyzed in 886 men and 640 women in relation to adult fibrinogen, high-sensitivity C-reactive protein (hsCRP) and plasminogen activator inhibitor (PAI-1) concentrations.
Results
All the pro-inflammatory/pro-thrombotic risk factors were higher in participants with higher adiposity. In women, BMI at birth and age 2 years was inversely related to fibrinogen (p=0.002 and 0.05) and, after adjusting for adult adiposity, to hsCRP (p=0.02 and 0.009). After adjusting for adult adiposity, BMI at 2 years was inversely related to hsCRP and PAI-1 concentrations (p<0.001 and p=0.02) in men. BMI gain between 2-11 years and/or 11 years to adulthood was positively associated with fibrinogen and hsCRP in women and with hsCRP and PAI-1 in men.
Conclusions
Thinness at birth or during infancy, and accelerated BMI gain during childhood/adolescence are associated with a pro-inflammatory/pro-thrombotic state in adult life. An altered inflammatory state could be one link between small newborn/infant size and adult cardiovascular disease. Associations between pro-inflammatory markers and childhood/adolescent BMI gain are probably mediated through adult adiposity.
Keywords: Birthweight, growth, C-reactive protein, fibrinogen, plasminogen activator inhibitor-1
India is experiencing high and rising rates of cardiovascular disease (CVD) and type 2 diabetes.1 Coronary heart disease has been estimated to affect 10% of adults over 35 years of age in Indian cities, and to have caused 1.5 million deaths in India in 2000.1 India is known as the diabetes capital of the world, because of the high burden of type 2 diabetes compared with other geographic regions; the number of patients with diabetes is predicted to rise from 50.8 million in 2010 to 87 million in 2030.2
Recent evidence suggests that the inflammation-coagulation interface may contribute to the development of these diseases. Inflammation may increase cardiovascular risk through increasing platelet activity and coagulation factors, and down-regulating anticoagulant mechanisms and fibrinolysis.3 Fibrinogen and CRP released in response to inflammation increase the risk of thrombosis by increasing the expression of tissue factor and PAI-1. The coagulation system can in turn increase inflammation by releasing mediators from platelets, activating cells and promoting cell-cell interaction. In prospective studies, fibrinogen, CRP and PAI-1 predict an increased risk of future atherosclerotic CVD4-7 and type 2 diabetes.8-12 Adipose tissue is a major source of pro-inflammatory cytokines and PAI-1, which could explain its role as a risk factor for the CVD and type 2 diabetes.13 Both pro-inflammatory cytokines and PAI-1 have been shown to be raised in South Asian populations compared with white Caucasians.14, 15
Environmental factors acting in early life influence the risk of developing adult CVD and type 2 diabetes. Low birthweight and/or infant weight, and accelerated weight gain during childhood, are associated with an increased risk of both disorders16-19 The underlying mechanisms are largely unknown, but activation of the innate immune system during critical periods of fetal/post natal development may predispose to a pro-inflammatory state. Studies have shown associations of lower birthweight or infant weight, and/or greater weight gain during childhood with increased adult concentrations of fibrinogen20-23, hsCRP24,25 and PAI-126 . We now report associations of these pro-inflammatory/pro-thombotic markers with BMI at birth and during infancy, childhood and adolescence in an Indian population.
METHODS
During 1969-1972, married women living in a 12 km2 area of Delhi (N=20,755) were followed up.27-29 There were 9,169 pregnancies, and 8,181 live births. Babies’ weight and length were recorded within 72 hours of birth and 6-monthly until 14-21 years. Measurements were interrupted in 1972-1973 and 1980-1982. At recruitment, 60% of families had incomes >50 rupees per month (national average 28 rupees) and 15% of parents were illiterate (national average 66%). 43% of families lived in one room. Hindus were the majority religious group (84%), followed by Sikhs (12%), Christians (2%), Muslims (1%) and Jains (1%).
Current Study
In 1998-2002 we retraced 2,584 (32%) of the cohort and 1,583 agreed to participate. They were visited at home by a social worker who administered a questionnaire to obtain data on schooling, occupation, household possessions, alcohol consumption, tobacco use, physical activity, family history and (in women) parity.27,28 Participants then attended a clinic, after an overnight fast. Weight, height, waist circumference and skinfold thicknesses (triceps and subscapular) were measured using standardized techniques.28 Participants were categorized as normal weight (BMI <25 kg/m2), overweight (BMI ≥25 kg/m2) or obese (BMI ≥30 kg/m2). Blood pressure was recorded using an automated device (Omron 711) after five minutes seated at rest (mean of two readings).
Blood samples were drawn in the fasting state for measurement of plasma fibrinogen, PAI-1, glucose and insulin; and serum hsCRP and lipids. For glucose, a further sample was drawn 120 minutes after a 75 gm oral glucose load. Glucose was measured on the day of collection. For all other parameters serum and plasma were aliquoted and stored at −70°C until analysis. Glucose, cholesterol and triglyceride concentrations were analyzed by enzymatic methods using Randox kits on a Beckman autoanalyser, and HDL-cholesterol using the same method after phosphotungstate precipitation. Insulin concentrations were measured by radioimmunoassay (Coat-a-Count insulin kit, Diagnostic Products, USA). IGT and diabetes were defined using WHO criteria.30 Metabolic syndrome was defined using NCEP-ATPIII (National Cholesterol Education Program, Adult Treatment Panel III) criteria.31,32 Fibrinogen was determined using the Clauss method.33 HsCRP and PAI-1 were measured using ELISA kits from BioCheck Inc., CA, USA and Diagnostica Stago, Asnieres, France respectively.
The study was approved by the All India Institute of Medical Sciences research ethics committee, and informed consent obtained.
