Abstract
Multiple factors control susceptibility of C57BL/6 mice to infection with the helminth Heligmosomoides polygyrus, including TGF-β signaling which inhibits immunity in vivo. However, mice expressing a T-cell-specific dominant negative TGF-β receptor II (TGF-βRII DN) show dampened Th2 immunity and diminished resistance to infection. Interestingly, H. polygyrus-infected TGF-βRII DN mice show greater frequencies of CD4+Foxp3+Helios+ Tregs than infected wild-type mice, but levels of CD103 are greatly reduced on both these cells and on the CD4+Foxp3+Helios− population. While Th9 and Th17 levels are comparable between infected TGF-βRII-DN and wild-type mice, the former develop exaggerated CD4+ and CD8+ T cell IFN-γ responses. Increased susceptibility conferred by TGF-βRII DN expression was lost in IFN-γ-deficient mice, although they remained unable to completely clear infection. Hence overexpression of IFN-γ negatively modulates immunity, and the presence of Helios+ Tregs may maintain susceptibility on the C57BL/6 background.
Introduction
Immunity to gastrointestinal helminth infection is mediated by Th2-dependent mechanisms (1, 2), which are impaired by regulatory T cells (Tregs) (3) and cross-regulated by conventional IFN-γ Th1 effector populations (4). In the case of the murine nematode parasite Heligmosomoides polygyrus, immunity is boosted by interference with TGF-β signaling associated with the induction and activation of Foxp3+ Tregs (3), a well-established property of this cytokine (5). H. polygyrus is a broadly immunomodulatory parasite which can alleviate colitis in the absence of IL-10 (6), but not when T cell responsiveness to TGF-β is abrogated (7). TGF-β also participates in generating IL-9- (8) and IL-17-producing (9) Th subsets in the presence of IL-4 and IL-6 respectively, although in the setting of H. polygyrus infection both IL-9-dependent mast cell responses (10) and Th17 cells (11) have previously been reported to be blocked.
As TGF-β signaling was found to promote Tregs and prolong H. polygyrus infection, it was surprising that mice whose T cells expressed a dominant negative TGF-β receptor II (TGF-βRII DN) were not more resistant to this parasite (7). Our laboratory not only confirmed this phenotype, but established that TGF-βRII DN mice are in fact more susceptible to infection than wild-type animals. However, TGF-β can actively down-regulate multiple cell types (5, 12), and among the conspicuous phenotypes of TGF-βRII DN mice is potentiation of IFN-γ and Th1 responses (7, 13). We therefore investigated, using mice lacking IFN-γ, whether ablation of TGF-β signaling increased susceptibility due to uninhibited Th1 cytokine release.
Our results show that when IFN-γ stimulation is abrogated in the absence of TGF-β signaling in T cells, the increased fecundity of H. polygyrus within the host is lost. Infected TGF-βRII DN and wild-type mice show equivalent differentiation of IL-9 or IL-17 producing CD4+ T cells, and display no differences in mast cell expansion following infection. Our data therefore support the hypothesis that the increased susceptibility of TGF-βRII DN mice to H. polygyrus is due to elevated IFN-γ release, and not to a loss of Th9 or Th17 effector responses.
In addition, TGF-β plays a central role in the induction and maintenance of regulatory T cells, particularly in the periphery (5, 14). Because of the importance of Tregs in modulating responses to pathogens in general (15), and H. polygyrus in particular (3), we also investigated the balance of Treg frequencies and subsets in the presence or absence of TGF-β signaling, and the consequent outcome of infection. These studies show that within the Foxp3+ Treg population of TGF-βRII DN mice, CD103 expression is low on both Helios+ and Helios− cells, but a compensatory increase in Helios+ Tregs may account for the continuing susceptibility of mice expressing the mutated receptor.
