Abstract
Background
Southern Africa is witnessing the emergence of an epidemic of long-term survivors of vertically- acquired HIV infection presenting with untreated HIV as adolescents. Dermatologic conditions, common in both HIV-infected adults and children, have not been described in this age-group. We investigated the prevalence and spectrum of skin conditions in adolescents admitted to hospitals in Zimbabwe.
Methods
Three hundred and one consecutive adolescents admitted to two central Harare hospitals, underwent a dermatologic examination. Clinical history, HIV serology and CD4 lymphocyte counts were obtained. HSV-2 serology was used as a surrogate marker for sexual activity.
Results
One hundred and thirty-nine (46%) patients were HIV-1 antibody positive, of whom only 2 (1.4%) were HSV-2 antibody positive. The prevalence of any skin complaint among HIV-infected and uninfected participants was 88% and 14%, respectively (OR 37.7, 95% CI=19.4-72). The most common HIV-related conditions were pruritic papular eruptions (42%) and plane warts >5% of body area (24%). Having three or more skin conditions, a history of recurrent skin rashes and angular cheilitis were each associated with CD4 counts <200 cells/μl (p<0.03, p<0.01 and p<0.05, respectively).
Conclusions
Skin disease was a common and striking feature of underlying HIV-infection in hospitalized HIV- infected adolescents in Zimbabwe. In resource-poor settings with maturing epidemics, the presence of skin disease should be regarded as a strong indication for HIV testing and especially as it may reflect advanced immunosuppression. The high frequency of multiple plane warts has not previously been described, and may be a feature that distinguishes vertically-infected from horizontally-infected adolescents.
Keywords: HIV infection, adolescents, skin disease
Introduction
Cutaneous manifestations are very common in human immunodeficiency virus (HIV)-infected individuals and are associated with significant morbidity [1, 2]. Greater than 90% of patients develop at least one skin or mucous membrane manifestation during the course of their infection [2]. Viral, fungal and bacterial infections as well as inflammatory dermatoses have all been reported with increased frequency in association with HIV infection [1, 2]. Several studies have reported a correlation between skin disease and underlying immune status [3-10], thus making diagnosis of certain skin conditions a valuable clinical tool in staging and predicting progression of disease [11]. Herpes zoster infection is recognized as an early manifestation of disease whereas Kaposi sarcoma and cryptococcosis reflect more advanced immunosuppression [7]. The prevalence of skin disease varies by age, ranging from 41-94% in adults and 36-52% in children [3-10, 12, 16]. The spectrum similarly varies with age, for example seborrhoeic dermatitis is relatively uncommon among HIV-infected children but is common among adults [3, 10-14, 16].
East and Southern Africa have been more severely affected by HIV than any other global region. In Zimbabwe, HIV prevalence in antenatal clinic attendees was greater than 30% during the late 1990s, so that approximately 10% of infants born in that period were infected with HIV from mother-to-child transmission [17]. Vertically-acquired HIV is associated with high mortality [18] and, until recently, it was assumed that few HIV-positive vertically-infected children survived beyond the age of 5 years. However, current understanding is that a substantial minority of HIV-infected infants have prolonged survival even in the absence of HIV care. A pooled cohort analysis estimated that at least 13% of such children survive to age 10 years [19] and, more recently, that 17% will survive to age 15 years [20]. Surrogate markers of vertical HIV infection include poor longitudinal growth, recurrent childhood illness and high prevalence of maternal orphan hood (26). HIV epidemics in these regions are now maturing and considerable numbers of HIV-infected older children are presenting for care [21-23].
Skin disease and subsequent scarring is both disfiguring and stigmatizing and the negative influence of social stigma on the well-being of HIV-infected adolescents has been previously described [24]. In communities with a high prevalence of HIV infection, there may be lay recognition of the clinical manifestations of HIV, including recognition that certain skin diseases are likely to indicate underlying HIV infection, which may then lead to further stigmatization [25]. There is, however, a paucity of data relating to the spectrum and prevalence of skin disease in HIV-infected individuals in this age-group. The aim of this study was to describe the prevalence and spectrum of dermatologic manifestations among HIV-infected adolescents admitted to hospital in Harare, Zimbabwe.
Materials and Methods
Patient Recruitment
Between September 2007 and April 2008 adolescents (10-19 years) admitted for any acute cause other than obstetrics were recruited consecutively from the inpatient wards of two public-sector general hospitals in Harare, Zimbabwe, as previously described [26].
