Abstract
Background
Maternal and child survival are highly correlated, but the contribution of HIV infection on this relationship, and in particular the impact of HIV treatment has not been quantified. We estimate the association between maternal HIV and treatment and under-5 child mortality in a rural population in South Africa.
Methods
All children born between January 2000-January 2007 in the Africa Centre Demographic Surveillance Area were included. Maternal HIV status information was available from HIV surveillance; maternal antiretroviral treatment (ART) from the HIV Treatment Programme database and linked to surveillance data. Mortality rates were computed as deaths per 1000 person-years observed. Time-varying maternal HIV effect (positive, negative, ART) on U5MR was assessed in Cox regression, adjusting for other factors associated with under-5 mortality.
Results
9,068 mothers delivered 12,052 children, of whom 947 (7.9%) died before age 5. Infant mortality rate (IMR) declined by 49% from 69.0 in 2000 to 35.5 in 2006 deaths per 1000 person-years observed; a significantly decline was observed post-ART (2004-2006). The estimated proportion of deaths across all age groups were higher among the children born to the HIV-positive and HIV-not reported status women than among children of HIV-negative women. Multivariably, mortality in children of mothers on ART was not significantly different from children of HIV-negative mothers (aHR 1.29, 0.53-3.17; p=0.572).
Conclusions
These findings highlight the importance of maternal HIV treatment with direct benefits of improved survival among all children under-5. Timely HIV treatment for eligible women is required to benefit both mothers and children.
Introduction
Maternal HIV infection affects child mortality directly through mother-to-child transmission or indirectly through maternal ill-health [1-4]. A mother’s death has a negative impact on child health, even in the absence of transmission to the child; uninfected children of infected mothers may be more likely to be orphaned and thus be at higher risk of child mortality than children brought up by their own mothers [5-7].
Sub-Saharan Africa is the most heavily HIV-affected region in the world, with 67% of global new HIV infections in 2008, and 72% of AIDS-related deaths [8]. In South Africa, home to the world’s largest population of people living with HIV (5.7 million), child mortality rates have continued to increase since 1990, coinciding with the rapid rise in HIV prevalence in pregnant women from 1% in 1990 to more than 29% in 2008 [9, 10]. South Africa will fall short of achieving Millennium Development Goal 4 (MDG4) to reduce under-5 mortality by two-thirds from baseline 60 deaths per 1000 live-births by year 2015 [11].
Antiretroviral therapy (ART), which substantially delays HIV disease progression, is contributing to a decline in adult HIV-related mortality at a population level [12, 13]. Recent evidence suggests that declining early child mortality at a population level is similarly associated with expanding HIV treatment services [14]; in an earlier study in this rural area we estimated that the effect of maternal ART provision was greater than the impact of programmes delivering single-dose nevirapine (sdNVP) to prevent mother-to-child transmission, presumably because survival of infected mothers benefits both infected and uninfected children [14]. However, the association between survival of HIV infected mothers on ART and child survival at an individual level remains unquantified.
We investigated under-5 mortality in a largely rural population of KwaZulu-Natal, South Africa, with high HIV prevalence [15, 16], and quantify the association between maternal survival and child mortality, through linkage of mother and child records from demographic surveillance and an HIV treatment programme.
Methods
The Africa Centre Demographic Surveillance Area (DSA), Umkhanyakude district, northern KwaZulu-Natal, covers 438 km2 and nearly 90,000 Zulu-speaking people, including 22,600 women of child-bearing age, in 11,000 households. The area is predominantly rural; the main sources of income are waged employment and state pensions [17].
Since 2000, data including births, deaths, migration, and vaccination history are supplied by a key informant on all resident and non-resident household members during bi-annual household visits [18]. Further, since 2003, annual HIV surveillance documents the HIV status of consenting resident women aged 15-49 years and men aged 15-54 years [19]. Written informed consent allowing use of routine data was obtained from all participants. Ethical approval was obtained from the Biomedical Research Ethics Committee of the University of KwaZulu-Natal.
All children born between 1 January 2000 and 1 January 2007 (to allow at least 3 years of observation for children born post-2004 and for mothers of children born pre-2004 to have initiated ART before their children turned 5 years) to women enrolled in the Africa Centre surveillance were included. Twin births are at increased mortality risk [7] as are first born children [20]. We classified pregnancy as singleton or twin and birth order into three groups: first, second/third or fourth/later born. A count of household assets, closest to date of birth, (classified as ≤5 or >5) was used to indicate economic household status [21]. Previous child death and previous fetal death have been associated with increased risk of death of subsequent children [14]; the former was defined as a child death (age <5 years) prior to the birth of index child. Last known maternal vital status at or before end of observation was classified as ‘alive’, ‘dead’ or ‘unknown’.
The Department of Health Hlabisa HIV Treatment and Care Programme, initiated in late 2004, is supported by the Africa Centre for Health and Population Studies (www.africacentre.ac.za), with funding from PEPFAR. The programme provides free services at the 17 local government primary health care clinics (PHC) [22, 23]; 6 of which are located in the DSA. We used data regarding maternal antiretroviral treatment (ART) from the ARTemis database (an operational database of HIV-infected people on antiretroviral treatment) [22]; independent data were linked to the HIV surveillance data using the national South African identification number, a unique identifier in both datasets, and other identifiers where needed. Ethical approval for this linkage was obtained. Some women have ART information but no HIV surveillance data, as they have accessed treatment but not the surveillance. By end-2006, the last birth date in the cohort, 396 women were initiated on ART, rising to 2,172 by the end of observation [22]. A PMTCT programme was introduced in 2001 and established at all primary health care clinics by end 2002 [24] with sdNVP provided to all HIV-infected women and their infants. In 2007, sdNVP uptake in babies born to infected women was estimated at 54% [25]. The PMTCT programme changed in late-2008 in the district to include Zidovudine (AZT) from 28 weeks of pregnancy. Further changes came into effect from April 2010, when all HIV-infected pregnant women with a CD4 count ≤350 cell/mm3 are started on life-long ART, while those not eligible for ART are provided with AZT during pregnancy from 14 weeks [26]. We previously estimated the median duration of exclusive breastfeeding in this setting as 177 (IQR 150-180) and 175 days (IQR 137-180) in HIV-negative and HIV-positive women respectively with exclusive breastfeeding support provided by well trained lay counselors for 6 months [27]. 14.1% of exclusively breastfed infants were infected with HIV by age 6 weeks and 19.5% by 6 months of age [28].