Statistical Analyses
The distributions of fibrinogen, hsCRP, PAI-1, triglyceride and glucose concentrations and insulin resistance were skewed and were transformed to normality using inverse square roots (hsCRP only) or logarithms. Using all recorded data from the original cohort, not just those for subjects recruited for the current study, we generated BMI and height standards so as to derive internal sex-specific SD scores.27 Participants had a mean (SD) of 23 (5.5) observations between birth and the age of 21 years. We modelled the progress of the median, spread, and skewness of the measurements as age increased. For each subject we interpolated values linearly between successive SD scores to estimate SD scores at 6 months and at birthdays from 1 to 21 years. The interpolated values were used if measurements were made within 6 months (up to 1 year), 1 year (age 2 years), 1.5 years (age 3 years), and 2 years (all older ages). Back-transformation provided estimates of measurements at these ages. To measure changes in early-life BMI and height (growth) we used the conditional SD scores method.34,35 We divided growth into 3 periods: birth-2 years (infancy), 2-11 years (pre-pubertal childhood growth) and 11 years to adulthood (adolescent growth). To describe growth during each interval, for example between 2 and 11 years, we regressed SD-scores at the end of the interval (11 years) on SD-scores at the beginning (2 years) and at all preceding time-points (birth, 6 months, 1 year), and expressed the residuals as SD-scores. This produces uncorrelated variables describing change between specific ages (‘conditional SD-scores’). Quadratic terms were included when relationships between measurements at different ages were non-linear.
Associations between size and conditional growth in early life and adult outcomes were examined using regression, adjusting for age in all models, and for potential confounding variables as described. There were a number of interactions between sex and BMI (both early-life and adult BMI) in relation to the outcomes; we therefore analysed the data separately for men and women. Analyses were carried out using SPSS version 15.0.
RESULTS
Of the 1583 men and women who agreed to participate, 57 were excluded (24 were pregnant, 2 left after recruitment and 31 were unreliably linked to earlier data) leaving 1526 (59% of those traced and 19% of the original cohort). Fibrinogen values were available for 1492, hsCRP for 1472 and PAI-1 for 1469. Compared with the original birth cohort, 7% more of the participants in this study were male, maternal literacy was 6% higher, and mean birthweight was 32 g heavier. BMI and height in infancy, childhood and adolescence were approximately 0.1 SD lower. Mean concentrations of the pro-inflammatory/pro-thrombotic variables are shown in Table 1. HsCRP concentrations were positively correlated with fibrinogen and PAI-1 (r=0.21 and r=0.14, p<0.001 for all, adjusted for age and sex).
TABLE 1.
Body measurements at birth, 2, 11 and 26-32 years, adult pro-inflammatory and pro-thrombotic parameters and other cardiovascular risk factors
| Men (n=886) |
Women (n=640) |
||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Mean | SD | n | Mean | SD | n | ||
| Birth | Length (cm) | 48.8 | 2.1 | 779 | 48.3 | 1.9 | 558 |
| Weight (kg) | 2.89 | 0.44 | 803 | 2.79 | 0.38 | 561 | |
| At 2 years | Height (cm) | 81.1 | 3.6 | 840 | 79.6 | 3.6 | 609 |
| Weight (kg) | 10.3 | 1.3 | 834 | 9.8 | 1.2 | 609 | |
| BMI (kg/m2) | 15.8 | 1.2 | 833 | 15.4 | 1.2 | 604 | |
| At 11 years | Height (cm) | 135.9 | 5.7 | 831 | 134.2 | 7.4 | 607 |
| Weight (kg) | 28.4 | 4.7 | 834 | 27.6 | 5.4 | 608 | |
| BMI (kg/m2) | 15.3 | 1.7 | 830 | 15.2 | 1.8 | 606 | |
| Adult | |||||||
| Age (years) | 29.2 | 1.3 | 886 | 29.2 | 1.4 | 640 | |
| Height (cm) | 169.7 | 6.4 | 886 | 154.9 | 5.7 | 638 | |
| BMI (kg/m2) | 24.9 | 4.3 | 886 | 24.6 | 5.1 | 638 | |
| Waist circumference (cm) | 90.2 | 12.1 | 886 | 79.6 | 12.4 | 640 | |
| Sum of triceps and subscapular skinfolds (mm)* |
39.6 | 27.6, 51.4 | 883 | 51.6 | 36.4, 66.4 | 635 | |
| Overweight (BMI ≥25) (%) | 47.4 | 886 | 45.5 | 638 | |||
| Obese (BMI ≥30) (%) | 9.5 | 886 | 13.0 | 638 | |||
| Any alcohol intake (%) | 56.2 | 886 | 1.4 | 640 | |||
| Ex-smokers (%) | 5.1 | 886 | 0.2 | 640 | |||
| Current smokers (%) | 29.8 | 886 | 0.2 | 640 | |||
| Fibrinogen (g/L)* | 2.5 | 2.2, 3.0 | 870 | 2.8 | 2.4, 3.2 | 622 | |
| hsCRP (mg/l)* | 2.0 | 1.0, 3.9 | 853 | 1.9 | 0.8, 4.6 | 618 | |
| PAI-1 (ng/ml)* | 95 | 56, 151 | 858 | 84 | 48, 136 | 611 | |
| Systolic blood pressure (mmHg) | 118 | 11 | 880 | 107 | 11 | 631 | |
| Diastolic blood pressure (mmHg) | 78 | 10 | 880 | 73 | 9 | 631 | |
| Fasting glucose (mmol/l)* | 5.4 | 4.9, 5.9 | 869 | 5.3 | 4.8, 5.8 | 623 | |
| insulin (pmol/l)* | 38 | 18, 66 | 868 | 32 | 14, 60 | 623 | |
| total cholesterol (mmol/l) | 5.2 | 1.1 | 869 | 4.8 | 0.9 | 623 | |
| HDL cholesterol (mmol/l) | 1.16 | 0.29 | 869 | 1.27 | 0.31 | 621 | |
| triglycerides (mmol/l)* | 1.48 | 1.07, 2.23 | 868 | 1.06 | 0.79, 1.37 | 623 | |
| 2-hour glucose (mmol/l)* | 5.9 | 5.1, 7.1 | 848 | 6.1 | 5.3, 7.1 | 591 | |
Median and IQR (inter-quartile range) for skewed variables
Associations of outcomes with current (adult) factors
All the pro-inflammatory/pro-thrombotic outcomes, especially hsCRP, were positively related to adult adiposity. Correlations coefficients (r) for BMI with fibrinogen, hsCRP and PAI-1 were 0.09, 0.26 and 0.20 in men and 0.13, 0.44, 0.09 in women respectively (p<0.05 for all), and there were similar correlations with sum of skinfolds and waist circumference. All outcomes were higher in participants with metabolic syndrome (N=435, 29%) than in the rest of the cohort: fibrinogen (2.94 g/L v 2.72 g/L; p=0.001) in women; PAI-1 in men (100.1 ng/ml v 83.3 ng/ml; p<0.001); and hsCRP in both sexes (2.25 mg/l v 1.86 mg/l; p=0.003 in men and 3.45 mg/l v 1.64 mg/l; p<0.001 in women).
