Abstract
The chromosomal passenger complex (CPC-INCENP, Aurora B kinase, Survivin and Borealin) is implicated in many mitotic processes. Here we describe how we generated DT40 conditional knockout cell lines for incenp1 and survivin1 to better understand the role of these CPC subunits in the control of Aurora B kinase activity. These lines enabled us to reassess current knowledge of Survivin function and to show that INCENP acts as a rheostat for Aurora B activity.
Keywords: DT40, Knockout, INCENP, Survivin
INTRODUCTION
Accurate chromosome segregation requires that kinetochores of sister chromatids bind microtubules that emanate from opposing spindle poles. During prometaphase, various aberrant kinetochore attachments occur which, if not corrected, can lead to improper chromosome segregation and aneuploidy. To avoid this, cells have a surveillance mechanism, called the spindle checkpoint, which delays anaphase until all sister kinetochores are properly captured and under tension. Kinetochore attachment and error correction are directly controlled by the Aurora B kinase, part of the chromosomal passenger complex (CPC).
In most organisms, the core CPC is composed of Aurora B kinase [1] and three non-enzymatic subunits, INCENP [2, 3], Survivin and Borealin/Dasra B [4-7] that control targeting, enzymatic activity and stability of Aurora B kinase [8]. The CPC controls many aspects of mitosis ranging from chromosome and spindle structure to the correction of kinetochore-microtubule attachment errors, regulation of mitotic progression and completion of cytokinesis [9]. Knockdown by RNA interference of any member of the complex disrupts mitotic progression [4, 7, 10-13]. Although INCENP has been shown to be responsible for the initial Aurora B activation through direct binding and a phosphorylation feedback loop [9, 14, 15], the mechanisms responsible for controlling the activity of this important kinase are still poorly understood.
The ability to shut down/off the expression of a gene by RNAi or knockout is widely used to study protein function in many eukaryotic systems. Whereas RNAi relies upon the destruction of the mRNA, which is continuously produced, conditional knockouts block production of the mRNA at its source. Another advantage of knockout strategies over RNAi is the absence of off-target effects [16]. Several cell types are used to generate knockouts including mouse ES cells, human RPE, Nalm-6, HT1080 and chicken DT40 cell lines [17-22].
DT40 cells are chicken B lymphoma with a very high rate of homologous recombination (up to 90% for some loci), greatly facilitating gene targeting. DT40s have been used to study many biological processes, including DNA damage pathways [23], transcriptional regulation [24], calcium signalling [25], apoptosis [26-29] and chromatin structure [30-32].
To better understand the role of the CPC in regulating Aurora B activity we studied the involvement of INCENP and Survivin by generating DT40 conditional knockout cell lines for their genes [33, 34].
1- Assessment of SURVIVIN function in mitosis and apoptosis
Survivin is a protein involved in mitotic progression and apoptotic regulation that is up-regulated in many human tumours. Survivin’s expression peaks during mitosis [8, 35, 36]. The protein is required for targeting the CPC to centromeres [8, 37]. Survivin is considered a member of the inhibitor of apoptosis protein (IAP) family, despite lacking some of the key features shared by other members of the family. The exact role of Survivin in mitosis and apoptosis remains unclear.
Our lab generated two DT40 conditional knockouts of the survivin gene. We used this system to study the role of Survivin both in mitosis and apoptosis and to assess the role of key residues of the proteins in these biological processes.
1-1 KNOCKOUT STRATEGY
Survivin−/− cells were generated by deleting the entire survivin1 gene (Acc. N°: ENSGALG00000008713, Figure 1A). Briefly, after targeting and replacement of the first allele by a resistance marker, the survivin cDNA, regulated by a Tet off system, was introduced in the heterozygous cells. The second allele was then targeted, generating a conditional Survivin knockout cell line which, upon addition of doxycycline exhibits the null phenotype. Stable transfection of this cell line with various Survivin cDNA-bearing mutations of interest allowed us to study their phenotypes in a null background.