Maternal HIV status based on data from the HIV surveillance was classified into known (to us, from surveillance) negative, not-reported, known positive (including unknown at birth of child but HIV-negative before pregnancy and positive after delivery) and ‘On ART (from the HIV Treatment clinical database)’ for children whose mothers were initiated on ART within 5 years post-delivery before 31st December 2009. Date of initiation is also recorded. The study included 12 mothers who began HAART before delivery; overall, an estimated maximum 12% of women would have been eligible for ART with a CD4 below the cut-off for eligibility at the time [24].
Statistical methods
Infant and child mortality rates were computed as deaths per 1000 person-years observed. For descriptive analysis, a chi-square test was used for categorical variables and two-sample test of proportions for independent proportions. Survival by age group was estimated by Kaplan-Meier method and Cox regression analysis was used to investigate the effect of maternal HIV status on U5MR, in a time-varying approach, adjusting for factors known to be associated with mortality. Observation time was censored at change of maternal HIV status to ‘On ART’ and at seroconversion from HIV-negative to positive status reflecting the end of the former category and the start of the latter. For mothers who were on ART since before birth time was set to 0. For variables with missing data, we included ‘unknown’ as a category in the model to retain overall denominators. For each child, the observation time was taken as time from birth until death within the five years, date of fifth birthday, end of observation (31 December 2009) or date last seen, whichever came first. In multivariable analysis, all variables with a statistically significant association univariately were included. Goodness of fit of the final models was assessed using the likelihood-based Akaike information criteria (AIC) [29]. Estimated mortality rates were obtained from the predicted survivor function of the Cox regression model. We used Stata (Version 11.0, Stata Corporation, College Station, Texas, USA), which allows the analysis of continuous and time-dependent covariates.
Results
Nine hundred and forty-seven children died before 5 years of age from 12,052 live-births to 9,068 women (Table 1). Total number of child-years’ follow-up was 47,482. A total of 307 (3.4%) mothers initiated ART within 5 years post-delivery; 12 (3.9%) women were initiated before delivery (and continued beyond) with median time to delivery of 1.8 months [lnterquartile range (IQR) 0.7-10.6]. 193 (7.5%) of 2,591 HIV-positive and 114 (2.3%) of 4,860 ‘HIV-not reported’ mothers initiated ART. 12 (3.9%) of mothers initiated ART within 6 months of delivery, 17 (5.5%) between 6 months and 1 year of age, 55 (17.9%) between 1-2 years, 60 (19.5%) between 2-3 years and 151 (49.2%) initiated between 3-5 years of the child’s age. Women who initiated ART were older, more educated, had the index child as their first born, and among those with more than one child, a significantly larger proportion (15.7% vs. 4.7%, p=0.018) reported previous history of child death (age <5 years) prior to birth of the index child compared to those not initiated on ART.
Table 1.
Distribution of demographic variables and risk factors of children born at ACDIS, 2000-2007
| Characteristic | Alive (n=11 105) Number (%) |
Dead(n=947) Number (%) |
P | Mortality rate per 1000 live births |
|---|---|---|---|---|
| Sex | ||||
| Male | 5,501 (49.5) | 446 (47.1) | 74.9 | |
| Female | 5,604 (52.8) | 501 (52.8) | 0.158 | 82.1 |
| Multipregnancy | ||||
| Singleton | 10,295 (92.7) | 888 (93.8) | 79.4 | |
| Twin | 352 (3.2) | 49 (5.2) | 122.2 | |
| Unknown | 458 (4.1) | 10 (1.0) | 0.006 | 21.4 |
| Birth order | ||||
| First born | 4,468 (40.2) | 387 (40.9) | 79.7 | |
| Second/third | 3,943 (35.6) | 377 (39.8) | 87.3 | |
| Fourth/later | 2,236 (20.1) | 173 (18.3) | 71.8 | |
| Unknown | 458 (4.1) | 10 (1.0) | <0.001 | 21.4 |