Materials and Methods
Animals and Parasites
C57BL/6, TGF-βRII DN (T cell-specific dominant negative TGF-βRII (16)), IFN-γ−/−, and doubly transgenic mice were housed in individually ventilated cages. Both transgenic lines were on a C57BL/6 background. Mice were infected by oral gavage with 200 H. polygyrus bakeri third stage larvae (L3), obtained from fecal cultures (3); 14 and 28 days later small intestinal adult worms and fecal pellet eggs were enumerated.
Restimulation, flow cytometry and cytokine measurements
Mesenteric lymph node cells (MLNC) were stained directly ex vivo (for Foxp3 and Helios measurements) or restimulated with 0.5 μg/ml PMA and 1 μg/ml Ionomycin for 3.5 hrs, with 10 μg/ml Brefeldin A included for the final 2.5 hrs (for intracellular cytokine measurements). Cells were stained with antibodies to surface CD4 (RM4-5; BD), CD8α (53-6.7; Biolegend), TCR-β (H57-597; eBioscience), and CD103 conjugated to biotin (M290; BD Pharmingen) followed by PerCP-streptavidin (BD). Cells were fixed by manufacturer’s instructions with Cytofix/Cytoperm (BD) or Fix/Perm (eBioscience;for Foxp3 and Helios staining), then stained with antibodies to intracellular IFN-γ (XMG1.2; Biolegend), IL-13 (eBio 13A; eBioscience), Foxp3 (FJK-16s; eBioscience) and Helios (22F6; Biolegend). Cells were analysed using FACSCanto or LSRII flow cytometers (BD) and FlowJo software (Tree Star). Serum cytokines were assayed by CBA flex set (BD) with a minimum detection limit of 2.5 pg/ml.
Histology
Transverse sections of jejunum were fixed in 4% formaldehyde and stained with hematoxylin, eosin and Toluidine Blue. Mast cell counts per μm of villus crypt were recorded.
Statistical analysis
Statistical tests were applied according to data normality and group numbers. Normally distributed two-way comparisons used unpaired T tests, and multiple comparisons One-way ANOVA followed by Tukey’s test). If normality was not achieved, Mann–Whitney (for two-way comparisons) and Kruskal-Wallis tests (for multiple comparisons, followed by Dunn’s test) were used. Data from multiple experiments were pooled only where no statistical differences existed between separate data sets. A p value of < 0.05 was considered significant and indicated by *; p < 0.01 by ** and p < 0.001 by ***.
Results and Discussion
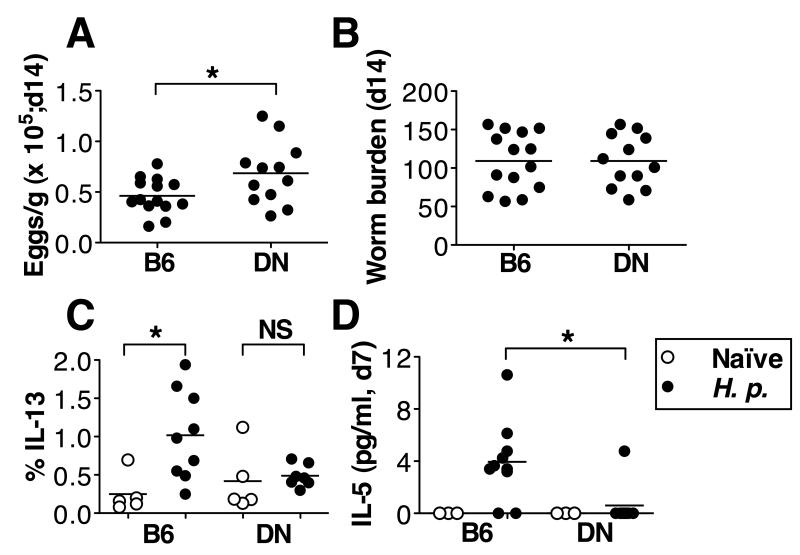