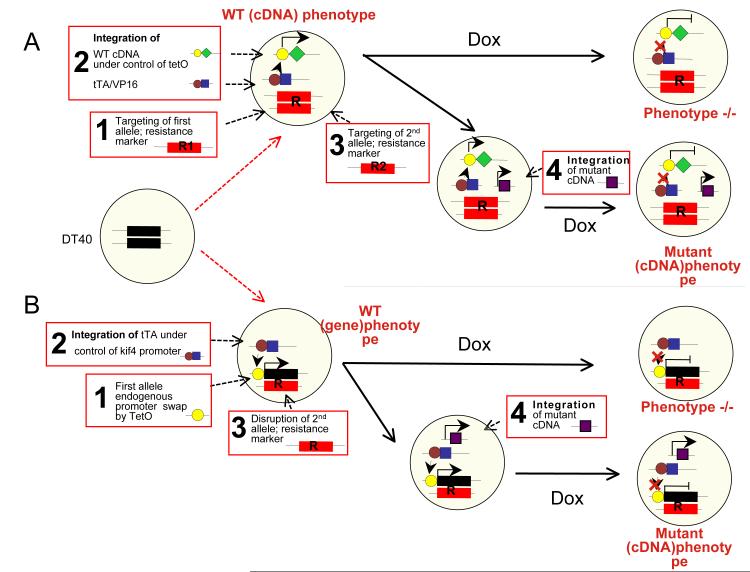
Figure 1. Schematic representation of the DT40 gene disruption and “conditional promoter”.
A, Gene disruption strategy: The first survivin1 allele is fully replaced with a resistance marker by homologous recombination (1). In a second step, a rescue construct containing, the transactivator (tTA/VP16) under control of either kif4a or CMV promoter and the Survivin cDNA under control of tet operator (TetO) are randomly integrated (2). Finally, the second survivin1 allele is targeted and replaced by a resistance marker (3). Addition of doxycycline (dox) turns off the expression of the exogenous cDNA giving the null phenotype. cDNA encoding Survivin mutants are randomly integrated (4). Addition of doxycycline allows the observation of the mutant phenotype in a null background. B, Conditionnal promoter strategy: In a first step, the promoter of the first incenp1 allele is swapped with a TetO by homologous recombination (1). The second step involves random integration of a construct containing the tTA under control of the kif4a promoter (2). In a third step, the second incenp1 allele is disrupted by a resistance marker (3). The addition of doxycycline turns off the expression of the allele regulated by the TetO promoter giving the null phenotype. Random integration of wild type or mutant INCENP cDNAs (4) enables, after addition of doxycycline, the observation of their phenotype in a null background.
1-2 THE ROLE of SURVIVIN in MITOSIS
Using our unique survivin−/− model, we confirmed some previous findings on Survivin function and revealed important features of key residues of the protein. For instance, we showed that Survivin is essential for both chromosome segregation and cytokinesis, concordant with observations from RNAi studies [12, 13]. A mutant previously shown to abolish interaction between Survivin and Aurora B (human SurvivinD70A/D71A, chicken SurvivinD72A/D73A) [38] introduced into the Survivin knockout cells was unable to concentrate normally at centromeres. This induced delocalization of the other passenger proteins in early mitosis, surprisingly without affecting subsequent transfer of the CPC to the spindle midzone. Furthermore, this mutant showed a compromised spindle checkpoint.
Unexpectedly, despite the presence of these defects, this mutant could progress through mitosis and sustain cell growth, suggesting that Survivin binding to Aurora B is not absolutely required for cell survival [34]. In contrast with RNAi studies, we didn’t observe any significant increase in defects in early phases of mitosis such as chromosome misalignment or abnormal spindles in the absence of survivin [12, 13, 39-41]. Survivin−/− cells exhibited mitotic arrest at high doses of taxol whereas at lower doses they could override the spindle checkpoint and progress through mitosis. This override was concomitant with increased apoptosis suggesting abnormal mitotic exit and subsequent cell death occurring in interphase [34].
1-3 THE ROLE of SURVIVIN in CELL DEATH
Similarly to what we observed for mitosis, survivin−/− cells enabled us to confirm some previously described results regarding Survivin’s role in apoptosis and to contradict others. For instance we could confirm that some mutations in specific domains of Survivin did induce a loss of function phenotype, such as point mutations in the BIR domain (SurvivinC59A or SurvivinC86A) [42]. On the other hand, loss of Survivin did not induce an increased sensitivity to pro-apoptotic stimuli. Furthermore, abolition of Survivin binding to Smac (SurvivinD55A), an antagonist of Survivin involved in apoptosis, did not prevent cell growth nor did it display a pro-apoptotic phenotype, contradicting previous reports about the protective role of Survivin in apoptosis [42-46].