Data collection
A structured questionnaire was administered to participants to ascertain demographic characteristics and history of HIV testing. A standardized clinical history was recorded and all participants underwent a full dermatologic examination by the study doctor, as part of a comprehensive clinical evaluation including WHO staging [11]. Skin conditions were classified a priori into 9 main categories: pruritic papular eruption, seborrhoeic dermatitis, Herpes zoster infection, molluscum contagiosum, warts covering >5% of the body, Kaposi sarcoma, angular cheilitis, onycholysis and ‘other’ (including all other viral, fungal and non-specific inflammatory dermatoses). Diagnosis of each skin condition was made according to WHO case definitions [11] and characteristic clinical appearance. Diagnostic biopsies were taken in two cases of extensive plane warts. Recurrent rashes were defined as eruptions occurring more than once in a 6 month period. Drug rashes were diagnosed when eruptions occurred within a month of commencement of a new drug, after exclusion of inter- current sepsis.
History and examination findings were cross checked with hospital clinical and patient-held records and relevant investigations were conducted to study the cause of admission. In all participants CD4 lymphocyte count and full blood count measurements were obtained and herpes simplex virus (HSV)-2 serology was performed as a surrogate for sexual activity [27, 28].
HIV testing was carried out in two ways; anonymous serologic HIV testing was performed with subsequent unlinking to personal identifiers, and each patient was also offered diagnostic HIV counseling and testing (CT). HIV serology was determined using a standardized algorithm. Abbott Determine (Abbott Diagnostics, Johannesburg, South Africa), was used as the primary screening test and all positive tests were confirmed using SD Bioline (Standard Diagnostics Inc, Kyonggi-do, Korea): CD4 counts were determined by flow cytometry (Partec CyFlow Counter, Partec GmbH, Munster, Germany).
Statistical analysis
Data was entered into Epi-Info (version 3.4) and analyzed using STATA version 10. Categorical variables were compared using the Chi-squared test. Continuous variables were compared using Student’s t-test for normally distributed variables and Kruskal-Wallis test for variables not normally distributed. For analysis of associations, odds ratios (OR) and 95% confidence intervals (CI) were calculated by univariate and logistic regression analyses. A p-value of 0.05 was considered statistically significant.
Ethical considerations
Written informed consent was obtained from all participants older than 16 years of age. For participants younger than 16 years written consent from the guardian and written assent from the participant was obtained. The study was approved by the Medical Research Council of Zimbabwe, and the Ethics Committees of the Biomedical Research and Training Institute, Harare, London School of Hygiene and Medicine, and both the hospitals.
Results
Baseline characteristics
Three hundred and one patients were recruited. The participation rate was 93%. Median age of participants was 13 years (IQR: 11-16). Participant characteristics are shown in Table 1. HIV prevalence was 46% (139 participants). A predominantly non-sexual mode of HIV transmission was suggested by the equal sex distribution among HIV infected and uninfected individuals (53% vs 59%, HIV-infected and HIV-uninfected men respectively), low prevalence of HSV-2 seropositivity among HIV-infected individuals ((1.4%), high prevalence of maternal orphans (63%) and lack of variation of HIV serologic status by age (p=0.96). One hundred and ten (79%) HIV-infected participants had WHO stage 3 or 4 disease and their median CD4 count was 151 cells/μL (IQR 57-328), with 23% of participants having a CD4 count <50 cells/μL. Forty five (32%) HIV-infected participants were taking anti-retroviral therapy (ART). Median treatment duration was 5 months (IQR: 1-15) and six had started ART within one month of the recruitment date.
Table 1.
Baseline characteristics of participants: N=301 unless specified
| No (%) of participants |
|||
|---|---|---|---|
| Characteristic | HIV+ N=139 |
HIV− N=162 |
P value |
| Male | 74 (53%) | 97 (59%) | 0.29 |
| Mean age (years: S.D) | 13.2 (2.4) | 13.7 (2.9) | 0.14 |
| Orphan (single or double) | 111 (80%) | 58 (36%) | <0.001 |
| Maternal Orphan | 87 (63%) | 23 (14%) | <0.001 |
| Known HIV positive | 103 (74%) | N/A | N/A |
| On ART | 45 (32%) | N/A | N/A |
| WHO Stage 3 or 4 | 110 (79%) | N/A | N/A |
| Median CD4 count (cells/μL:IQR) (n=125) |
151 (57-328) | N/A | N/A |
Skin Disease
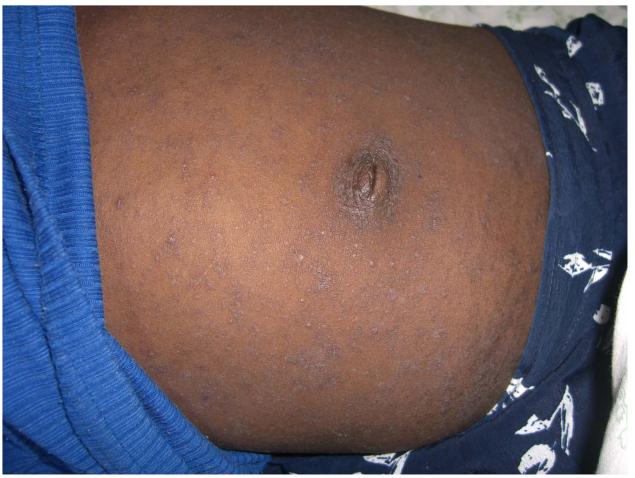
One hundred and twenty two (88%) HIV-infected and 22 (14%) HIV negative participants had skin disease (Table 2). Seventy-one (51%) HIV-infected patients had a history of recurrent skin rashes. The most common diagnosis was papular pruritic eruption, occurring in 42% of participants. A spectrum of papular pruritic eruption was diagnosed ranging from current papular pruritic vesicles (Figure 1A, online only) to evidence of previous disease with widespread post-inflammatory pigmentation. Thirty-five (25%) patients reported a past history of herpes zoster infection; scars were visible in thirty three. Twelve (36%) had recurrent or multi-dermatomal infection.