The C57BL/6 mouse strain has a high level of susceptibility to the gastrointestinal helminth H. polygyrus (17), but immunity can be enhanced by pharmaceutical inhibition of TGF-β signaling (3). As TGF-βRII DN mice have deficient TGF-β signaling in T cells, they may be expected to be more resistant to H. polygyrus than their wild-type littermates, and may lack inducible Tregs to inhibit effector responses against the worm. Surprisingly however, H. polygyrus shows heightened fecundity in TGF-βRII DN mice (Fig. 1A), and mice have similar adult worm burdens to wild-type mice (Fig. 1B), consistent with an earlier report (7). Furthermore, TGF-βRII DN mice show diminished Th2 cytokine responses, failing to generate a significant population of IL-13+ CD4+ T cells in the MLNC (Fig. 1C). While a degree of IL-4 responsiveness was maintained in TGF-βRII DN mice (data not shown), we also found that serum IL-5 responses to infection were absent in all but one gene-targetted animal (Fig. 1 D). The IL-5 serum response at day 7 of infection in wild-type mice was transient, and had returned to naïve levels by day 14 of infection (data not shown). No IL-4 or IL-13 was detectable in sera of either mouse strain. Reduced IL-10 production measured by antigen-specific recall responses to adult worm excretory-secretry antigens (HES) in vitro was also found in TGF-βRII DN mice (data not shown), consistent with its role in promoting Th2 responsiveness in gastrointestinal helminth infections (2), and with a report that IL-10 release by lamina propria cells is inhibited in H. polygyrus-infected TGF-βRII DN mice (7). Hence T cell-specific ablation of TGF-β signaling does not recapitulate the effects of global pharmaceutical inhibition (3), and the phenotype of the TGF-βRII DN mice does not equate to the Th2-boosting effects of broader interference with Treg function in nematode infection (18-20).
Figure 1. Ablation of TGF-β signaling in T cells does not confer resistance in mice to H. polygyrus infection.
A. Fecal H. polygyrus egg counts after 14 days of infection in C57BL/6 and TGF-βRII DN mice. Data are pooled from 3 experiments, with mice aged 7-18 wks. Mice were age-matched between groups and no age effect on H. polygyrus susceptibility was seen.
B. Adult H. polygyrus counts from the same experiments and time point as (A).
C. % IL-13+ cells of CD4+ lymphocytes in MLNC from naïve (open circles) and 14-day-post-infected (closed circles) C57BL/6 and TGF-βRII DN mice. MLNC were stimulated with PMA/Ionomycin, and stained for flow cytometry. Data are pooled from 2 experiments with 6-9 wk old mice.
D. Levels of serum IL-5 in naïve (open circles) and 7-day-post infected (closed circles) mice. Data are pooled from 2 experiments; naïve mice were examined in one of these experiments. Mice were 7-12 wks old and age matched between groups.
Abbreviations used in figure: B6= C57BL/6; DN= TGF-βRII DN; H. p.= H. polygyrus-infected.
(A-B) were analysed by unpaired T test; and (C-D) by Kruskal-Wallis test.