1-4 CONCLUSIONS
Generation of DT40 survivin−/− cells expressing various mutants, allowed us to reassess Survivin functions both in mitosis and in apoptosis in complete absence of the endogenous wild-type protein. The null background proved to be essential to study Survivin function. Discrepancies observed between our study and previous reports demonstrate the importance of a genetically-clean system to dissect the protein functions. Our study ultimately proved that cell death induced by loss of Survivin is linked to cell cycle defects (primarily a failure in cytokinesis) rather than disregulation of apoptosis.
2- Functionnal analysis of INCENP-Aurora B interactions
INCENP is the scaffolding protein of the CPC [2, 3]. Its N-terminus is required for centromere targeting of the complex whereas its C-terminus contains the IN box where Aurora B binds and gets activated. To study in more detail the interactions between INCENP and Aurora B, we generated a conditional DT40 knockout for incenp1 (Acc. N°: ENSGALG00000007537), expressing INCENP bearing specific point mutations in the IN box. We chose residues predicted to be important for activation (W766) or binding of Aurora B (F802) to INCENP [15].
2-1 KNOCKOUT STRATEGY
To generate an incenp1 knockout, we used a promoter hi-jack strategy (Figure 1B) [33, 47]. Here, the first allele was placed under the control of a Tet off system, which was achieved by replacing the endogenous promoter with a minimal CMV/TetO promoter, together with stable expression of the tTA/VP16 transactivator under the control of the kif4A promoter. The other incenp1 allele had its ORF disrupted, potentially allowing expression of only the 28 first amino acids of the protein. In these cells, the addition of doxycycline shuts off expression from the endogenous incenp1 allele (Figure 2A, B). This strategy enabled the conditional expression of the multiple spliced isoforms of INCENP (class I and class II) [48].
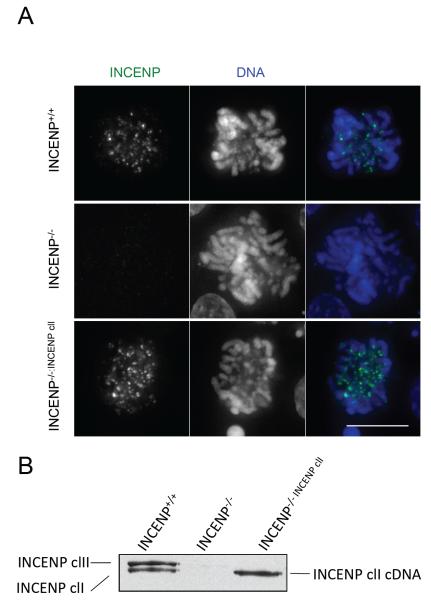
Figure 2. The conditional INCENP knockout model.
A, Immunofluorescence of DT40 (INCENP+/+), DT40 INCENP−/− or DT40 INCENP−/− expressing INCENP class I after 24h of doxycycline stained for INCENP (green) and DNA (blue). Bar 10 μm. B, Immunoblot comparing the levels of INCENP protein in INCENP+/+ cells, INCENP−/− and in the INCENP−/− cells transfected with INCENP classI cDNA 24h after addition of doxycycline. Note the presence of two isoforms, class I and II in the wild type cells and the total absence of the protein in the knockout cells.
2-2 Regulation of Aurora B activity through INCENP binding
We used this system to study how interactions between INCENP and Aurora B affect the activity of the kinase along with CPC function and localisation. The two mutants generated had different binding capacity to Aurora B leading to different levels of kinase activity. Expression of INCENPW766G in absence of wild-type INCENP abolished Aurora B interaction with INCENP, reducing Aurora B activity by 70%. INCENPF802A mutant bound Aurora B in a normal fashion but kinase activity was reduced by 50% contrasting with predictions from previous structural analysis [15]. Expression of either INCENPW766G or INCENPF802A in a null background induced cytokinesis failure and rapid cell death [33].
Both INCENPW766G and INCENPF802A were properly localised at centromeres in early mitosis but failed to transfer to the spindle midzone in anaphase. We could thus demonstrate that mitotic entry and sister chromatid separation are independent of CPC formation or Aurora B activity whereas both are required for proper transfer of the CPC to the spindle midzone and completion of cytokinesis [33]. Despite reports from RNAi experiments showing that Aurora B activity is required for outer kinetochore assembly [49, 50], we were able to detect CENP-A, -H, -O, -T, -E and the Hec1/Ndc80 complex at kinetochores of prometaphase incenp1−/− cells suggesting that kinetochore assembly is not affected by the lack of CPC at centromeres [33]. Moreover, CENP-E and PRC-1 localisation to the spindle midzone of incenp1−/− anaphase cells demonstrated that a spindle midzone was present despite the absence of CPC [33].