In men, fibrinogen and PAI-1 rose with increasing socio-economic status (p=0.05 and 0.01) and PAI-1 rose with increasing alcohol intake (p=0.005). In women, hsCRP rose with increasing parity (p=0.008), PAI-1 was lower in those reporting more physical activity (p=0.05). HsCRP and PAI-1 were higher in men, and hsCRP was higher in women with a positive family history of CVD or diabetes (p<0.01 for all). None of the outcomes was related to tobacco use.
Associations of outcomes with BMI in early life
In women, BMI at birth and 2 years was inversely related to adult fibrinogen and hsCRP concentrations (Table 2 and Figure 1). Similar associations to those with BMI at birth were present with birthweight (fibrinogen p=0.01; hsCRP [after adjustment for adult BMI] p=0.003). There were no associations between size at birth and adult outcomes in men. None of the findings were changed after adjusting for gestational age at birth.
Table 2.
Mean plasma fibrinogen, serum CRP and plasma TPAI-1 concentrations according to body mass index (BMI) at birth, at age 2 years, 11 years and in adulthood
| Men | Women | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Fifths of BMI at |
BMI (kg/m2) limits |
N min,max |
Plasma Fibrinogen (g/dl) |
Serum CRP (mg/l) |
Plasma TPAI-1 (ng/ml) |
BMI (kg/m2) limits |
N min,max |
Plasma Fibrinogen (g/dl) |
Serum CRP (mg/l) |
Plasma TPAI-1 (ng/ml) |
| Birth | ||||||||||
| 1 | 8.6,11.0 | 146,150 | 2.54 | 1.93 | 87.2 | 8.2,11.0 | 107,109 | 2.86 | 1.95 | 81.6 |
| 2 | 11.0,11.8 | 153,153 | 2.53 | 1.97 | 89.0 | 11.0,11.6 | 109,111 | 2.80 | 2.04 | 80.7 |
| 3 | 11.8,12.3 | 151,153 | 2.60 | 2.00 | 98.3 | 11.6,12.2 | 106,108 | 2.78 | 1.77 | 74.2 |
| 4 | 12.3,13.1 | 150,155 | 2.54 | 1.99 | 86.6 | 12.2,12.9 | 103,106 | 2.72 | 2.08 | 78.0 |
| 5 | 13.1,16.6 | 147,153 | 2.49 | 2.00 | 90.0 | 12.9,16.4 | 107,109 | 2.62 | 1.74 | 78.4 |
| −0.01 | 0.00 | 0.01 | −0.13 | −0.03 | −0.04 | |||||
| (95% CI) | −0.08 to 0.06 | −0.07 to 0.07 | −0.06 to 0.08 | −0.22 to –0.05 | −0.12 to 0.05 | −0.13 to 0.04 | ||||
| p-value | 0.7 | 0.9 | 0.7 | 0.002 | 0.4 | 0.3 | ||||
| B* adjusted for adult BMI | −0.02 | −0.02 | 0.00 | −0.14 | −0.09 | −0.06 | ||||
| (95% CI) | −0.09 to 0.05 | −0.09 to 0.05 | −0.07 to 0.07 | −0.23 to –0.06 | −0.17 to –0.01 | −0.14 to 0.3 | ||||
| p-value | 0.6 | 0.5 | 0.9 | 0.001 | 0.02 | 0.2 | ||||
| Age 2 years | ||||||||||
| 1 | 12.4,14.7 | 154,160 | 2.48 | 2.16 | 95.3 | 11.7,14.4 | 115,116 | 2.87 | 1.87 | 84.0 |
| 2 | 14.7,15.4 | 164,167 | 2.52 | 2.03 | 85.9 | 14.4,15.1 | 116,120 | 2.68 | 1.85 | 79.7 |
| 3 | 15.4,16.1 | 162,165 | 2.52 | 1.98 | 87.3 | 15.1,15.7 | 119,119 | 2.80 | 1.96 | 74.4 |
| 4 | 16.1,16.8 | 161,164 | 2.58 | 1.95 | 94.5 | 15.7,16.4 | 115,117 | 2.81 | 1.87 | 74.3 |
| 5 | 16.8,20.4 | 161,164 | 2.56 | 1.84 | 82.8 | 16.4,19.8 | 115,119 | 2.65 | 2.02 | 80.2 |
| 0.05 | −0.04 | −0.03 | −0.08 | 0.00 | −0.03 | |||||
| (95% CI) | −0.02 to 0.12 | −0.11 to 0.03 | −0.10 to 0.04 | −0.16 to 0.00 | −0.08 to 0.09 | −0.11 to 0.06 | ||||
| p-value | 0.1 | 0.2 | 0.4 | 0.05 | 0.9 | 0.5 | ||||
| B* adjusted for adult BMI | 0.04 | −0.12 | −0.08 | −0.11 | −0.10 | −0.05 | ||||
| (95% CI) | −0.03 to 0.11 | −0.19 to –0.05 | −0.15 to –0.01 | −0.19 to –0.02 | −0.18 to –0.03 | −0.13 to 0.04 | ||||
| p-value | 0.3 | <0.001 | 0.02 | 0.01 | 0.009 | 0.3 | ||||
| Age 11 years | ||||||||||