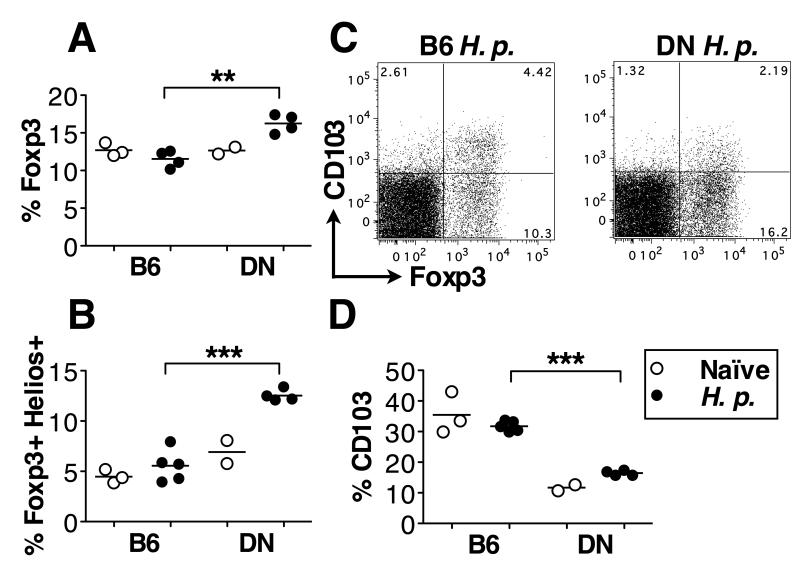
As TGF-β signaling promotes Treg differentiation, particularly in the periphery, we next examined Treg frequencies in H. polygyrus-infected TGF-βRII DN mice. Surprisingly, we found a significantly higher proportion of CD4+Foxp3+ T cells in MLNC of infected TGF-βRII DN mice compared to wild-type C57BL/6 animals (Fig. 2A). The increased frequency of Foxp3+ cells was accounted for by a greater proportion of CD4+ cells expressing the transcription factor Helios (Fig. 2B), which is associated with thymic or natural Tregs (21), while the frequencies of Foxp3+ Helios− T cells (considered to be peripherally induced Tregs, known to be more dependent on TGF-β signaling) were not significantly different between the two genotypes (data not shown). Although the Treg compartment was not thus numerically diminished in TGF-βRII DN mice, their expression of CD103 (an activation/memory marker known to be inducible by TGF-β (22)) was substantially reduced (Fig. 2C, 2D), with low levels in both Helios+ and Helios− subsets (data not shown).
Figure 2. TGF-βRII DN mice have more T regulatory cells but fewer CD103+ Tregs than C57BL/6 animals during H. polygyrus infection.
C57BL/6 and TGF-βRII DN mice were left naïve (open circles) or H. polygyrus-infected for 14 days (closed circles), and MLNC were isolated and stained directly ex vivo. Data are representative of the results from 2 experiments each with 2-5 mice per group. Mice were 7-9 wks old.
A. % Foxp3+ cells among CD4+ lymphocytes.
B. % Foxp3+Helios+ cells among CD4+ lymphocytes.
C. Representative CD103 and Foxp3 staining among CD4+ lymphocytes, from 14-day-H. polygyrus-infected C57BL/6 and TGF-βRII DN mice.
D. % CD103 among CD4+Foxp3+ lymphocytes.
Abbreviations used as in Figure 1. H. polygyrus-infected groups in (A,B,D) were analysed by unpaired T test.
We next addressed the question of whether a loss of TGF-β signaling impacts on other effector functions in the immune response. As TGF-β signaling promotes differentiation of Th17 cells in the presence of IL-6 (9), and Th9 in the presence of IL-4 (8), we investigated the generation of these cell types following infection. Few Th17 cells identified by intracellular IL-17 staining develop in the MLNC in either genotype (Fig. 3A), suggesting that the conditions for optimal Th17 expansion are not generated at this site during H. polygyrus infection.
Figure 3. TGF-βRII DN mice have exaggerated IFN-γ responses yet Th17, Th9 and mast cell responses are not compromised.
A-B, E-F. MLNC were isolated from 14-day-H. polygyrus-infected C57BL/6 and TGF-βRII DN mice, stimulated with PMA/Ionomycin, and stained for flow cytometry.
A-B. Data are pooled from 3 experiments, with 6-18 wks old mice age-matched between groups. % IL-17A+ cells (A) or % IL-9+ cells (B) of CD4+ lymphocytes.
C. Jejunums were sectioned and stained for mast cells with Toluidine Blue. Number of mast cells per μm of villus crypt are shown in naïve (open circles) and 14-day-H. polygyrus infected (closed circles) mice. Data shown are from 7-9 wk old mice and are representative of 2 experiments each with 4-5 mice per group for infected mice; naïve mice were included in one of these experiments.