Table 2.
Frequency of skin disease by HIV status, and Odds Ratios (OR) for having skin disease among HIV patients (adjusted for age and sex)
| Skin Disease | No. (%) of participants | OR (95% C.I) | P values | |
|---|---|---|---|---|
|
|
||||
| HIV+ | HIV− | |||
| Any skin condition | 120 (86%) | 22 (14%) | 7.1 (4.5-11.2) | <0.0001 |
| Number of skin conditions | ||||
| 1 | 24 (17%) | 17 (10%) | ||
| 2 | 31 (22%) | 5(3%) | ||
| >3 | 65 (47%) | 1(1%) | ||
| Recurrent skin rashes | 71 (51%) | 4 (2%) | 42 (14.6-120.2) | <0.0001 |
| Papular pruritic eruption | 59 (42%) | 1 (1%) | 115.2 (15.7-847.5) | <0.0001 |
| Angular cheilitis | 54 (39%) | 6 (4%) | 16.1 (6.6-39.1) | <0.0001 |
| Warts > 5% body surface | 34 (24%) | 0 (0%) | N/A | |
| Previous Herpes Zoster: | ||||
| Scar seen | 33 (24%) | 0 (0%) | N/A | |
| Previously reported | 35 (25%) | 0 (0%) | N/A | |
| Seborrhoeic dermatitis | 16 (12%) | 3 (2%) | 7.9 (2.2-28.3) | <0.0001 |
| Onycholysis | 12 (9%) | 1 (1%) | 13.0 (1.6-102.2) | <0.0001 |
| Kaposi Sarcoma | 4 (3%) | 0 (0%) | N/A | |
| Molluscum contagiosum | 4 (3%) | 0 (0%) | N/A | |
| Other a | 48 (35%) | 15 (9%) | 4.7 (2.5-9) | <0.0001 |
| Other fungal b | 15 | 7 | ||
| Other viral c | 18 | 2 | ||
| Adverse drug reaction | 2 | 1 | ||
| Other d | 11 | 1 | ||
| Non specific Pigmentation e | 13 | 4 | ||
11 patients had 2 “other” conditions;
fungal infections include tinea corporis, tinea capitis, tinea manuum and pityriasis versicolor;
viral infections include HSV and HPV covering <5% of body surface;
Eczema =4, folliculitis =3, Scabies =3, pellagra =2(1 HIV-infected patient);
Non-specific hypo- and hyper-pigmentation
Figure 1.