| 1 | 12.2,14.1 | 152,161 | 2.46 | 1.91 | 83.4 | 10.4,13.8 | 113,115 | 2.73 | 1.50 | 79.4 |
| 2 | 14.1,14.8 | 160,163 | 2.53 | 2.02 | 88.4 | 13.8,14.6 | 119,120 | 2.81 | 1.80 | 81.7 |
| 3 | 14.8,15.5 | 162,163 | 2.53 | 1.80 | 86.2 | 14.6,15.3 | 117,119 | 2.72 | 1.98 | 77.4 |
| 4 | 15.5,16.4 | 159,162 | 2.52 | 1.90 | 88.9 | 15.3,16.3 | 112,116 | 2.74 | 1.91 | 79.5 |
| 5 | 16.4,28.5 | 163,165 | 2.59 | 2.22 | 103.8 | 16.3,23.9 | 116,118 | 2.81 | 2.25 | 81.2 |
| 0.08 | 0.08 | 0.11 | 0.03 | 0.12 | 0.03 | |||||
| (95% CI) | 0.01 to 0.15 | 0.01 to 0.15 | 0.04 to 0.18 | −0.05 to 0.11 | 0.04 to 0.20 | −0.06 to 0.11 | ||||
| p-value | 0.02 | 0.02 | 0.003 | 0.5 | 0.003 | 0.5 | ||||
| B* adjusted for adult BMI | 0.07 | −0.11 | −0.01 | −0.05 | −0.21 | −0.04 | ||||
| (95% CI) | −0.02 to 0.16 | −0.19 to –0.03 | −0.09 to 0.08 | −0.15 to 0.05 | −0.30 to –0.12 | −0.14 to 0.06 | ||||
| p-value | 0.1 | 0.01 | 0.9 | 0.3 | <0.001 | 0.4 | ||||
| Adult Life | ||||||||||
| 1 | 14.5,21.2 | 164,171 | 2.46 | 1.43 | 73.4 | 13.9,20.1 | 121,123 | 2.68 | 0.96 | 74.2 |
| 2 | 21.2,23.7 | 169,173 | 2.52 | 1.83 | 80.9 | 20.1,23.2 | 125,127 | 2.68 | 1.52 | 76.7 |
| 3 | 23.7,25.7 | 175,178 | 2.59 | 1.95 | 87.6 | 23.2,25.7 | 123,126 | 2.86 | 1.98 | 79.5 |
| 4 | 25.7,28.3 | 173,174 | 2.55 | 2.24 | 98.0 | 25.7,28.4 | 121,125 | 2.76 | 2.54 | 77.0 |
| 5 | 28.3,46.2 | 171,174 | 2.53 | 2.71 | 108.6 | 28.4,45.4 | 120,123 | 2.84 | 3.34 | 86.7 |
| 0.07 | 0.29 | 0.21 | 0.08 | 0.41 | 0.07 | |||||
| (95% CI) | 0.00 to 0.14 | 0.22 to 0.36 | 0.14 to 0.28 | 0.01 to 0.16 | 0.35 to 0.48 | 0.00 to 0.15 | ||||
| p-value | 0.05 | <0.001 | <0.001 | 0.02 | <0.001 | 0.04 | ||||
The means presented are unadjusted. The summary effect size estimates (regression coefficient (B) and 95% confidence intervals) and p values are derived from linear regression analyses, using all variables (measures of BMI in early-life and adult pro-inflammatory markers) as continuous standardised variables. These estimates are presented adjusted first for age alone, and then adjusted for adult BMI and other covariates as follows: alcohol consumption (four levels from none to heavy), tobacco use (never, ex-user, current user), socio-economic status in childhood (father’s occupation, ranging from 1 (low class) to 6 (high class)), adult socio-economic status derived from education level, household possessions and occupation (ranging from 1 (low class) to 17 (high class)) and family history of high blood pressure, angina, myocardial infarction, stroke or diabetes in a first degree relative.
Figure 1.
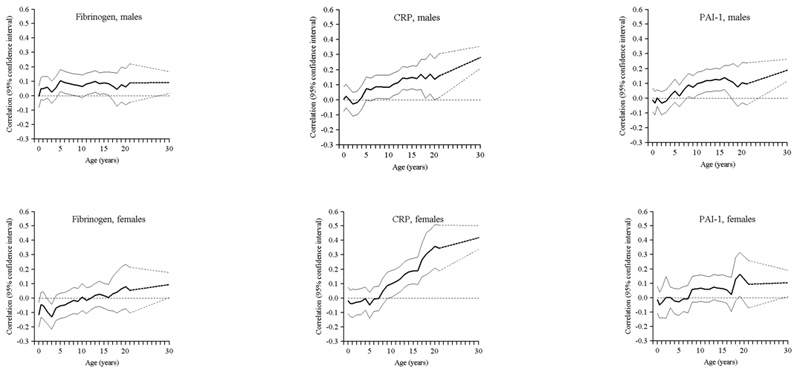
Age-adjusted correlations between BMI in earlier life and adult fibrinogen, hsCRP and PAI-1
In men, BMI at 2 years was inversely related to hsCRP and PAI-1 concentrations and BMI at 11 years was positively related to adult hsCRP concentration in both sexes after adjusting for adult BMI (Table 2 and Figure 1). Adult BMI was positively related to all outcomes in both sexes.
Conditional regression analyses showed that greater BMI gain from 2-11 years and/or 11 years to adulthood was associated with higher adult hsCRP concentrations (both sexes), higher PAI-1 in men, and higher fibrinogen in women (Table 3). These positive associations were attenuated after adjusting for adult adiposity (BMI or sum of skinfolds and waist circumference).
TABLE 3.