Expressing INCENP mutants in a null background enabled us to analyse the relationship between Aurora B kinase activity and the spindle checkpoint response induced by taxol. We established that various Aurora B activity levels yielded different spindle checkpoint responses when cells were treated with low doses of taxol. A minimal Aurora B activity (~50%, INCENPF802A) was sufficient to trigger a checkpoint response whereas lower activity (~30%, INCENPW766G or 15% with Aurora B inhibitor) only induced a weak checkpoint response [33]. This probably reflects the ability of the kinase to correct kinetochore-microtubules mis-attachments.
2-3 CONCLUSION
We mutated residues of INCENP that were predicted by crystallography studies to affect Aurora B binding or activity. Using incenp1−/− cells, we could assess the in vivo effects of mutating these residues. We were able to show that modulation of Aurora B activity regulates CPC localisation and function during mitosis. Ultimately, the use of DT40 incenp1−/− cells expressing various mutants allowed us to strengthen the model in which INCENP acts as a rheostat for Aurora B activity [33].
3- CONCLUSIONS
The DT40 cell line is a powerful system to study protein function. The ability to perform conditional knockouts of essential, multiply-spliced cell cycle-regulated genes makes it an ideal system to screen for and characterise functional domains or residues through the analysis of specific mutants against a null background.
Acknowledgments
SR is funded by “Ligue contre le Cancer du Grand-Ouest” comity, (districts: 22, 29, 56 and 79). Work in the Earnshaw Laboratory is supported by the Wellcome trust of which he is a Principal Research Fellow.
ABBREVIATIONS
- CPC
Chromosomal Passenger Complex
- IAP
Inhibitor of Apoptosis Protein
- ORF
Open Reading Frame
- TetO
tet operator
- tTA
Transactivator
Footnotes
NOTE Amino acid numbering corresponds to chicken proteins.
REFERENCES
- 1.Terada Y, Tatsuka M, Suzuki F, Yasuda Y, Fujita S, Otsu M. AIM-1: a mammalian midbody-associated protein required for cytokinesis. The EMBO journal. 1998;17:667–676. doi: 10.1093/emboj/17.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooke CA, Heck MM, Earnshaw WC. The inner centromere protein (INCENP) antigens: movement from inner centromere to midbody during mitosis. The Journal of cell biology. 1987;105:2053–2067. doi: 10.1083/jcb.105.5.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams RR, Wheatley SP, Gouldsworthy AM, Kandels-Lewis SE, Carmena M, Smythe C, Gerloff DL, Earnshaw WC. INCENP binds the Aurora-related kinase AIRK2 and is required to target it to chromosomes, the central spindle and cleavage furrow. Curr Biol. 2000;10:1075–1078. doi: 10.1016/s0960-9822(00)00673-4. [DOI] [PubMed] [Google Scholar]
- 4.Honda R, Korner R, Nigg EA. Exploring the functional interactions between Aurora B, INCENP, and survivin in mitosis. Molecular biology of the cell. 2003;14:3325–3341. doi: 10.1091/mbc.E02-11-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sampath SC, Ohi R, Leismann O, Salic A, Pozniakovski A, Funabiki H. The chromosomal passenger complex is required for chromatin-induced microtubule stabilization and spindle assembly. Cell. 2004;118:187–202. doi: 10.1016/j.cell.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 6.Klein UR, Nigg EA, Gruneberg U. Centromere targeting of the chromosomal passenger complex requires a ternary subcomplex of Borealin, Survivin, and the N-terminal domain of INCENP. Molecular biology of the cell. 2006;17:2547–2558. doi: 10.1091/mbc.E05-12-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gassmann R, Carvalho A, Henzing AJ, Ruchaud S, Hudson DF, Honda R, Nigg EA, Gerloff DL, Earnshaw WC. Borealin: a novel chromosomal passenger required for stability of the bipolar mitotic spindle. The Journal of cell biology. 2004;166:179–191. doi: 10.1083/jcb.200404001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lens SM, Rodriguez JA, Vader G, Span SW, Giaccone G, Medema RH. Uncoupling the central spindle-associated function of the chromosomal passenger complex from its role at centromeres. Molecular biology of the cell. 2006;17:1897–1909. doi: 10.1091/mbc.E05-08-0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruchaud S, Carmena M, Earnshaw WC. Chromosomal passengers: conducting cell division. Nature reviews. 2007;8:798–812. doi: 10.1038/nrm2257. [DOI] [PubMed] [Google Scholar]