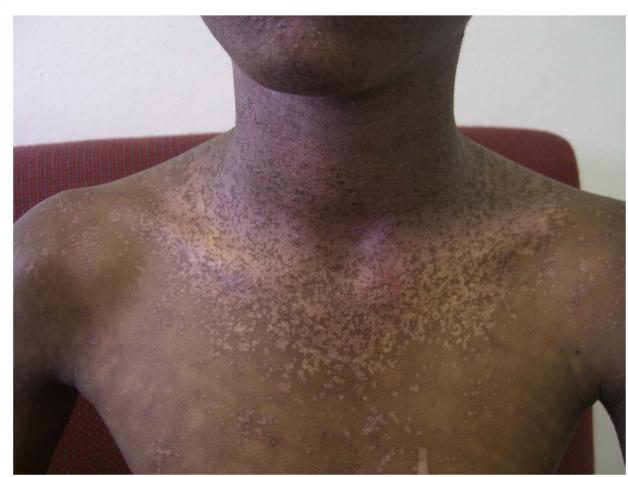
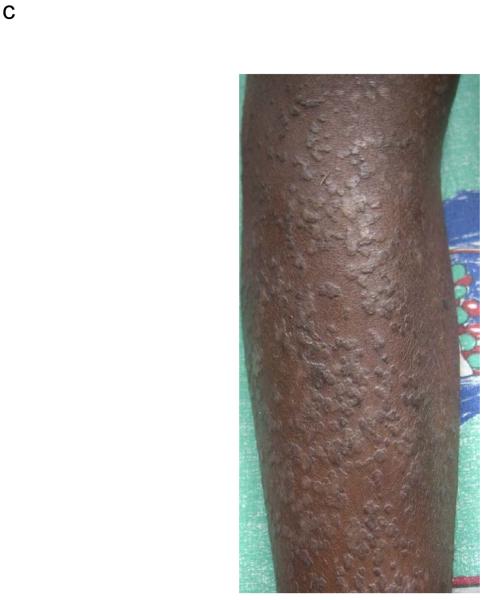
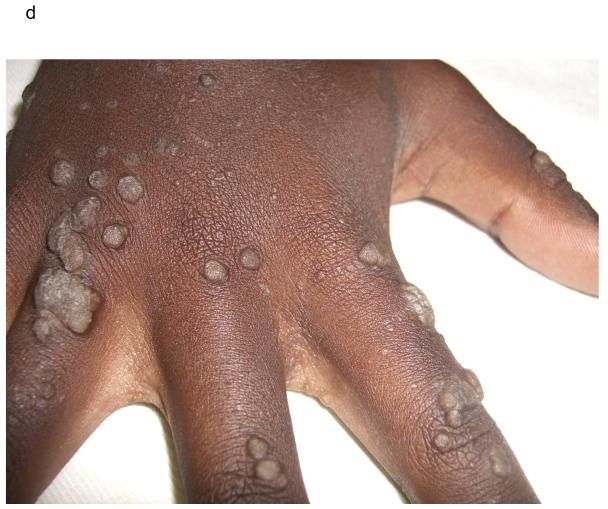
Thirty four patients (24%) had plane warts covering more than 5% of the body surface. A spectrum of distribution was noted from plane warts observed on the arms and legs (Figure 1B, online only) to the more frequently observed, extensive plane warts observed in a photosensitive distribution covering the forearms, head and neck (Figure 1C, online only). Cutaneous biopsies from lesional skin of 2 patients with extensive plane warts were histologically analyzed; this demonstrated moderate hyperkeratosis with a prominent granular layer, acanthosis and numerous koiliocytes consistent with the clinical diagnosis of extensive plane warts. Five patients had verruca vulgaris lesions covering <5% of body surface (Figure 1D,online only), included in the category of “other viral” infections.
In nine patients (seven HIV-infected), skin disease was the primary reason for admission. Four had Kaposi sarcoma with extensive skin disease, lymphedema and lymphadenopathy. In these four patients the CD4 lymphocyte counts were >200 cells/μl (median 298: IQR 230-417). Adverse drug reactions to trimethroprim-sulfamethoxazole occurred in two HIV-infected patients. The seventh patient was admitted with extensive peri-oral herpes simplex virus infection and severe tinea capitis with superimposed bacterial infection. Of the two non-infected adolescents, one had erythema multiforme secondary to penicillin and the other had cerebral palsy and presented with pellagra and infected bedsores.
The presence of any skin disease was strongly associated with being HIV-infected (age, sex adjusted OR =37.7 (95% CI: 19.4-72.0, p<0.001). Sixty five (47%) HIV-infected adolescents had three or more skin conditions. The presence of an increasing number of skin conditions was associated with increasing odds of being HIV-infected (age, sex adjusted OR for two skin conditions =22.9 (95% C.I: 8.1-64.9, p<0.001) and OR for ≥three skin conditions =234 (95% C.I: 31.3-1754, p<0.001).
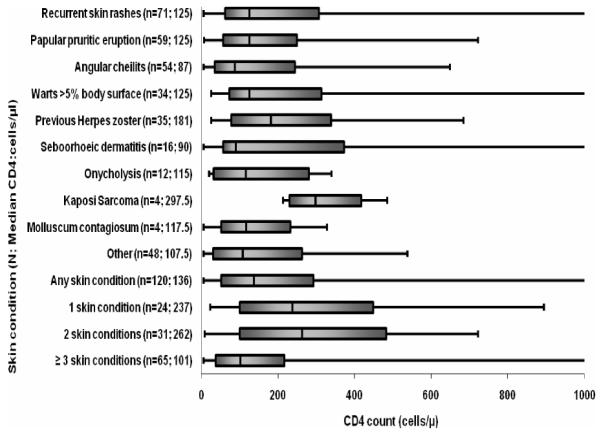
The CD4 counts for all categories of skin conditions are shown in Figure 2. Having ≥3 skin conditions ( adjusted OR =3.7 (95% C.I 1.5-8.9, p<0.003), history of recurrent skin infections (adjusted OR =2.7 (95% C.I 1.3-5.7, p<0.01) and angular cheilitis (adjusted OR =2.1 (95% C.I 1-4.5), p<0.05) were associated with advanced immunosuppression (CD4 counts <200cells/μl) in HIV-infected patients, after adjusting for age, sex and antiretroviral therapy.