D. Levels of circulating IFN-γ in the sera of naïve (open circles) or 7-day-H. polygyrus-infected C57BL/6 and TGF-βRII DN mice (closed circles). Data are pooled from 2 experiments with 7-12 wk old mice.
E-F. % IFN-γ+ cells among CD4+ (E) or CD8α+ (F) TCR-β+ lymphocytes. Data shown are from mice 7-9 wk old mice and are representative of 4 experiments each with 2-5 mice per group.
Abbreviations used as in Figure 1. (A) was analysed by unpaired T test; (B, D) by Mann Whitney; (C) by Kruskal-Wallis; and (E-F) by unpaired T test between H. polygyrus-infected groups.
The frequency of CD4+ T cells producing IL-9 was, however, altered in TGF-βRII DN mice, with a significantly greater, rather than lower, proportion of IL-9+ T cells compared to wild-type mice (Fig. 3B). IL-9 is important for mast cell survival and proliferation (23) and mast cells have been suggested as an effector population for H. polygyrus expulsion (10, 24, 25). We therefore quantified the extent of jejunal mast cells but found their numbers increased significantly and equivalently after H. polygyrus infection in both C57BL/6 and TGF-βRII DN mice (Fig. 3C).
By day 14 post-infection, effector responses in wild-type mice are predominantly Th2 type (26). TGF-βRII DN mice however, display strong Th1 IFN-γ production (Fig. 3). Notably, a high proportion of splenic CD4+ and CD8+ T cells develop into IFN-γ producing cells in vitro (16), and constitutive IFN-γ levels in naïve animals are markedly elevated (7). Infection is hence initiated in an environment intrinsically unfavorable to Th2. Following H. polygyrus-infection, this trend is exacerbated with TGF-βRII DN mice showing elevated serum levels of IFN-γ compared to C57BL/6 mice (Fig. 3D), with an approximately five-fold higher frequency of IFN-γ production among CD4+ T cells in the MLNC (Fig. 3E) and a substantial increase in CD8+ IFN-γ + T cells in the same mice (Fig. 3F).
To investigate whether the substantial IFN-γ in TGF-βRII DN mice inhibits Th2 cytokines required to control H. polygyrus, we bred double transgenic mice with the TGF-βRII DN mutation together with the IFN-γ−/− genotype on the C57BL/6 background (TGF-βRII DN IFN-γ−/− mice).
After 14 days of infection, TGF-βRII DN mice lacking IFN-γ had a lower fecal egg burden than IFN-γ-sufficient TGF-βRII DN mice (Fig. 4A) and by day 28, lower worm counts (Fig. 4B). However, the double-transgenic mice were not able to fully clear infection. Thus, while over-expression of IFN-γ is responsible for the heightened susceptibility of TGF-βRII DN mice, IFN-γ itself is not solely responsible for the failure of mice to expel the parasite. In this manner, control of H. polygyrus appears to be more complex than Trichus muris, in which neutralization of IFN-γ is sufficient to convert a susceptible genotype to a resistant phenotype (4). Hence, the reported greater susceptibility of TGF-βRII DN mice to T. muris may be due to high intrinsic IFN-γ in this model rather than lack of Th9-driven mast cell responses (8).
Figure 4. Increased susceptibility of TGF-βRII DN mice is reversed in the absence of IFN-γ.
A. Fecal egg counts after 14 days of infection in C57BL/6, TGF-βRII DN, TGF-βRII DN IFN-γ−/− and IFN-γ−/− mice. Data are pooled from 3 experiments with 6-14 wk old mice age-matched between groups.
B. Adult worm counts from the same experiments after 28 days of infection.
C-D. Levels of circulating IFN-γ (C) and IL-5 (D) in the same experiments after 7 days of infection. Data are pooled from two experiments with 7-14 wk old mice.
E. % Foxp3+ T cells among total CD4+ lymphocytes. After 28 days of infection, MLNC were stained directly ex vivo. Data are pooled from 2 experiments with 7-14 wk old mice.