Multiple linear regression analyses using conditional BMI SD-scores in earlier life to predict adult outcomes
| BMI at birth (SD score) |
BMI change birth – 2 years (SD) * |
BMI change 2-11 years (SD) * |
BMI change1 11-adult (SD)* |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Risk Factors | ||||||||||||
| B | 95% CI | p | B | 95% CI | p | B | 95% CI | p | B | 95% CI | p | |
| MEN | ||||||||||||
| Fibrinogen (SD) | −0.01 | −0.09, 0.07 | 0.8 | 0.08 | −0.00, 0.15 | 0.06 | 0.07 | −0.01, 0.14 | 0.1 | 0.03 | −0.04, 0.11 | 0.4 |
| hsCRP (SD) | 0.01 | −0.06, 0.08 | 0.8 | −0.03 | −0.11, 0.04 | 0.4 | 0.14 | 0.07, 0.22 | <0.001 | 0.29 | 0.22, 0.36 | <0.001 |
| PAI-1 (SD) | −0.03 | −0.10, 0.05 | 0.5 | −0.05 | −0.12, 0.03 | 0.2 | 0.10 | 0.03, 0.18 | 0.008 | 0.14 | 0.07, 0.22 | <0.001 |
| WOMEN | ||||||||||||
| Fibrinogen (SD) | −0.14 | −0.23, -0.05 | 0.003 | −0.06 | −0.15, 0.03 | 0.2 | 0.04 | −0.05, 0.13 | 0.4 | 0.12 | 0.03, 0.21 | 0.01 |
| hsCRP (SD) | −0.01 | −0.09, 0.07 | 0.9 | 0.00 | −0.08, 0.08 | 1.0 | 0.14 | 0.06, 0.22 | 0.001 | 0.44 | 0.35, 0.52 | <0.001 |
| PAI-1 (SD) | −0.03 | −0.12, 0.06 | 0.5 | −0.00 | −0.10, 0.09 | 0.9 | 0.07 | −0.03, 0.16 | 0.2 | 0.07 | −0.02, 0.17 | 0.1 |
BMI changes are calculated as conditional measures (see Statistical Methods). The continuous outcome variables were normalised so that B (regression coefficient) values indicate the SD change in the outcome per SD change in the predictor. All analyses are adjusted for age and alcohol consumption (four levels from none to heavy), tobacco use (never, ex-user, current user), socio-economic status in childhood (father’s occupation, ranging from 1 (low class) to 6 (high class)), adult socio-economic status derived from education level, household possessions and occupation (ranging from 1 (low class) to 17 (high class)) and family history of high blood pressure, angina, myocardial infarction, stroke or diabetes in a first degree relative.
We analysed the outcomes in relation to combinations of BMI at birth or during infancy with adult BMI or conditional adult BMI, to determine whether the adverse effects of adult BMI were greater in individuals with lower early BMI. The highest fibrinogen, hsCRP and PAI-1 values tended to be in participants who were small at birth (women) or two years (both sexes) and adipose as adults (Supplementary Table 1). However, for fibrinogen and hsCRP, there were no interactions of either BMI at birth or 2-years with adult BMI or conditional BMI gain (11 years-adult). For PAI-1, there was a greater increase in concentrations with conditional adult BMI in men of lower 2 year BMI.
Associations of outcomes with height in early life
In comparison with BMI, there were only weak associations of height in early life with the adult pro-inflammatory markers (Online Supplementary Tables 2 and 3). Height at birth, 2 and 11 years, was inversely related to hsCRP in women, and height at 11 years was inversely related to hsCRP in men, after adjusting for adult BMI. Height at 2 years and 11 years was positively related to PAI-1 in men, but these associations were attenuated after BMI adjustment. Shorter adult height was associated with higher fibrinogen concentrations in women and higher CRP concentrations in men after BMI adjustment. Less height gain between 11 years and adulthood was associated with higher PAI-1 concentrations in women.
All analyses were repeated after excluding participants on medication that could alter inflammatory markers (corticosteroids N=1, oral contraceptives N=23, aspirin and other antiplatelet medications N=4, non-steroidal anti-inflammatory drugs N=33) and antibiotics (indicating current infection, N=12). The findings were unchanged.
DISCUSSION
The study reports an inverse association of birthweight and BMI at birth and 2 yrs with hsCRP concentration in both sexes and with fibrinogen in women, and BMI at 2 yrs with PAI-1 concentration in men. In contrast, greater BMI gain after infancy (between 2 and 11 years and/or 11 years and adulthood) was associated with an increase in all pro-inflammatory/pro-thrombotic markers, associations that diminished after adjusting for adult adiposity. Participants who were thin at birth (women) or 2yrs (both sexes) and had a high BMI as adults had the highest hsCRP, fibrinogen and PAI-1 levels.
C-reactive protein
An inverse relation of birthweight with adult hsCRP concentrations has been reported in the MIDSPAN study from the UK24 after adjusting for adult BMI, and in the Northern Finland birth cohort study.25 The Finnish study also showed, consistent with our findings, that greater weight gain from birth-14 years, and 14-31 years, was associated with higher hsCRP concentrations. In the Pelotas birth cohort (Brazil), there were positive associations between weight gain at all ages >1 year and adult hsCRP concentrations.22 In contrast, the Barry-Caerphilly study (Wales) showed no association with weight velocity between 1 and 5 years.23
Fibrinogen
Our finding of an association of lower birthweight with higher fibrinogen in women and not men is consistent with the ARIC study 36; the associations were stronger for white Caucasians than African Americans suggesting ethic differences. Other reports of associations between birth weight and fibrinogen have been inconsistent.20,21,23,37,38 Lower birthweight is associated with higher clottable fibrinogen concentrations within dizygotic but not monozygotic twins39 suggesting that genetic factors could contribute to the association. The inconsistencies among studies could be due to ethnic genetic differences. Low weight at one year in men (but not women) was associated with higher adult fibrinogen concentrations in the Hertfordshire study.20,40 In the Caerphilly study, weight velocity in infancy was not related to adult fibrinogen concentrations.23
Plasminogen activator inhibitor
In the only published study of birthweight in relation to PAI-1, a strong inverse association after adjusting for adult BMI was found in 70-year old men.26 Genetic determinants are a stronger influence on concentrations of PAI-1 in South Asian population which could explain weaker associations in our study.41
Potential Mechanisms
Associations of lower BMI at birth and during infancy with higher hsCRP, fibrinogen and PAI-1 may indicate persisting effects of the intra-uterine and early post-natal environment or genetic effects. Small-for-gestational-age newborns have high cord blood hsCRP concentrations (after excluding known infected pregnancies) perhaps supporting a programming effect.42 Associations with fibrinogen, hsCRP and PAI-1 (factors released from the liver in response to interleukin-6 [IL-6] and chiefly cleared by the kidneys), may indicate impaired development of hepatic and/or renal tissue due to in-utero or early post-natal influences. Up-regulation of inflammatory cytokines has been proposed as a mechanism of in utero ‘thriftiness’, inducing muscular insulin resistance in order to preserve the glucose supply to the brain.43 Increased maternal circulating glucocorticoids, due to stressful environmental factors could influence the fetus via transplacental transfer of glucocorticoids and other hormones, or stimulation of the fetal adrenal axis, causing a persistent inflammatory response. Post-natal stressors could have a similar effect during infancy; smaller newborns are more prone to infection, and maltreated children have higher inflammatory markers in adult life.44 Impaired intrauterine growth followed by infection postnatally could further augment the inflammatory response.