| Delivery location | ||||
| Homestead | 1,149 (10.4) | 139 (14.7) | 107.9 | |
| Clinic | 2,773 (24.9) | 266 (28.1) | 87.5 | |
| Hospital | 5,889 (53.0) | 465 (49.1) | 73.2 | |
| Unknown | 1, 294 (11.7) | 77 (8.1) | <0.001 | 56.2 |
| Area of birth | ||||
| Rural | 6,961 (62.7) | 602 (63.6) | 79.6 | |
| Peri-urban | 3,305 (29.8) | 314 (33.2) | 86.8 | |
| Urban | 508 (4.6) | 29 (3.1) | 54.0 | |
| Unknown | 331 (2.9) | 2 (0.2) | <0.001 | 6.0 |
| Household assets | ||||
| <=5 | 2,001 (18.0) | 211 (22.3) | 95.4 | |
| >5 | 8,556 (77.1) | 718 (75.8) | 77.4 | |
| Unknown | 548 (4.9) | 18 (1.9) | <0.001 | 31.8 |
|
Maternal characteristics Age (years) |
||||
| <20 | 2,532 (22.8) | 179 (18.9) | 66.0 | |
| 20-30 | 5,243 (47.2) | 526 (55.5) | 91.2 | |
| >30 | 2,519 (22.7) | 203 (21.4) | 74.6 | |
| Unknown | 811 (7.3) | 39 (4.1) | 0.001 | 45.9 |
| Education | ||||
| At least Primary | 2,751 (24.8) | 266 (28.1) | 88.2 | |
| Post primary | 7,708 (69.4) | 624 (65.9) | 74.9 | |
| Unknown | 646 (5.8) | 57 (6.0) | 0.065 | 81.1 |
|
Previous Fetal death |
||||
| No | 10,413 (93.8) | 906 (95.7) | 80.0 | |
| Yes | 361 (3.3) | 39 (4.1) | 97.5 | |
| Unknown | 331 (2.9) | 2 (0.2) | <0.001 | 6.0 |
|
Previous Child death |
||||
| No | 10,524 (94.8) | 866 (91.5) | 76.0 | |
| Yes | 250 (2.3) | 79 (8.3) | 240.1 | |
| Unknown | 331 (2.9) | 2 (0.2) | <0.001 | 6.0 |
| Vital status | ||||
| Alive | 10,386 (93.5) | 788 (83.2) | 70.5 | |
| Died | 367 (3.3) | 144 (15.2) | 281.8 | |
| Unknown | 352 (3.2) | 15 (1.6) | <0.001 | 40.8 |
| HIV Status | ||||
| HIV-negative | 4, 146 (37.3) | 148 (15.5) | 34.5 | |
| HIV-not reported | 4,418 (39.8) | 442 (46.7) | 90.9 | |
| HIV-positive | 2,242 (20.2) | 349 (36.9) | 134.7 | |
| On ART | 299 (2.7) | 8 (0.8) | <0.001 | 26.1 |
ACDIS stands for Africa Centre Demographic Information System. P is for the difference between the children who died and those who were alive.
Median age of mothers was 24 years (IQR 20-30); most had post primary education. Although only a small percentage (4.2%) of children had mothers who had died, these children had higher mortality than those whose mothers were alive (Table 1). Similarly, mortality rate was higher in children of infected women who never initiated ART than those whose mothers were initiated (Table 1).
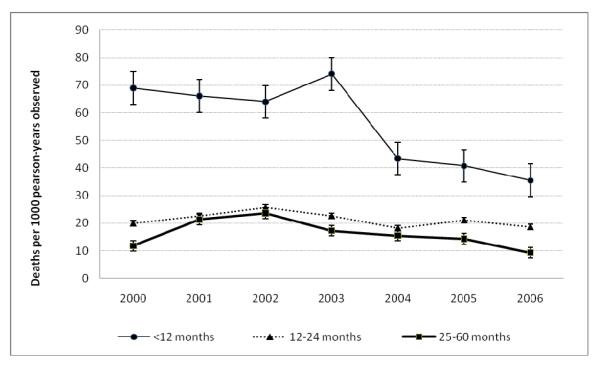
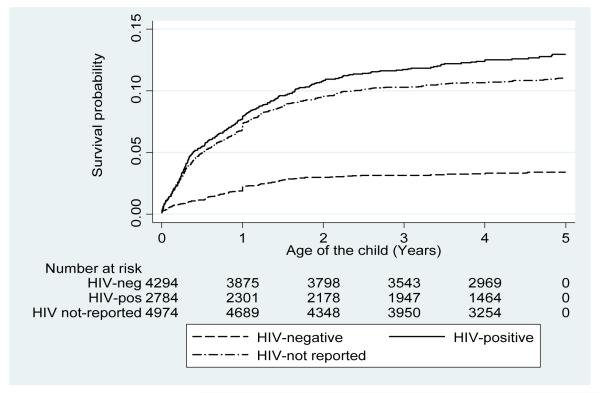
Trends in all-cause age-standardized mortality rates are shown in Figure 1. The infant mortality rate (IMR; <12 months) declined by 49% from 69.0 in 2000 to 35.5 in 2006 deaths per 1000 person-years observed. A significantly decline in the IMR was observed after roll-out of ART (2004-2006). Mortality in the age group 12-24 months declined by 27% and among the children aged 25-60 months by 60%, from a peak in 2002 to 2006, with slight declines observed in the latter age group after roll-out of ART (Figure 1). Crude mortality rates among children born to HIV-negative mothers remained relatively stable averaging 10.5 deaths per 1000 person-years from 2000 to 2006. Mortality rates among children born to HIV-positive mothers declined by 33% from 53.1 in 2003 (at the start of HIV surveillance) to 35.8 in 2006 deaths per 1000 person-years observed and averaged 41.3 deaths per 1000 person-years over the period. In further Kaplan-Meier survival analysis, we estimate that 3.9% of children of HIV-negative, 11% of HIV-not reported and 12.8% of HIV-positive mothers would have died by 5 years of age (Figure 2).
Figure 1.
All-cause age-standardized child mortality rates by age group, KwaZulu-Natal, South Africa, 2000-2006
Figure 2. Estimated unadjusted under-5 mortality by maternal HIV status at child birth.
Overall, the estimated proportion of children dying decreased with increasing child age (Table 2). The estimated proportions of deaths across all age groups were higher among the children born to HIV-positive and HIV-not reported status women than among the children of HIV-negative women.
Table 2.