Figure 2.
Box and whisker plot showing median and range of CD4 count (up to a maximum CD4 count of 1 000cells/μl) according to type and number of skin condition in HIV-infected participants. Lower and upper bars show the minimum and maximum CD4 counts for each category. The lower, middle and upper points on the solid bars denote the 25th percentile, median, and 75th percentile respectively. Median values are also shown in the parentheses.
Discussion
This study shows that dermatologic conditions were extremely common in a case-series of HIV-infected adolescents recruited systematically at the time of admission to hospital for any acute cause. Skin complaints were, however, incidental to the need for admission in all but a small minority of cases. Eighty eight percent of HIV-infected adolescents had evidence of skin disease at the time of examination. Recurrent skin rashes were reported in greater than 50% of participants, with most reporting longstanding or recurrent skin complaints from early childhood. This is a much higher burden than reported from adult and pediatric studies [3-10, 12-16 ], although selection bias resulting from exclusive inpatient recruitment should be considered. In the present study, 70% of adolescents had two or more skin manifestations by comparison with 40% of adults recruited from an outpatient HIV dermatology clinic in a recent study from Singapore [3] and 13% of HIV-infected Thai children recruited consecutively from an outpatient pediatric HIV clinic [16]. The range of immunosuppression was similar in all three study populations, although greater than 50% of the adults from Singapore were taking ART [3, 16].
Long-term survivors of vertically acquired HIV infection, often presenting de novo in adolescence, are now recognized as an emerging group following earlier severe adult and pediatric epidemics in Southern Africa [19-23]. Late presentations with adolescent AIDS can affect African migrants to the UK, often with delayed diagnosis that highlights the need for increased global awareness of this phenomenon [29]. The low prevalence of HSV-2 infection is consistent with our assumption that most participants in this study were vertically infected, as HSV-2 prevalence is extremely high among young people with sexually-acquired HIV infection in Southern Africa [27, 28]. Furthermore, as separately reported, our participants had other features to suggest vertical transmission in the majority of cases, notably a high prevalence of maternal orphan hood or AIDS, and growth failure [26].
Our study highlights the value of skin disease in adolescents admitted to hospital as a strong indicator for diagnostic HIV testing. This is important in a setting where diagnosis in this group is often delayed and routine testing, although strongly recommended, is not usually possible. There is increasing evidence, in both adults and children, that the prevalence and type of skin disease is significantly associated with the degree of immunosuppression [3-5, 7-9, 16]. Seborrheic dermatitis, papular pruritic eruption, molluscum contagiosum and adverse drug reactions have all been reported to occur more commonly at lower CD4 counts [3-5, 7-9, 14, 30]. A trend of association between the number of skin conditions and immunosuppression has also been reported [3, 14]. In this study, three or more different skin complaints, reported history of recurrent skin rashes, and angular cheilitis were each significantly associated with advanced immunosuppression. However, no other single skin condition was associated with having a CD4 count of <200cells/μl , lending support to other suggestions that CD4 counts in adolescent survivors of vertically acquired HIV infection may be less well correlated with clinical manifestations of immunosuppression than in other age groups. [26, 31]. For example, all four participants with Kaposi sarcoma in this study had CD4 counts of >200 cells/μl, despite having extensive disease.
Nearly a quarter of the HIV-infected adolescents in this study had disseminated flat hypochromic, versicolorlike patches. Extensive plane warts have previously been described in the context of HIV. However, the clinical appearance in our patients was strikingly similar to the cutaneous manifestations of the genodermatosis epidermodysplasia verruciformis [32]. To our knowledge, the high frequency of this clinical presentation in HIV-infected adolescents has not previously been described, but has significance first as an indication for HIV testing, and secondly because of the potential for HPV-associated epithelial neoplasia in advanced immunosuppression. Currently there are no reports of malignant transformation but close dermatological surveillance appears prudent.
In this study papular pruritic eruption was the most common HIV-related skin disease with a high prevalence, as previously reported for HIV-infected African adults [7] that contrasts with much lower prevalence rates in other global regions. For example, prevalence rates of only 2-4% were reported from India and Taiwan [4, 14]. A pediatric study in Thailand also reported a low prevalence of 5% [16]. A Ugandan study investigating the etiology of papular pruritic eruption suggested that it may be an arthropod-induced pruritic skin eruption and that arthropod antigens on the African continent may simply differ from those in areas with a lower prevalence of papular pruritic eruption [33]. Currently symptomatic treatment of active papular pruritic eruption is limited, steroids and antihistamines having minimal effects. An 87% reduction of symptomatic papular pruritic eruption has been observed in patients started on ART [34]. It has also been suggested that recurrence of papular pruritic eruption is associated with virologic failure and may be a useful marker to access underlying immune status and for treatment monitoring in resource poor settings [34].