Adipose tissue is a major source of IL-6, the main stimulus for hsCRP and fibrinogen production. Our data suggest that the positive relationships of the adult outcomes to earlier BMI gain (especially BMI gain from 11 years) is observed because BMI gain at this stage of the life course is strongly related to the development of adult adiposity.28 Alternatively, a sub-clinical inflammatory state could cause BMI gain.45,46 Regulation of metabolism and food intake by cytokines, regulation of the hypothalamo-pituitary-adrenal axis by cytokines, leptin deficiency and resistance to leptin are possible mechanisms. Thus, an inflammatory state caused by early life factors could potentially cause excessive adipose tissue gain during childhood and adolescence. In the absence of measures of inflammatory markers in early life, it is impossible to ascertain whether inflammation drives excess adipose tissue acquisition or vice versa. Sex differences in the relative deposition of lean and adipose tissue during childhood could account for the sex differences we observed in associations between early BMI growth and adult pro-inflammatory markers. Another possible explanation for the sex differences is a greater impact on liver development in the growth-restricted female fetus, than in the male. Studies in the Finnish birth cohort showed an association between short birth length and coronary heart disease mortality in women but not men47.
Strengths and limitations of the study
Strengths of the study were that it was population-based and children were measured by trained personnel, with frequent follow-up throughout childhood. Like other birth cohorts, there was considerable loss to follow-up and participants are likely to be unrepresentative of the original sample. Only 19% of the original cohort participated. There was a slightly higher percentage of men among the participants than in the original cohort, probably because women are more likely to move away when they marry, and maternal education was higher in the study sample than in the original cohort, which probably reflects the influence of social factors on where people settle in adult life. The differences in their mean size at birth and during infancy and childhood, however, were trivial. In a within-sample analysis, loss to follow-up would introduce bias only if associations between early BMI/weight and later disease differed between those studied and not studied, which seems unlikely because inclusion was based only on subjects’ availability. Another limitation is that we did not collect data on infection at the time of the data collection.
In summary, thinness at birth and/or in infancy is associated with higher fibrinogen, hsCRP and PAI-1 in adulthood. Both in-utero influences and greater adiposity due to BMI gain in childhood/adolescence could be implicated consolidating the need to prevent excessive BMI gain in childhood.
Supplementary Material
Supplementary Table 1: Mean fibrinogen, hsCRP and PAI-1 according to thirds of BMI at 2 yrs and adulthood
Supplementary Table 2: Mean plasma fibrinogen, serum CRP and plasma TPAI-1 concentrations according to height at birth, at age 2 years, 11 years and in adulthood
Supplementary Table 3: Multiple linear regression analyses using conditional Height SD-scores in earlier life to predict adult outcomes
Key messages.
Thinness at birth and accelerated BMI gain during childhood are associated with an increased risk of adult cardiovascular disease. Several studies have suggested that these associations may be mediated by inflammation.
We have examined the relationship of BMI and height growth in early life to adult pro-inflammatory/pro-thrombotic markers (fibrinogen, CRP and PAI-1 concentrations) in a birth cohort from Delhi, India.
Lower BMI at birth (women) and during infancy (both sexes), and accelerated BMI gain during childhood were associated with higher adult fibrinogen, CRP and PAI-1 concentrations.
Acknowledgements
We acknowledge Rajeshwari Verma and Bhaskar Singh for maintaining liaison with the cohort.
Funding sources: This work was supported by the British Heart Foundation [grant RG98001].