Estimated proportion of children dying by infant age and maternal HIV status at child birth
| < 12 months | 12-24 months | 25-60 months | ||||
|---|---|---|---|---|---|---|
| Group | n | Proportion | n | Proportion | n | Proportion |
| All | 601 | 232 | 114 | |||
| HIV-negative | 79 | 13.14 | 41 | 17.67 | 12 | 10.52 |
| HIV-not reported | 317 | 52.74 | 102 | 43.96 | 54 | 47.36 |
| HIV-positive | 205 | 34.10 | 89 | 38.36 | 48 | 42.10 |
Factors associated with under-5 mortality
The 12,052 children were followed for a total of 48,396 child-years of follow up; median follow-up time was 3.9 years (IQR 3.1-4.9). Children who died were more likely to have been born at home, born in peri-urban or rural areas, to somewhat older mothers and mothers with primary school education only, and to mothers not initiated on ART (Table 1). In univariate and multivariable analyses of the overall cohort, compared to the relevant reference categories, children from multiple births were at a significantly increased hazard of death, as were first born children, those born at home, born in peri-urban or rural areas, to mothers between 20 and 30 years old, with primary education only, and with a history of child death (Table 3). Children born in households with fewer assets were at increased hazard of death in unadjusted, but not adjusted analyses. Overall, children whose mothers had died were at a 3-fold increased hazard of death, compared to children whose mothers were alive in both univariate and multivariate analyses.
Table 3.
Risk of mortality before age 5 years by maternal and child characteristics
| Events/total | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| Characteristic | 947/12,052 | HR (95% CI) P |
HR (95% CI) | P |
| Multipregnancy | ||||
| Singleton | 888/11,183 | 1.00 | 1.00 | |
| 1.62 (1.21-2.16) | ||||
| Twin | 49/401 | 0.001 | 1.60 (1.20-2.14) | 0.001 |
| 0.31 (0.16-0.57) | ||||
| Unknown | 10/468 | <0.001 | 0.30 (0.09-1.06) | 0.057 |
| Birth order | ||||
| Second/third | 377/4,320 | 1.00 | 1.00 | |
| 0.91 (0.79-1.05) | ||||
| First born | 387/4,855 | 0.207 | 1.28 (1.09-1.51) | 0.003 |
| 0.81 (0.67-0.97) | ||||
| Fourth/later | 173/2,409 | 0.020 | 0.85 (0.69-1.06) | 0.154 |
| 0.28 (0.15-0.52) | ||||
| Unknown | 10/468 | <0.001 | - | |
| Delivery location | ||||
| Hospital | 465/6,354 | 1.00 | 1.00 | |
| 1.21 (1.04-1.40) | ||||
| Primary health Clinic | 266/3,039 | 0.015 | 1.18 (1.01-1.37) | 0.035 |
| 1.49 (1.23-1.79) | ||||
| Homestead | 139/1,288 | <0.001 | 1.36 (1.12-1.67) | 0.002 |
| 0.80 (0.62-1.01) | ||||
| Unknown | 77/1,371 | 0.065 | 0.93 (0.72-1.20) | 0.523 |
| Area of birth | ||||
| Urban | 29/537 | 1.00 | 1.00 | |
| 1.42 (0.98-2.07) | ||||
| Peri-urban | 602/7,563 | 0.063 | 1.48 (1.01-2.17) | 0.043 |
| 1.60 (1.09-2.34) | ||||
| Rural | 314/3,619 | 0.015 | 1.50 (1.02-2.21) | 0.043 |
| 0.13 (0.03-0.55) | ||||
| Unknown | 2/333 | 0.006 | - | |
|
Environmental variables Household assets |
||||
| >5 | 718/9,274 | 1.00 | 1.00 | |
| 1.24 (1.06-1.44) | ||||
| ≤5 | 211/2,212 | 0.007 | 1.10 (0.94-1.29) | 0.221 |
| 0.46 (0.29-0.75) | ||||
| Unknown | 18/566 | 0.220 | 0.74 (0.44-1.21) | 0.216 |
| Birth season | ||||
| Apr-Sept | 470/6,173 | 1.00 | 1.00 | |
| 1.15 (1.01-1.30) | ||||
| Oct-Mar | 477/5,546 | 0.037 | 1.12 (0.99-1.27) | 0.080 |
|
Mothers characteristics Age (years) |
||||
| <20 | 179/2,711 | 1.00 | 1.00 | |
| 1.43 (1.20-1.69) | ||||
| 20-30 | 526/5,769 | <0.001 | 1.28 (1.07-1.56) | 0.009 |
| 1.13 (0.93-1.39) | ||||
| >30 | 203/2,722 | 0.221 | 1.26 (0.96-1.63) | 0.089 |
| 0.76 (0.54-1.07) | ||||
| Unknown | 39/850 | 0.117 | 1.15 (0.76-1.74) | 0.511 |
| Education | ||||
| Post primary | 624/8,332 | 1.00 | 1.00 | |
| 1.17 (1.01-1.35) | ||||
| At least primary | 266/3,017 | 0.031 | 1.21 (1.04-1.42) | 0.017 |
| 1.31 (0.99-1.72) | ||||
| Unknown | 57/703 | 0.051 | 2.74 (2.00-3.75) | <0.001 |
| Previous Fetal death | ||||
| No | 906/11,319 | 1.00 | - | |
| 1.24 (0.90-1.71) | ||||
| Yes | 39/400 | 0.185 | - | |
| 0.09 (0.02-0.36) | ||||
| Unknown | 2/333 | 0.001 | - | |
| Previous Child death | ||||
| No | 866/11,390 | 1.00 | 1.00 | |
| 3.39 (2.69-4.27) | ||||
| Yes | 79/329 | <0.001 | 2.69 (2.12-3.42) | <0.001 |
| 0.09 (0.02-0.38) | ||||
| Unknown | 2/333 | 0.001 | - | |
| Vital status | ||||
| Alive | 788/11,174 | 1.00 | 1.00 | |
| 4.61 (3.86-5.51) | ||||
| Died | 144/525 | <0.001 | 3.05 (2.54-3.67) | <0.001 |
| 0.58 (0.35-0.97) | ||||
| Unknown | 15/353 | 0.036 | 1.29 (0.54-3.13) | 0.534 |
| HIV Status | ||||
| HIV-negative | 148/4,294 | 1.00 | 1.00 | |
| 2.93 (2.43-3.55) | ||||
| HIV-not reported | 442/4,860 | <0.001 | 2.52 (2.07-3.06) | <0.001 |
| 4.88 (4.02-5.92) | ||||
| HIV-positive | 349/2,591 | <0.001 | 4.22 (3.46-5.15) | <0.001 |
| 1.12 (0.49-2.53) | ||||
| On ART | 8/307 | 0.456 | 1.29 (0.53-3.17) | 0.570 |
Children of mothers reported to be HIV infected, at birth or who seroconverted postpartum, or with HIV-not reported status were at four-fold and two-fold respectively significantly increased hazard of dying compared to children of HIV-uninfected mothers in both univariate and multivariate analyses. The mortality in children while their mother was on ART was not significantly different from that of HIV-negative mothers (Table 3).