The introduction of ART has been shown to have a significant impact on the spectrum and severity of skin disease [34]. Previous studies have shown an overall decrease in the prevalence of mucocutaneous manifestations with ART [36]. Paradoxically, initiation of ART has been also been associated with dermatologic immune restoration syndrome (IRIS) resulting in worsening or reappearance of certain skin diseases and drug rashes [37]. Only 32% of study participants were receiving antiretroviral therapy at the time of recruitment, but with a median treatment length of 5 months it is possible that a proportion of the skin disease diagnosed was as a result of IRIS. This may become increasingly clinically important as ART becomes more widely available in these settings, particularly in this group who are often significantly immunosuppressed at the onset of treatment.
This study has several limitations. First it is part of a larger cross-sectional study with broader objectives; the study doctor was, therefore, a general physician not a dermatologist and diagnoses were made using clinical definitions. Second, the population studied were all hospitalized patients introducing bias towards sicker patients with more severe immunosuppression and, for the 9 patients where this was the cause of admission, more severe skin disease.
Although not commonly life-threatening, the wider effects of skin disease can be devastating for the individual. The negative effects of living with chronic skin disorders and permanent scarring in HIV negative adults have a significant social, psychological and also occupational impact [38]. This has also been demonstrated in HIV infected adults in South Africa by the validated Dermatology Life Quality Index (DLQI) scores in HIV [39]. Low mood, poor self esteem, decreased school attendance and a general reduction in quality of life have all been described in HIV negative children with chronic skin disease [40]. In communities with a high burden of HIV, social isolation is compounded by the strong association of skin disease with HIV and subsequent stigmatization [25].
Skin disease was a prominent and striking clinical feature amongst HIV-infected adolescents in Zimbabwe. Further research is needed to investigate the response of skin lesions to ART, and better define the natural history of the unusual epidermodysplasia verruciformis-like presentation of plane warts that was observed with such high frequency in this case series. Previously undiagnosed or untreated HIV presenting in late childhood and adolescence is becoming increasingly common in countries with maturing HIV epidemics. The presence of cutaneous manifestations in adolescents born in high HIV prevalence settings should be recognized, first, as a strong indicator for HIV testing and, secondly, as a marker for immunosuppression.
Acknowledgments
We thank all the staff of Parirenyatwa and Harare Central Hospitals for their help with data collection.
Footnotes
Potential conflicts of interest. All authors: no conflict.
References
- 1.Tschachler E, Bergstrasser PR, Stingl G. HIV-related skin diseases. Lancet. 1996;348:659–63. doi: 10.1016/S0140-6736(96)01032-X. [DOI] [PubMed] [Google Scholar]
- 2.Coldiron BM, Bergstresser PR. Prevalence and clinical spectrum of skin disease in patients infected with the human immunodeficiency virus. Arch Dermatol. 1988;125:357–61. [PubMed] [Google Scholar]
- 3.Goh BK, Chan R, Sen P, et al. Spectrum of skin disorders in human immunodeficiency virus infected patients in Singapore and their relationship to CD4 lymphocyte counts. Int J Dermatol. 2007;46:695–699. doi: 10.1111/j.1365-4632.2007.03164.x. [DOI] [PubMed] [Google Scholar]
- 4.Raju PV, Rao GR, Ramani TV, Vandana S. Skin disease: clinical indicator of immune status in human immunodeficiency virus (HIV) infection. Int J Dermatol. 2005;44:646–649. doi: 10.1111/j.1365-4632.2004.02067.x. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein B, Berman B, Sukenik E, Frankel SJ. Correlation of skin disorders with CD4 lymphocyte count in patients with HIV/AIDS. J Am Acad Dermatol. 1997;36:262–264. doi: 10.1016/s0190-9622(97)70295-0. [DOI] [PubMed] [Google Scholar]
- 6.Shah I. Correlation of CD4 count, CD4% and HIV viral load with clinical manifestations of HIV in infected Indian children. Ann Trop Pediatr. 2006;26:115–119. doi: 10.1179/146532806X107458. [DOI] [PubMed] [Google Scholar]