The original cohort studies were supported by the National Center for Health Statistics and the Indian Council of Medical Research
Footnotes
Disclosures: None
REFERENCES
- 1.Ghaffar AA, Reddy KS, Singhi M. Burden of non-communicable diseases in South Asia. BMJ. 2004;328:807–810. doi: 10.1136/bmj.328.7443.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Unwin N, Gan D, Whiting D. The IDF diabetes Atlas: Providing evidence, raising awareness and promoting action. Diabetes Res Clin Pract. 2009 doi: 10.1016/j.diabres.2009.11.006. Epub ahead of rpint. [DOI] [PubMed] [Google Scholar]
- 3.Esmon CT. The interactions between inflammation and coagulation. British J Haematol. 2005;131:417–430. doi: 10.1111/j.1365-2141.2005.05753.x. [DOI] [PubMed] [Google Scholar]
- 4.Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 5.Casas JP, Shah T, Hingorani AD, Danesh J, Pepys MB. C-reactive protein and coronary heart disease; a critical review. J Intern Med. 2008;264:295–314. doi: 10.1111/j.1365-2796.2008.02015.x. [DOI] [PubMed] [Google Scholar]
- 6.Fibrinogen Studies Collaboration Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. JAMA. 2005;294:1799–809. doi: 10.1001/jama.294.14.1799. [DOI] [PubMed] [Google Scholar]
- 7.Danesh J, Collins R, Appleby P, Peto R. Association of Fibrinogen, C-reactive Protein, Albumin, or Leukocyte Count With Coronary Heart Disease; Meta-analyses of Prospective Studies. JAMA. 1998;279:1477–1482. doi: 10.1001/jama.279.18.1477. [DOI] [PubMed] [Google Scholar]
- 8.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 9.Barzilay JI, Abraham L, Heckbert SR, et al. The relation of markers of inflammation to the development of glucose disorders in the elderly: the Cardiovascular Health Study. Diabetes. 2001;50:2384–2389. doi: 10.2337/diabetes.50.10.2384. [DOI] [PubMed] [Google Scholar]
- 10.Freeman DJ, Norrie J, Caslake MJ, et al. West of Scotland Coronary Prevention Study. C-reactive protein is an independent predictor of risk for the development of diabetes in the West of Scotland Coronary Prevention Study. Diabetes. 2002;51:1596–1600. doi: 10.2337/diabetes.51.5.1596. [DOI] [PubMed] [Google Scholar]
- 11.Lowe GDO, Danesh J, Lewington S, et al. Tissue plasminogen activator antigen and coronary heart disease. Eur Heart J. 2004;25:252–259. doi: 10.1016/j.ehj.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Ridker PM, Brown NJ, Vaughan DE, Harrison DG, Mehta JL. Established and emerging plasma biomarkers in the prediction of first atherothrombotic events. Circulation. 2004;109(Suppl 1):IV6–IV19. doi: 10.1161/01.CIR.0000133444.17867.56. [DOI] [PubMed] [Google Scholar]
- 13.Forouhi NG, Sattar N. CVD risk factors and ethnicity – a homogeneous relationship? Atheroscl Supplements. 2006;7:11–19. doi: 10.1016/j.atherosclerosissup.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Anand SS, Yusuf S, Vuksan V, et al. Differences in risk factors, atherosclerosis, and cardiovascular disease between ethnic groups in Canada: the Study of Health Assessment and Risk in Ethnic groups (SHARE) Lancet. 2000;356:279–284. doi: 10.1016/s0140-6736(00)02502-2. [DOI] [PubMed] [Google Scholar]
- 15.Kain K, Catto AJ, Grant PJ. Impaired fibrinolysis and increased fibrinogen levels in South Asian subjects. Atherosclerosis. 2001;156:457–461. doi: 10.1016/s0021-9150(00)00684-5. [DOI] [PubMed] [Google Scholar]
- 16.Osmond C, Barker DJP, Winter PD, Fall CHD, Simmonds SJ. Early growth and death from cardiovascular disease in women. BMJ. 1993;307:1519–1524. doi: 10.1136/bmj.307.6918.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eriksson JG, Forsen T, Tuomilehto HJ, Barker DJP. Early growth and coronary heart disease in later life: longitudinal study. BMJ. 2001;322:949–53. doi: 10.1136/bmj.322.7292.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huxley R, Owen CG, Whincup PH, et al. Is birth weight a risk factor for ischemic heart disease in later life? Am J Clin Nutr. 2007;85:1244–1250. doi: 10.1093/ajcn/85.5.1244. [DOI] [PubMed] [Google Scholar]
- 19.Whincup PH, Kaye SJ, Owen CG, et al. Birthweight and risk of type 2 diabetes: a quantitative systematic review of published evidence. JAMA. 2008;300:2885–2897. [Google Scholar]
- 20.Barker DJP, Meade TW, Fall CHD, et al. Relation of fetal and infant growth to plasma fibrinogen and factor VII concentrations in adult life. BMJ. 1992;304:148–152. doi: 10.1136/bmj.304.6820.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martyn CN, Meade TW, Stirling Y, Barker DJ. Plasma concentrations of fibrinogen and Factor VII in adult life and their relation to intra-uterine growth. British J Haematol. 1995;89:142–146. doi: 10.1111/j.1365-2141.1995.tb08920.x. [DOI] [PubMed] [Google Scholar]
- 22.Nazmi A, Gonzalez DC, Oliveira IO, Horta BL, Gigante DP, Victora C. Life course weight gain and C-reactive protein levels in young adults: findings from a Brazilian birth cohort. Am J of Hum Biol. 2009;21:192–199. doi: 10.1002/ajhb.20852. [DOI] [PubMed] [Google Scholar]
- 23.Fraser A, Hughes R, McCarthy A, et al. Early life growth and hemostatic factors; the Barry Caerphilly Study. Am J Epidemiol. 2008;168:179–187. doi: 10.1093/aje/kwn106. [DOI] [PubMed] [Google Scholar]
- 24.Sattar N, McConnachie A, O’Reilly D, et al. Inverse association between birth weight and C-reactive protein concentrations in the MIDSPAN family study. Arterioscler Thromb Vas Biol. 2004;24:583–587. doi: 10.1161/01.ATV.0000118277.41584.63. [DOI] [PubMed] [Google Scholar]
- 25.Tzoulaki I, Jarvelin M-R, Hartikainen A-L, et al. Size at birth, weight gain over the life course, and low-grade inflammation in young adulthood: northern Finland 1966 birth cohort study. Eur Heart J. 2008;29:1049–1056. doi: 10.1093/eurheartj/ehn105. [DOI] [PubMed] [Google Scholar]
- 26.Byberg L, McKeigue P, Zethelius B, Lithell HO. Birthweight and the insulin resistance syndrome; association of low birthweight with truncal obesity and raised plasminogen activator inhibitor-1 but not with abdominal obesity or plasma lipid disturbances. Diabetologia. 2000;43:54–60. doi: 10.1007/s001250050007. [DOI] [PubMed] [Google Scholar]