F. % CD103 among Foxp3+ CD4+ lymphocytes, in the same experiments as E.
Abbreviations used in figure: B6= C57BL/6; DN= TGF-βRII DN; DNγ−/− = TGF-βRII DN IFN-γ−/−; γ−/− = IFN-γ−/−; H. p.= H. polygyrus-infected. (A,B,E,F) were analysed by ANOVA; (C-D) by Kruskal-Wallis test.
Serum cytokine analysis confirmed the absence of IFN-γ in gene-targetted mice (Fig. 4C) and showed that serum IL-5 levels were partially restored (Fig. 4D). However, intracellular staining of MLNC showed broadly similar levels of Th2 cytokine production by day 28 of infection in C57BL/6 and double-transgenic genotypes (data not shown), indicating that the suppression of Th2 responses is only partly relieved in the absence of both IFN-γ and TGF-β signaling in T cells. HES-specific IgG1 responses were equivalent in all strains at day 28 of infection.
Analysis of Treg populations showed that the proportion of Foxp3+ CD4+ T cells are increased in TGF-βRII DN mice irrespective of their IFN-γ status (Fig. 4E). Moreover, CD103 expression is reduced on both Helios+ and Helios−Foxp3+CD4+ T cells in both IFN-γ-deficient and –sufficient TGF-βRII DN mice (Fig. 4F), confirming that CD103 expression is regulated by TGF-β signaling (27). Levels of CD103 therefore do not correspond to the susceptibility of the mouse strain, suggesting that CD103 is not required for functional suppression of the anti-helminth response. However, as CD103 is important for effector T cell migration and retention in the gut (28), and as the TGF-βRII DN Foxp3− effector population also fails to express high CD103 levels (Fig. 2C and data not shown), the susceptibility of this genotype could reflect a diminished presence of effector cells at the site of infection.
Overall, these data argue that neither Th1 nor TGF-β-induced adaptive Tregs are essential for repression of the protective Th2 response to H. polygyrus. Several interesting alternatives can now be considered. First, the greater expansion of natural Tregs in TGF-βRII DN mice may account for their continued susceptibility. The outgrowth of natural or Helios+ Tregs in vivo may result from a homeostatic compensation for the paucity of CD103+ adaptive Tregs (moderated by cytokines such as IL-2) and/or outgrowth to control a greater mucosal inflammatory response in the absence of TGF-β-inducible CD103+ adaptive Tregs (29). Secondly, although Th2 effectors would be inured from TGF-β mediated inhibition (30), Tregs operate through other suppressive pathways including co-inhibitors such as CTLA-4 and PD-1 known to be important in other helminth systems (18). Thirdly, many other regulatory subsets are known to arise in H. polygyrus infection including DCs, macrophages and regulatory B cells (reviewed in (2)), which may account for the susceptibility of these mice. Finally, it should noted that significant non-lymphoid populations are responsive to TGF-β, and the efficacy of global TGF-β inhibition (3) and the TGF-β dependent effects in H. polygyrus-infected RAG-deficient hosts (6), implies that there are critical non-T cell targets of this suppressive cytokine.
In conclusion, although inducible Tregs control mucosal inflammation (29), our data support the idea that control of protective immunity in the intestinal setting may be regulated by natural and not inducible Tregs. This scenario has been suggested by recent studies of the IL-6-deficient BALB/c mouse, which are highly resistant to H. polygyrus infection (K A Smith and R M Maizels, manuscript submitted for publication). This intriguing and unexpected division of labor between Treg subsets remains to be further explored.
Acknowledgments
We are grateful to David Gray for maintaining IFN-y −/− mice, to Yvonne Harcus for genotyping single- and double-transgenic lines, and to James Hewitson, Henry McSorley, Katie Smith and Matt Taylor for critical reading of the manuscript.