- 7.Nnoruka EN, Chukwuka JC, Anisuiba B. Correlation of mucocutaneous manifestations of HIV/AIDS infections with CD4 counts and disease progression. Int J Dermatol. 2007;46(Suppl 2):14–18. doi: 10.1111/j.1365-4632.2007.03349.x. [DOI] [PubMed] [Google Scholar]
- 8.Muhammad B, Eligius L, Mugusi F, et al. The prevalence and pattern of skin disease in relation to CD4 counts among HIV-infected police officers in Dar es Salaam. Trop Doct. 2003;33:44–8. doi: 10.1177/004947550303300122. [DOI] [PubMed] [Google Scholar]
- 9.Josephine M, Issac E, George A, Ngole M, Albert SF. Patterns of skin manifestations and their relationships with CD4 counts among HIV/AIDS patients in Cameroon. Int J Dermatol. 2006;45:280–284. doi: 10.1111/j.1365-4632.2004.02529.x. [DOI] [PubMed] [Google Scholar]
- 10.Munoz-Perez MA, Rodriguez-Pichardo A, Camacho F, Colmenero MA. Dermatological findings correlated with CD4 lymphocyte count in a prospective 3 year study of 1161 patients with human immunodeficiency virus disease predominantly acquired through intravenous drug abuse. Br J Dermatol. 1998;139:33–9. doi: 10.1046/j.1365-2133.1998.02310.x. [DOI] [PubMed] [Google Scholar]
- 11.WHO Case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV related disease in adults and children. WHO. 2006:15–38. [Google Scholar]
- 12.Freytes DM, Arroyo-Novoa CM, Figueroa-Ramos MI, Ruiz-Lebrón RB, Stotts NA, Busquets A. Skin disease in HIV-positive persons living in Puerto Rico. Adv Skin Wound Care. 2007;20:149–156. doi: 10.1097/01.ASW.0000262711.97411.a1. [DOI] [PubMed] [Google Scholar]
- 13.Emodi IJ, Okafor GO. Clinical Manifestations of HIV infection in Children at Enugu, Nigeria. J Trop Pediatr. 1998;44:73–76. doi: 10.1093/tropej/44.2.73. [DOI] [PubMed] [Google Scholar]
- 14.Tzung T, Yang C, Chao S, Lee J. Cutaneous Manifestations of Human immunodeficiency virus infection in Taiwan. Kaohsiung J Med Sci. 2004;20:216–223. doi: 10.1016/S1607-551X(09)70109-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hira SK, Wadhawan D, Kamanga J, et al. Cutaneous manifestations of human immunodeficiency virus in Lusaka, Zambia. J Am Acad Dermatol. 1988;19:451–7. doi: 10.1016/s0190-9622(88)70197-8. [DOI] [PubMed] [Google Scholar]
- 16.Wananukul S, Deekajorndech T, Panchareon C, Thisyakorn U. Mucocutaneous finding in pediatric AIDS related to degree of immunosupression. Pediatr Dermatol. 2003;20:289–294. doi: 10.1046/j.1525-1470.2003.20401.x. [DOI] [PubMed] [Google Scholar]
- 17.UNAIDS AIDS Epidemic. [Update, November 2007].
- 18.Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F, Ghent International AIDS Society (IAS)Working Group in HIV Infection in Women and Children Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;369(9441):1236–43. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- 19.Marston M, Zaba B, Saloman JA, Brahmbatt H, Bagenda D. Estimating the Net effect of HIV on child mortality in African populations affected by generalized HIV epidemics. J Acquir Immune Defic Syndr. 2005;38:219–227. doi: 10.1097/00126334-200502010-00015. [DOI] [PubMed] [Google Scholar]
- 20.Stover J, Walker N, Grassly NC, Marston M. Projecting the demographic impact of AIDS and the number of people in need of treatment: updates to the Spectrum projection package. Sex Transm Inf. 2006;82(Suppl 3):iii45–50. doi: 10.1136/sti.2006.020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walker S, Mulenga V, Sinyinza F, et al. Determinants of survival without antiretroviral therapy after infancy in HIV-1 infected Zambian children in the CHAP trial. J Acquir Immune Defic Syndr. 2006 Aug 15;42(5):637–45. doi: 10.1097/01.qai.0000226334.34717.dc. [DOI] [PubMed] [Google Scholar]
- 22.Ferrand R, Luethy R, Bwakura F, Mujuru H, Miller RF, Corbett EL. HIV infection presenting in older children and adolescents: A case series from Harare, Zimabwe. Clin Infect Dis. 2007;44:874–878. doi: 10.1086/511873. [DOI] [PubMed] [Google Scholar]
- 23.Bagenda D, Nassali A, Kalyesubula I, et al. Health, neurolgic, and cognitive status of HIV-infected, long-surviving and antiretroviral-naive Ugandan children. Paediatrics. 2006;117(3):729–740. doi: 10.1542/peds.2004-2699. [DOI] [PubMed] [Google Scholar]