- 27.Bhargava SK, Sachdev HPS, Fall CHD, et al. Relation of serial changes in childhood body mass index to impaired glucose tolerance in young adulthood. New Eng J Med. 2004;350:865–875. doi: 10.1056/NEJMoa035698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sachdev HPS, Fall CHD, Osmond C, et al. Anthropometric indicators of body composition in young adults: relation to size at birth and serial measurements of body mass index in childhood; the New Delhi birth cohort. Am J Clin Nutr. 2005;82:456–466. doi: 10.1093/ajcn.82.2.456. [DOI] [PubMed] [Google Scholar]
- 29.Fall CHD, Sachdev HPS, Osmond C, et al. Adult metabolic syndrome and impaired glucose tolerance are associated with different patterns of body mass index gain during infancy; data from the New Delhi birth cohort. Diabetes Care. 2008;31:2349–2356. doi: 10.2337/dc08-0911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization . Report of a WHO Consultation. World Health Organization, WHO/NCD/NCS/99.2; Geneva: 1999. Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications. [Google Scholar]
- 31.National Cholesterol Education Program Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults. 3rd report of the National Cholesterol Education Program (NCEP) (Adult treatment panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 32.Grundy S. Metabolic Syndrome Scientific Statement by the American Heart Association and the National Heart, Lung, and Blood Institute. Arterioscler Thromb Vasc Biol. 2005;25:2243–2244. doi: 10.1161/01.ATV.0000189155.75833.c7. [DOI] [PubMed] [Google Scholar]
- 33.Von Clauss A. Gerinnungsphysiologische Schnellmethode zur Bestimmung des Fibrinogens. Acta Haematol. 1957;17:237–246. doi: 10.1159/000205234. [DOI] [PubMed] [Google Scholar]
- 34.Gale CR, O’Callaghan FJ, Bredow M, Martyn CN, and the ALSPAC Study Team The influence of head growth in fetal life, infancy and childhood on intelligence at the ages of 4 and 8 years. Pediatrics. 2006;118:1486. doi: 10.1542/peds.2005-2629. [DOI] [PubMed] [Google Scholar]
- 35.Adair LS, Martorell R, Stein AD, Hallal PC, Sachdev HPS, Prabhakaran D, Wills AK, Norris SA, Dahly DL, Lee NR, Victora CG, COHORTS group Size at birth, weight gain in infancy and childhood, and adult blood pressure in five low and middle income country cohorts: When does weight gain matter? AJCN. 2009;89:1383–92. doi: 10.3945/ajcn.2008.27139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pellanda LC, Duncan BB, Vigo A, Rose K, Folsom AR, Erlinger TP, ARIC investigators Low birthweight and indicators of inflammation and endothelial activation in adulthood; the ARIC study. International J Cardiol. 2009;134:371–377. doi: 10.1016/j.ijcard.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frankel S, Elwood P, Sweetnam P, Yarnell J, Smith GD. Birthweight, adult risk factors and incident coronary heart disease: the Caerphilly Study. Public Health. 1996;110:139–143. doi: 10.1016/s0033-3506(96)80066-7. [DOI] [PubMed] [Google Scholar]
- 38.Fall CHD, Osmond C, Barker DJP, et al. Fetal and infant growth and cardiovascular risk factors in women. BMJ. 1995;310:428–432. doi: 10.1136/bmj.310.6977.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tuya C, Mutch WJ, Broom I, Mcneill G. The effect of birthweight on clottable and intact fibrinogen; a twin study. J Thromb Hemostasis. 2005;3:1143–1148. doi: 10.1111/j.1538-7836.2005.01431.x. [DOI] [PubMed] [Google Scholar]
- 40.Henry JA, Bolla M, Osmond C, Fall C, Barker DJ, Humphries SE. The effects of genotype and infant weight on plasma levels of fibrinogen, factor VII and LDL-cholesterol are additive. BMJ. 1997;34:553–558. doi: 10.1136/jmg.34.7.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chetty N Naran, Crowther NJ. The influence of metabolic syndrome components on plasma PAI-1 concentrations is modified by the PAI-1 4G/5G genotype and ethnicity. Atherosclerosis. 2008;196:155–163. doi: 10.1016/j.atherosclerosis.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 42.Trevisanuto D, Doglioni N, Ainier S, Zaninotto M, Plebani M, Zanardo V. High sensitivity C-reactive protein in umbilical cord of small-for-gestational-age neonates. Neontatology. 2007;91:186–9. doi: 10.1159/000097451. [DOI] [PubMed] [Google Scholar]
- 43.Despina D Briana, Ariadne Malamitsi-Puchner. Intrauterine growth restriction and adult disease: the role of adipocytokines. Eur J Endocrin. 2009:337–347. doi: 10.1530/EJE-08-0621. [DOI] [PubMed] [Google Scholar]
- 44.Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. PNAS. 2007;104:1319–24. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duncan BB, Schmidt MI, Chambless LE, Folsom AR, Carpenter M, Heiss G, ARIC investigators Fibrinogen, other putative markers of inflammation, and weight gain in middle-aged adults; the ARIC Study. Obesity Research. 2000;8:279–86. doi: 10.1038/oby.2000.33. [DOI] [PubMed] [Google Scholar]
- 46.Engstrom G, Hedblad G, Stavenow L, Lind P, Janzon L, Lindgarde F. Inflammation-Sensitive Plasma Proteins Are Associated With Future Weight Gain. Diabetes. 2003;52:2097–2101. doi: 10.2337/diabetes.52.8.2097. [DOI] [PubMed] [Google Scholar]
- 47.Forsen T, Eriksson JG, Tuomilehto J, Osmond C, Barker DJP. Growth in utero and during childhood among women who develop coronary heart disease: longitudinal study. BMJ. 1999;319:1403–1407. doi: 10.1136/bmj.319.7222.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Mean fibrinogen, hsCRP and PAI-1 according to thirds of BMI at 2 yrs and adulthood
Supplementary Table 2: Mean plasma fibrinogen, serum CRP and plasma TPAI-1 concentrations according to height at birth, at age 2 years, 11 years and in adulthood
Supplementary Table 3: Multiple linear regression analyses using conditional Height SD-scores in earlier life to predict adult outcomes