Additionally, in a separate analysis in children born to HIV-positive mothers only, children whose mothers initiated ART had a significantly reduced hazard of death than children whose mothers did not initiate ART (adjusted hazard ratio 0.32, 95% CI 0.13-0.78; 0=0.012).
Discussion
We present all-cause age-standardized mortality rates among children in a largely rural South African surveillance site and show significantly declining infant mortality rates after ART roll-out. Slight declines in mortality were observed in the children aged 25-60 months after ART roll-out. The peak in mortality in 2003 in the <12 months group is due to a drop in the number of live-births that year, rather than an increase in the number of deaths, with a net decrease observed in the resident population that year, especially among women [30].
The decline in child mortality occurred despite continuing high HIV incidence and prevalence [16, 17, 19]. The contribution of HIV infection to child mortality has been previously reported, with child mortality rates expected to rise proportionately to the prevalence of HIV [31-34]. It has been suggested that the effect of HIV on child mortality would be more marked in Southern than other parts of Africa because of lower underlying (non HIV) mortality rates [6, 31, 35], which makes the reversal of the trend in child mortality rates despite continued high HIV prevalence and incidence in our study all the more remarkable.
Children born after 2004 will have been observed only for 3-4 years and under-5 mortality for them is thus incompletely assessed in the Cox regression analysis. The declining trend in recent years may be partially explained by this, although given that most under-5 mortality is in the first year of life the extent of this will be limited. Further, as there will have been more women on ART from 2006 onwards [22], the effect of maternal survival associated with ART would have been under-estimated.
Children born to HIV-infected mothers are more vulnerable compared to those of uninfected mothers [3, 20]. We show here that children of women who were HIV infected and not initiated on ART were four times more likely to die than children of uninfected mothers. We also show that women initiated on ART postpartum more likely reported previous child death than those not intiated, indicating vulnerability. Overall, HIV-related adult mortality at a population level declined in the area by 39% between 2003 and 2006 following ART roll out [36]. This, coupled with the decline in estimated proportion of children dying after birth with increasing number of mothers on ART, is in line with a maternal ART effect at an individual level in an area where adult deaths attributable to HIV declined from 83% to 70% after ART roll out [36]. As maternal ART keeps an infected mother alive, there will be an indirect effect of reducing child mortality in both her infected and uninfected children [14, 36]. Additionally, in a separate analysis, we confirm the effect of maternal survival on child survival, allowing for other factors known to be associated with mortality, with children of infected mothers on ART having a significantly reduced hazard of death than those without ART.
Access to ART could have impacted on household assets, for instance when people in the lower socio-economic status dispose household assets to cover substantial expenditure for health care [37]. However, in this setting with high unemployment, and high HIV prevalence, the impact of HIV on mortality is greater than the impact of household wealth on mortality [14].
Although not all mothers participated in the HIV surveillance, and we thus did not have information on the maternal HIV status during the early years for all children, children of HIV infected mothers, as well as those of not reported HIV status were less likely to die if their mothers were initiated on ART. This, coupled with the fact that children of the HIV-not reported status mothers had high hazards of death, with a similar trend to those of the HIV infected mothers, would suggest that a substantial number of the women in this group were likely to have been HIV-positive during the index pregnancy in this high HIV setting.
We also show that children whose mothers had died were three times more likely to have died than children of surviving mothers, independent of maternal HIV status. A mother’s vital status may be associated with child mortality as present mothers may spend more time caring for their children than absent mothers; without the protection of mothers or dependable caregivers, children may be disadvantaged [38]. Further, the effect of maternal survival has been shown to impact on child mortality independent of maternal HIV infection status [2], with the year before and following a mother’s death being particularly risky times for their children [39, 40].
We show that one-third of the mothers initiated ART during the breastfeeding period. This, coupled with the high rates of exclusive breastfeeding in the setting account for the relatively low rates of post-natal transmission [25, 27]. Further, mortality risk in children infected through breastfeeding is substantially less than in those infected perinatally [3].
Our results confirm the negative effect of twin births [2, 7] on child mortality as well as first born births [7], rural and peri-urban births [24, 41] and low maternal education [2]. Additionally, our findings draw attention to the importance of place of delivery to the survival of children. We show that children born at home are at significantly increased risk of death compared to those born in a health facility, though this is largely related to the first 2 years of life [42]. Increasing access to care is important in rural areas where a tenth of children are born at home and two-thirds of the population travel an hour or more to the nearest facility [12].