- 10.Adams RR, Maiato H, Earnshaw WC, Carmena M. Essential roles of Drosophila inner centromere protein (INCENP) and aurora B in histone H3 phosphorylation, metaphase chromosome alignment, kinetochore disjunction, and chromosome segregation. The Journal of cell biology. 2001;153:865–880. doi: 10.1083/jcb.153.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vader G, Kauw JJ, Medema RH, Lens SM. Survivin mediates targeting of the chromosomal passenger complex to the centromere and midbody. EMBO reports. 2006;7:85–92. doi: 10.1038/sj.embor.7400562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carvalho A, Carmena M, Sambade C, Earnshaw WC, Wheatley SP. Survivin is required for stable checkpoint activation in taxol-treated HeLa cells. Journal of cell science. 2003;116:2987–2998. doi: 10.1242/jcs.00612. [DOI] [PubMed] [Google Scholar]
- 13.Lens SM, Medema RH. The survivin/Aurora B complex: its role in coordinating tension and attachment. Cell cycle (Georgetown, Tex. 2003;2:507–510. doi: 10.4161/cc.2.6.559. [DOI] [PubMed] [Google Scholar]
- 14.Bishop JD, Schumacher JM. Phosphorylation of the carboxyl terminus of inner centromere protein (INCENP) by the Aurora B Kinase stimulates Aurora B kinase activity. The Journal of biological chemistry. 2002;277:27577–27580. doi: 10.1074/jbc.C200307200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sessa F, Mapelli M, Ciferri C, Tarricone C, Areces LB, Schneider TR, Stukenberg PT, Musacchio A. Mechanism of Aurora B activation by INCENP and inhibition by hesperadin. Molecular cell. 2005;18:379–391. doi: 10.1016/j.molcel.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 16.Hubner NC, Wang LH, Kaulich M, Descombes P, Poser I, Nigg EA. Re-examination of siRNA specificity questions role of PICH and Tao1 in the spindle checkpoint and identifies Mad2 as a sensitive target for small RNAs. Chromosoma. 2010;119:149–165. doi: 10.1007/s00412-009-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dovey OM, Foster CT, Cowley SM. Histone deacetylase 1 (HDAC1), but not HDAC2, controls embryonic stem cell differentiation. Proc Natl Acad Sci U S A. 2010;107:8242–8247. doi: 10.1073/pnas.1000478107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burkard ME, Randall CL, Larochelle S, Zhang C, Shokat KM, Fisher RP, Jallepalli PV. Chemical genetics reveals the requirement for Polo-like kinase 1 activity in positioning RhoA and triggering cytokinesis in human cells. Proc Natl Acad Sci U S A. 2007;104:4383–4388. doi: 10.1073/pnas.0701140104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adachi N, Kurosawa A, Koyama H. Highly proficient gene targeting by homologous recombination in the human pre-B cell line Nalm-6. Methods in molecular biology (Clifton, N.J. 2008;435:17–29. doi: 10.1007/978-1-59745-232-8_2. [DOI] [PubMed] [Google Scholar]
- 20.Adachi N, So S, Iiizumi S, Nomura Y, Murai K, Yamakawa C, Miyagawa K, Koyama H. The human pre-B cell line Nalm-6 is highly proficient in gene targeting by homologous recombination. DNA and cell biology. 2006;25:19–24. doi: 10.1089/dna.2006.25.19. [DOI] [PubMed] [Google Scholar]
- 21.Hudson DF, Morrison C, Ruchaud S, Earnshaw WC. Reverse genetics of essential genes in tissue-culture cells: ‘dead cells talking’. Trends in cell biology. 2002;12:281–287. doi: 10.1016/s0962-8924(02)02281-x. [DOI] [PubMed] [Google Scholar]
- 22.Itzhaki JE, Gilbert CS, Porter AC. Construction by gene targeting in human cells of a “conditional’ CDC2 mutant that rereplicates its DNA. Nat Genet. 1997;15:258–265. doi: 10.1038/ng0397-258. [DOI] [PubMed] [Google Scholar]