Footnotes
Funded by Wellcome Trust PhD studentship and Programme Grant funding
References
- 1.Anthony RM, Rutitzky LI, Urban JF, Jr., Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nat. Rev. Immunol. 2007;7:975–987. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen JE, Maizels RM. Diversity and dialogue in immunity to helminths. Nat Rev Immunol. 2011;11:375–388. doi: 10.1038/nri2992. [DOI] [PubMed] [Google Scholar]
- 3.Grainger JR, Smith KA, Hewitson JP, McSorley HJ, Harcus Y, Filbey KJ, Finney CAM, Greenwood EJD, Knox DP, Wilson MS, Belkaid Y, Rudensky AY, Maizels RM. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-β pathway. J Exp Med. 2010;207:2331–2341. doi: 10.1084/jem.20101074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Else KJ, Finkelman FD, Maliszewski CR, Grencis RK. Cytokine-mediated regulation of chronic intestinal helminth infection. J. Exp. Med. 1994;179:347–351. doi: 10.1084/jem.179.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li MO, Flavell RA. TGF-β: a master of all T cell trades. Cell. 2008;134:392–404. doi: 10.1016/j.cell.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hang L, Setiawan T, Blum AM, Urban J, Stoyanoff K, Arihiro S, Reinecker HC, Weinstock JV. Heligmosomoides polygyrus infection can inhibit colitis through direct interaction with innate immunity. J Immunol. 2010;185:3184–3189. doi: 10.4049/jimmunol.1000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ince MN, Elliott DE, Setiawan T, Metwali A, Blum A, Chen HL, Urban JF, Flavell RA, Weinstock JV. Role of T cell TGF-β signaling in intestinal cytokine responses and helminthic immune modulation. Eur J Immunol. 2009;39:1870–1878. doi: 10.1002/eji.200838956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J, Martin B, Wilhelm C, Stockinger B. Transforming growth factor-β ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008;9:1341–1346. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 9.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 10.Behnke JM, Wahid FN, Grencis RK, Else KJ, Ben-Smith AW, Goyal PK. Immunological relationships during primary infection with Heligmosomoides polygyrus (Nematospiroides dubius ): downregulation of specific cytokine secretion (IL-9 and IL-10) correlates with poor mastocytosis and chronic survival of adult worms. Parasite Immunol. 1993;15:415–421. doi: 10.1111/j.1365-3024.1993.tb00626.x. [DOI] [PubMed] [Google Scholar]
- 11.Elliott DE, Metwali A, Leung J, Setiawan T, Blum AM, Ince MN, Bazzone LE, Stadecker MJ, Urban JF, Jr., Weinstock JV. Colonization with Heligmosomoides polygyrus suppresses mucosal IL-17 production. J Immunol. 2008;181:2414–2419. doi: 10.4049/jimmunol.181.4.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsunawaki S, Sporn M, Ding A, Nathan C. Deactivation of macrophages by transforming growth factor-β. Nature. 1988;334:260–262. doi: 10.1038/334260a0. [DOI] [PubMed] [Google Scholar]
- 13.Gorelik L, Constant S, Flavell RA. Mechanism of transforming growth factor β-induced inhibition of T helper type 1 differentiation. J Exp Med. 2002;195:1499–1505. doi: 10.1084/jem.20012076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-β1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:1061–1067. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maizels RM, Smith KA. Regulatory T cells in infection. Adv Immunol. 2011;112:73–136. doi: 10.1016/B978-0-12-387827-4.00003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorelik L, Flavell RA. Abrogation of TGFβ signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–181. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- 17.Prowse SJ, Mitchell GF, Ley PL, Jenkin CR. The development of resistance in different inbred strains of mice to infection with Nematospiroides dubius. Parasite Immunol. 1979;1:277–288. doi: 10.1111/j.1365-3024.1979.tb00713.x. [DOI] [PubMed] [Google Scholar]