Only a limited number of women known to have been initiated on ART could be linked to the surveillance data, partially because the largest primary health care clinic in the programme is positioned near the edge of the surveillance area and accessed by people not covered by the demographic and HIV surveillance. For instance, of a total of 2,172 women initiated on ART in this clinic, only 774 (35.6%) were linked to the surveillance, 307 (39.7%) of whom met the inclusion criteria of having initiated ART within 5 years post-delivery of the index child. 153 (19.8%) of 774 children died before their HIV infected mothers initiated on ART; median time from child death to mother initiation was 3.6 months (IQR 1.9-5.4). Only 7 (0.9%) of the 774 mothers died after their child had died and before they could be initiated on ART. These 153 children would have been correctly allocated to the mother not on ART group; in the mothers on ART, especially when initiated sometime after delivery, we may have some selection bias with higher likelihood of child survival as the child would have to survive until that time. As mortality risk declines with age of child, we thus may overall underestimate the potential effect of maternal ART on child mortality. Although we may thus have underestimated the effect of maternal ART, there is no evidence to suggest that people outside of the surveillance area are substantially different from those within. Further, only about half of eligible adults participate in each HIV surveillance round, although for the household surveillance coverage is nearly 100%.[17] Possible reasons for non-consent include survey fatigue and knowledge of HIV status from voluntary counseling and testing in the public health services freely available in the community. Therefore, although we are confident about the reliability of the overall denominator and the number of deaths in either children or mothers, we are less confident about the HIV infection status of all women; hence the inclusion of the not-reported category.
Infants less than one year of age on HIV treatment were rare with only 1 in 2006 and 1 in 2007 [25]. The poor ART coverage of children in this setting due to a number of reasons: firstly, the programme was only started in late-2004 and although there has been rapid scale up of adult initiations [43], the numbers of infants on ART has experienced a slow growth. Further, infant diagnosis remains a problem even now, partly attributable to the slow turnaround of PCR results from the centralized provincial laboratory, lack of information on the antenatal HIV status of the mother on the child’s immunization card leading to missed opportunities of identifying HIV exposed children during immunization uptake, poor integration of PMTCT, TB and ART services, and lack of clear HIV testing guidelines for children until very recently.
We lack information on child HIV infection status and individual access to PMTCT could thus not directly be controlled for in our analyses. However, we do have information as to the service delivery at the primary health care clinic level, and know that coverage was not complete and the only PMTCT available was sdNVP which has a limited effect, especially since some infants, for example those born outside health care facilities, may not have received the infant component of this PMTCT [14]. PMTCT, with sdNVP, is estimated to reduce HIV transmission from 20 to 12% [44], and can potentially affect child mortality through reducing the number of infected children. Assuming a 30% HIV prevalence, PMTCT with sdNPV would avoid infection in 2 out of 30 infants born to HIV-positive mothers and thus prevent 2 HIV deaths in these children, although not necessarily in the first five years of life. Treatment of infected children with ART would have kept infected children alive by delaying disease progression [45]. However, very few children were on ART by end-December 2009 [23].
In conclusion, with a functional HIV treatment programme and accurate linkage with longitudinal surveillance data, benefits for survival of children at individual level can be shown. These findings confirm and expand the previous indirect evidence of an association between HIV treatment programme and population early child mortality, and highlight the importance of maternal HIV treatment with direct benefits of improved survival among all their children under-5 years. Therefore, timely introduction of HIV treatment in all eligible women is of utmost importance if further significant reductions in under-5 mortality are expected, which is necessary if MDG4 is to be realised.
Acknowledgements
Wellcome Trust supported the Africa Centre Demographic Information System (GR065377/Z/01/H) and Claire Thorne (Research Career Development Fellowship). The Hlabisa HIV Treatment and Care Programme receives support through the United States Agency for International Development (USAID) and the President’s Emergency Plan (PEPFAR) under the terms of Award No. 674-A-00-08-00001-00. The opinions expressed herein are those of the authors and do not necessarily reflect the views of USAID or the United States Government. We are grateful to the fieldworkers and supervisors for their excellent work in the Demographic Information System, Colin Newell for assisting in data management, the Africa Centre community for their participation in the survey and the Department of Health for their partnership in the HIV treatment and care programme. Presented at the XVIII International AIDS Conference, Vienna, July 18-23, 2010, abstract no. MOAE0104.
Footnotes
Disclosure statement
We declare that we have no conflict of interest.