- 24.Fielden SJ, Sheckter L, Chapman GE, et al. Growing up: perspectives of children, families and service providers regarding the needs of older children with perinatally-acquired HIV. AIDS Care. 2006;18:1050–3. doi: 10.1080/09540120600581460. [DOI] [PubMed] [Google Scholar]
- 25.Ankrah ME. The impact of HIV/AIDS on the family and other significant relationships, the African clan revisited. AIDS Care. 1993;5:5–22. doi: 10.1080/09540129308258580. [DOI] [PubMed] [Google Scholar]
- 26.Ferrand RA, Mafukidze A, Mangeya N, et al. Causes of acute hospitalization during adolescence the burden and spectrum of HIV related morbidity in a country with an early and severe HIV epidemic. Poster 19, Meeting of Royal Society of Tropical Medicine and Hygiene; Brighton, U.K.. September 2008; Abstract. [Google Scholar]
- 27.Auvert B, Ballard R, Campbell C, et al. HIV infection among youth in a South African mining town is associated with herpes simplex virus-2 seropositivity and sexual behaviour. AIDS. 2001;15:931–4. doi: 10.1097/00002030-200105040-00009. [DOI] [PubMed] [Google Scholar]
- 28.Obasi A, Mosha F, Quigley M, et al. Antibody to herpes simplex virus type 2 as a marker of sexual risk behavior in rural Tanzania. J Infect Dis. 1999;179:16–24. doi: 10.1086/314555. [DOI] [PubMed] [Google Scholar]
- 29.Judd A, Ferrand R, Jungmann E, et al. Vertically acquired HIV diagnosed in adolescence and early adulthood: findings from national surveillance. HIV Medicine. doi: 10.1111/j.1468-1293.2008.00676.x. in Press. [DOI] [PubMed] [Google Scholar]
- 30.Boonchai W, Laohasrisakul R, Manonukul J, Kulthanan K. Pruritic papular eruption in HIV seropositive patients: a cutaneous marker for immunosuppression. Int J Dermatol. 1999;38:348–350. doi: 10.1046/j.1365-4362.1999.00694.x. [DOI] [PubMed] [Google Scholar]
- 31.Mangeya N, Mafukidze A, Makunike-Mutasa R, Makoni A, Miller RF, Corbett EL, Ferrand RA. Chronic knee stiffness and swelling in a Zimbabwean adolescent. Int J STD AIDS. 2009;20:63–64. doi: 10.1258/ijsa.2008.008239. [DOI] [PubMed] [Google Scholar]
- 32.Carre D, Dompmartin A, Verneuil L, et al. Epidermodysplasia verruciformis in a patient with HIV infection: no response to highly active antiretroviral therapy. Int J Derm. 2003;42:296–300. doi: 10.1046/j.1365-4362.2003.01707_2.x. [DOI] [PubMed] [Google Scholar]
- 33.Resneck JS, Van Beek M, Furmanski L, et al. Etiology of Pruritic Eruption with HIV infection in Uganda. JAMA. 2004;292:2614–2621. doi: 10.1001/jama.292.21.2614. [DOI] [PubMed] [Google Scholar]
- 34.Castelnuovo B, Byakwaga H, Menten J, Schaefer P, Kamya M, Colebunders R. Can response of a pruritic papular eruption to antiretroviral therapy be used as a clinical parameter to monitor virological outcome? AIDS. 2008;22:269–273. doi: 10.1097/QAD.0b013e3282f313a9. [DOI] [PubMed] [Google Scholar]
- 35.Zancanaro PC, McGirt LY, Mamelak AJ, Nguyen R, Martins C. Cutaneous manifestations of HIV in an era highly active antiretroviral therapy: An institutional urban clinic experience. J Am Acad Dermatol. 2006;54:581–588. doi: 10.1016/j.jaad.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 36.Seoane Reula E, Bellon JM, Gurbindo D, Mun̊oz-Fernandez MA. Role of antiretroviral therapies in mucocutaneous manifestations in HIV-infected children over a period of two decades. Br J Dermatol. 2005;153:382–389. doi: 10.1111/j.1365-2133.2005.06758.x. [DOI] [PubMed] [Google Scholar]
- 37.French MA, Lenzo N, John M, et al. Immune restoration disease after the treatment of immune-deficient HIV-infected patients with highly active antiretroviral therapy in an HIV-infected patient. HIV Med. 2000;1:107–115. doi: 10.1046/j.1468-1293.2000.00012.x. [DOI] [PubMed] [Google Scholar]
- 38.Hong J, Koo B, Koo J. The psychosocial and occupational impact of chronic skin disease. Dermatol Ther. 2008;21:54–59. doi: 10.1111/j.1529-8019.2008.00170.x. [DOI] [PubMed] [Google Scholar]
- 39.Reid D, Mosam A, Nelson R. Quality of life in patients with HIV and skin disease. J Am Acad Dermatol. 2006;53(3):AB60. [Google Scholar]
- 40.Beattie PE, Lewis-Jones MS. A Comparative study of impairment of quality of life in children with skin disease and children with other chronic childhood diseases. Br J Dermatol. 2006;155:145–151. doi: 10.1111/j.1365-2133.2006.07185.x. [DOI] [PubMed] [Google Scholar]