- 18.Taylor MD, Harris A, Babayan S, Bain O, Culshaw A, Allen JE, Maizels RM. CTLA-4+ and CD4+CD25+ regulatory T cells inhibit protective immunity to filarial parasites in vivo. J. Immunol. 2007;179:4626–4634. doi: 10.4049/jimmunol.179.7.4626. [DOI] [PubMed] [Google Scholar]
- 19.Rausch S, Huehn J, Loddenkemper C, Hepworth MR, Klotz C, Sparwasser T, Hamann A, Lucius R, Hartmann S. Establishment of nematode infection despite increased Th2 responses and immunopathology after selective depletion of Foxp3+ cells. Eur J Immunol. 2009;39:3066–3077. doi: 10.1002/eji.200939644. [DOI] [PubMed] [Google Scholar]
- 20.Blankenhaus B, Klemm U, Eschbach ML, Sparwasser T, Huehn J, Kuhl AA, Loddenkemper C, Jacobs T, Breloer M. Strongyloides ratti infection induces expansion of Foxp3+ regulatory T cells that interfere with immune response and parasite clearance in BALB/c mice. J Immunol. 2011;186:4295–4305. doi: 10.4049/jimmunol.1001920. [DOI] [PubMed] [Google Scholar]
- 21.Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, Shevach EM. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huehn J, Siegmund K, Lehmann JCU, Siewert C, Haubold U, Feuerer M, Debes GF, Lauber J, Frey O, Przybylski GK, Niesner U, de la Rosa M, Schmidt CA, Bräuer R, Buer J, Scheffold A, Hamann A. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J Exp Med. 2004;199:303–313. doi: 10.1084/jem.20031562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Townsend MJ, Fallon PG, Matthews DJ, Smith P, Jolin HE, McKenzie ANJ. IL-9-deficient mice establish fundamental roles for IL-9 in pulmonary mastocytosis and goblet cell hyperplasia but not T cell development. Immunity. 2000;13:573–583. doi: 10.1016/s1074-7613(00)00056-x. [DOI] [PubMed] [Google Scholar]
- 24.Hashimoto K, Uchikawa R, Tegoshi T, Takeda K, Yamada M, Arizono N. Immunity-mediated regulation of fecundity in the nematode Heligmosomoides polygyrus--the potential role of mast cells. Parasitology. 2010;137:881–887. doi: 10.1017/S0031182009991673. [DOI] [PubMed] [Google Scholar]
- 25.Morimoto M, Utsumiya K. Enhanced protection against Heligmosomoides polygyrus in IL-2 receptor lygyrusumiya. 2011. Enhanced protection against N. Arizono 2010. IJ Vet Med Sci. 2011;73:849–851. doi: 10.1292/jvms.10-0566. [DOI] [PubMed] [Google Scholar]
- 26.Finney CAM, Taylor MD, Wilson MS, Maizels RM. Expansion and activation of CD4+CD25+ regulatory T cells in Heligmosomoides polygyrus infection. Eur. J. Immunol. 2007;37:1874–1886. doi: 10.1002/eji.200636751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson PW, Green SJ, Carter C, Coadwell J, Kilshaw PJ. Studies on transcriptional regulation of the mucosal T-cell integrin αEβ7 (CD103) Immunology. 2001;103:146–154. doi: 10.1046/j.1365-2567.2001.01232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El-Asady R, Yuan R, Liu K, Wang D, Gress RE, Lucas PJ, Drachenberg CB, Hadley GA. TGF-β-dependent CD103 expression by CD8+T cells promotes selective destruction of the host intestinal epithelium during graft-versus-host disease. J Exp Med. 2005;201:1647–1657. doi: 10.1084/jem.20041044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y, Umetsu DT, Rudensky AY. Extrathymically generated regulatory T cells control mucosal Th2 inflammation. Nature. 2012;482:395–399. doi: 10.1038/nature10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fahlen L, Read S, Gorelik L, Hurst SD, Coffman RL, Flavell RA, Powrie F. T cells that cannot respond to TGF-β escape control by CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:737–746. doi: 10.1084/jem.20040685. [DOI] [PMC free article] [PubMed] [Google Scholar]