References
- 1.Nakiyingi JS, Bracher M, Whitworth JA, et al. Child survival in relation to mother’s HIV infection and survival: evidence from a Ugandan cohort study. AIDS. 2003 Aug 15;17(12):1827–34. doi: 10.1097/00002030-200308150-00012. [DOI] [PubMed] [Google Scholar]
- 2.Ng’weshemi J, Urassa M, Isingo R, et al. HIV impact on mother and child mortality in rural Tanzania. J Acquir Immune Defic Syndr. 2003 Jul 1;33(3):393–404. doi: 10.1097/00126334-200307010-00015. [DOI] [PubMed] [Google Scholar]
- 3.Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004 Oct 2-8;364(9441):1236–43. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- 4.Brahmbhatt H, Kigozi G, Wabwire-Mangen F, et al. Mortality in HIV-infected and uninfected children of HIV-infected and uninfected mothers in rural Uganda. J Acquir Immune Defic Syndr. 2006 Apr 1;41(4):504–8. doi: 10.1097/01.qai.0000188122.15493.0a. [DOI] [PubMed] [Google Scholar]
- 5.Taha TE, Miotti P, Liomba G, Dallabetta G, Chiphangwi J. HIV, maternal death and child survival in Africa. AIDS. 1996 Jan;10(1):111–12. doi: 10.1097/00002030-199601000-00021. [DOI] [PubMed] [Google Scholar]
- 6.Newell ML, Brahmbhatt H, Ghys PD. Child mortality and HIV infection in Africa: a review. AIDS. 2004 Jun;18(Suppl 2):S27–34. doi: 10.1097/00002030-200406002-00004. [DOI] [PubMed] [Google Scholar]
- 7.Becher H, Muller O, Jahn A, Gbangou A, Kynast-Wolf G, Kouyate B. Risk factors of infant and child mortality in rural Burkina Faso. Bull World Health Organ. 2004 Apr;82(4):265–73. [PMC free article] [PubMed] [Google Scholar]
- 8.UNAIDS . AIDS epidemic update. UNAIDS, the Joint United Nations Programme on HIV/AIDS; 2009. [PubMed] [Google Scholar]
- 9.Department of Health . National antenatal sentinel HIV and syphilis prevalence survey 2008, South Africa. Pretoria, Department of Health; [accessed 20 April 2010]. 2009. Available from www.info.gov.za/view/DownloadFileAction?id=109007. [Google Scholar]
- 10.Jones G, Steketee RW, Black RE, Bhutta ZA, Morris SS. How many child deaths can we prevent this year? Lancet. 2003 Jul 5;362(9377):65–71. doi: 10.1016/S0140-6736(03)13811-1. [DOI] [PubMed] [Google Scholar]
- 11.Chopra M, Daviaud E, Pattinson R, Fonn S, Lawn JE. Saving the lives of South Africa’s mothers, babies, and children: can the health system deliver? Lancet. 2009 Sep 5;374(9692):835–46. doi: 10.1016/S0140-6736(09)61123-5. [DOI] [PubMed] [Google Scholar]
- 12.Tanser F, Gijsbertsen B, Herbst K. Modelling and understanding primary health care accessibility and utilization in rural South Africa: an exploration using a geographical information system. Soc Sci Med. 2006 Aug;63(3):691–705. doi: 10.1016/j.socscimed.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 13.Tanser F, Hosegood V, Benzler J, Solarsh G. New approaches to spatially analyse primary health care usage patterns in rural South Africa. Trop Med Int Health. 2001 Oct;6(10):826–38. doi: 10.1046/j.1365-3156.2001.00794.x. [DOI] [PubMed] [Google Scholar]
- 14.Ndirangu J, Newell ML, Tanser F, Herbst AJ, Bland R. Decline in early life mortality in a high HIV prevalence rural area of South Africa: evidence of HIV prevention or treatment impact? AIDS. 2010 Feb 20;24(4):593–602. doi: 10.1097/QAD.0b013e328335cff5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rice BD, Batzing-Feigenbaum J, Hosegood V, et al. Population and antenatal-based HIV prevalence estimates in a high contracepting female population in rural South Africa. BMC Public Health. 2007;7:160. doi: 10.1186/1471-2458-7-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Welz T, Hosegood V, Jaffar S, Batzing-Feigenbaum J, Herbst K, Newell ML. Continued very high prevalence of HIV infection in rural KwaZulu-Natal, South Africa: a population-based longitudinal study. AIDS. 2007 Jul 11;21(11):1467–72. doi: 10.1097/QAD.0b013e3280ef6af2. [DOI] [PubMed] [Google Scholar]
- 17.Tanser F, Hosegood V, Barnighausen T, et al. Cohort Profile: Africa Centre Demographic Information System (ACDIS) and population-based HIV survey. Int J Epidemiol. 2008 Oct;37(5):956–62. doi: 10.1093/ije/dym211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Africa Centre for Health and Population Studies . Current Studies: Africa Centre Demographic Information Systems (ACDIS) [accessed 26 February 2009]. Available from http://www.africacentre.ac.za/Default.aspx?tabid=89. [Google Scholar]
- 19.Barnighausen T, Tanser F, Gqwede Z, Mbizana C, Herbst K, Newell ML. High HIV incidence in a community with high HIV prevalence in rural South Africa: findings from a prospective population-based study. AIDS. 2008 Jan 2;22(1):139–44. doi: 10.1097/QAD.0b013e3282f2ef43. [DOI] [PubMed] [Google Scholar]
- 20.Ndirangu J, Barnighausen T, Tanser F, Tint K, Newell ML. Levels of childhood vaccination coverage and the impact of maternal HIV status on child vaccination status in rural KwaZulu-Natal, South Africa*. Trop Med Int Health. 2009 Nov;14(11):1383–93. doi: 10.1111/j.1365-3156.2009.02382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barnighausen T, Hosegood V, Timaeus IM, Newell ML. The socioeconomic determinants of HIV incidence: evidence from a longitudinal, population-based study in rural South Africa. AIDS. 2007 Nov;21(Suppl 7):S29–38. doi: 10.1097/01.aids.0000300533.59483.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Houlihan CF, Bland RM, Mutevedzi PC, et al. Cohort Profile: Hlabisa HIV Treatment and Care Programme. Int J Epidemiol. 2010 Feb 12; doi: 10.1093/ije/dyp402. doi: 101093/ije/dyp402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janssen N, Ndirangu J, Newell ML, Bland RM. Successful paediatric HIV treatment in rural primary care in Africa. Arch Dis Child. 2009 Jun;95(6):414–21. doi: 10.1136/adc.2009.169367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rollins NC, Coovadia HM, Bland RM, et al. Pregnancy outcomes in HIV-infected and uninfected women in rural and urban South Africa. J Acquir Immune Defic Syndr. 2007 Mar 1;44(3):321–8. doi: 10.1097/QAI.0b013e31802ea4b0. [DOI] [PubMed] [Google Scholar]
- 25.Cooke GS, Little KE, Bland RM, Thulare H, Newell ML. Need for timely paediatric HIV treatment within primary health care in rural South Africa. PLoS One. 2009;4(9):e7101. doi: 10.1371/journal.pone.0007101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Department of Health . Clinical guidelines: PMTCT (Prevention of Mother-to-Child transmission), South Africa. Department of Health; Pretoria: [accessed 20 April 2011]. 2010. Available from http://www.doh.gov.za/docs/factsheets/guidelines/pmtct.pdf. [Google Scholar]
- 27.Bland RM, Little KE, Coovadia HM, Coutsoudis A, Rollins NC, Newell ML. Intervention to promote exclusive breast-feeding for the first 6 months of life in a high HIV prevalence area. AIDS. 2008 Apr 23;22(7):883–91. doi: 10.1097/QAD.0b013e3282f768de. [DOI] [PubMed] [Google Scholar]
- 28.Coovadia HM, Rollins NC, Bland RM, et al. Mother-to-child transmission of HIV-1 infection during exclusive breastfeeding in the first 6 months of life: an intervention cohort study. Lancet. 2007 Mar 31;369(9567):1107, 16. doi: 10.1016/S0140-6736(07)60283-9. [DOI] [PubMed] [Google Scholar]
- 29.Bruin J. newtest: command to compute new test. Academic Technology Services, Statistical Consulting Group; UCLA: [accessed 09 November 2009]. 2006. Available from http://www.ats.ucla.edu/stat/stata/ado/analysis/ [Google Scholar]
- 30.Muhwava W, Nyirenda M. Demographic and Socio-Economic trends in the ACDIS, Monograph No 2. Africa Centre for Health and Population Studies; Mtubatuba, South Africa: 2007. [Google Scholar]
- 31.Nicoll A, Timaeus I, Kigadye RM, Walraven G, Killewo J. The impact of HIV-1 infection on mortality in children under 5 years of age in sub-Saharan Africa: a demographic and epidemiologic analysis. AIDS. 1994 Jul;8(7):995–1005. doi: 10.1097/00002030-199407000-00019. [DOI] [PubMed] [Google Scholar]
- 32.Zaba B, Marston M, Floyd S. [accessed 15 March 2009];The effect of HIV on child mortality trends in sub-Saharan Africa. UN Population Bulletin. 2003 Available from http://www.un.org/esa/population/publications/adultmort/Zaba.pdf.
- 33.UNAIDS . AIDS epidemic update: November 2009. UNAIDS, the Joint United Nations Programme on HIV/AIDS; 2009. [Google Scholar]
- 34.Macro International Inc . MEASURE DHS STATcompiler. [accessed 11 April 2010]. 2010. Available from http://www.measuredhs.com. [Google Scholar]
- 35.Rollins N, Little K, Mzolo S, Horwood C, Newell ML. Surveillance of mother-to-child transmission prevention programmes at immunization clinics: the case for universal screening. AIDS. 2007 Jun 19;21(10):1341, 7. doi: 10.1097/QAD.0b013e32814db7d4. [DOI] [PubMed] [Google Scholar]
- 36.Herbst AJ, Cooke GS, Barnighausen T, KanyKany A, Tanser F, Newell ML. Adult mortality and antiretroviral treatment roll-out in rural KwaZulu-Natal, South Africa. Bull World Health Organ. 2009 Oct;87(10):754–62. doi: 10.2471/BLT.08.058982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bachmann MO, Booysen FL. Health and economic impact of HIV/AIDS on South African households: a cohort study. BMC Public Health. 2003 Apr 1;3:14. doi: 10.1186/1471-2458-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muller O, Sen G, Nsubuga A. HIV/AIDS, orphans, and access to school education in a community of Kampala, Uganda. AIDS. 1999 Jan 14;13(1):146–7. [PubMed] [Google Scholar]
- 39.Zaba B, Whitworth J, Marston M, et al. HIV and mortality of mothers and children: evidence from cohort studies in Uganda, Tanzania, and Malawi. Epidemiology. 2005 May;16(3):275–80. doi: 10.1097/01.ede.0000155507.47884.43. [DOI] [PubMed] [Google Scholar]
- 40.Dabis F, Ekpini ER. HIV-1/AIDS and maternal and child health in Africa. Lancet. 2002 Jun 15;359(9323):2097–104. doi: 10.1016/S0140-6736(02)08909-2. [DOI] [PubMed] [Google Scholar]
- 41.Habib NA, Lie RT, Oneko O, Shao J, Bergsjo P, Daltveit AK. Sociodemographic characteristics and perinatal mortality among singletons in North East Tanzania: a registry-based study. J Epidemiol Community Health. 2008 Nov;62(11):960–5. doi: 10.1136/jech.2007.062828. [DOI] [PubMed] [Google Scholar]
- 42.Ndirangu J, Newell ML, Tanser F, Herbst AJ, Bland R. Decline in early life mortality in a high HIV prevalence rural area of South Africa: evidence of HIV prevention or treatment impact? AIDS. Feb 20;24(4):593–602. doi: 10.1097/QAD.0b013e328335cff5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mutevedzi PC, Lessells RJ, Heller T, Barnighausen T, Cooke GS, Newell ML. Scale-up of a decentralized HIV treatment programme in rural KwaZulu-Natal, South Africa: does rapid expansion affect patient outcomes? Bull World Health Organ. 2010 Aug 1;88(8):593–600. doi: 10.2471/BLT.09.069419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guay LA, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999 Sep 4;354(9181):795–802. doi: 10.1016/S0140-6736(99)80008-7. [DOI] [PubMed] [Google Scholar]
- 45.Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008 Nov 20;359(21):2233–44. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]