- 23.Zachos G, Rainey MD, Gillespie DA. Chk1-dependent S-M checkpoint delay in vertebrate cells is linked to maintenance of viable replication structures. Molecular and cellular biology. 2005;25:563–574. doi: 10.1128/MCB.25.2.563-574.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsudo H, Otsuka A, Ozawa Y, Ono M. Disruption of the PU.1 gene in chicken B lymphoma DT40 cells and its effect on reported target gene expression. Gene. 2003;322:169–174. doi: 10.1016/j.gene.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 25.Lievremont JP, Numaga T, Vazquez G, Lemonnier L, Hara Y, Mori E, Trebak M, Moss SE, Bird GS, Mori Y, Putney JW., Jr The role of canonical transient receptor potential 7 in B-cell receptor-activated channels. The Journal of biological chemistry. 2005;280:35346–35351. doi: 10.1074/jbc.M507606200. [DOI] [PubMed] [Google Scholar]
- 26.Ageichik AV, Samejima K, Kaufmann SH, Earnshaw WC. Genetic analysis of the short splice variant of the inhibitor of caspase-activated DNase (ICAD-S) in chicken DT40 cells. The Journal of biological chemistry. 2007;282:27374–27382. doi: 10.1074/jbc.M704307200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Samejima K, Tone S, Earnshaw WC. CAD/DFF40 nuclease is dispensable for high molecular weight DNA cleavage and stage I chromatin condensation in apoptosis. The Journal of biological chemistry. 2001;276:45427–45432. doi: 10.1074/jbc.M108844200. [DOI] [PubMed] [Google Scholar]
- 28.Ruchaud S, Korfali N, Villa P, Kottke TJ, Dingwall C, Kaufmann SH, Earnshaw WC. Caspase-6 gene disruption reveals a requirement for lamin A cleavage in apoptotic chromatin condensation. The EMBO journal. 2002;21:1967–1977. doi: 10.1093/emboj/21.8.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Korfali N, Ruchaud S, Loegering D, Bernard D, Dingwall C, Kaufmann SH, Earnshaw WC. Caspase-7 gene disruption reveals an involvement of the enzyme during the early stages of apoptosis. The Journal of biological chemistry. 2004;279:1030–1039. doi: 10.1074/jbc.M306277200. [DOI] [PubMed] [Google Scholar]
- 30.Hudson DF, Vagnarelli P, Gassmann R, Earnshaw WC. Condensin is required for nonhistone protein assembly and structural integrity of vertebrate mitotic chromosomes. Developmental cell. 2003;5:323–336. doi: 10.1016/s1534-5807(03)00199-0. [DOI] [PubMed] [Google Scholar]
- 31.Vagnarelli P, Hudson DF, Ribeiro SA, Trinkle-Mulcahy L, Spence JM, Lai F, Farr CJ, Lamond AI, Earnshaw WC. Condensin and Repo-Man-PP1 co-operate in the regulation of chromosome architecture during mitosis. Nature cell biology. 2006;8:1133–1142. doi: 10.1038/ncb1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ribeiro SA, Vagnarelli P, Dong Y, Hori T, McEwen BF, Fukagawa T, Flors C, Earnshaw WC. A super-resolution map of the vertebrate kinetochore. Proc Natl Acad Sci U S A. 2010;107:10484–10489. doi: 10.1073/pnas.1002325107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu Z, Ogawa H, Vagnarelli P, Bergmann JH, Hudson DF, Ruchaud S, Fukagawa T, Earnshaw WC, Samejima K. INCENP-aurora B interactions modulate kinase activity and chromosome passenger complex localization. The Journal of cell biology. 2009;187:637–653. doi: 10.1083/jcb.200906053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yue Z, Carvalho A, Xu Z, Yuan X, Cardinale S, Ribeiro S, Lai F, Ogawa H, Gudmundsdottir E, Gassmann R, Morrison CG, Ruchaud S, Earnshaw WC. Deconstructing Survivin: comprehensive genetic analysis of Survivin function by conditional knockout in a vertebrate cell line. The Journal of cell biology. 2008;183:279–296. doi: 10.1083/jcb.200806118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li F, Ambrosini G, Chu EY, Plescia J, Tognin S, Marchisio PC, Altieri DC. Control of apoptosis and mitotic spindle checkpoint by survivin. Nature. 1998;396:580–584. doi: 10.1038/25141. [DOI] [PubMed] [Google Scholar]
- 36.Wheatley SP, McNeish IA. Survivin: a protein with dual roles in mitosis and apoptosis. International review of cytology. 2005;247:35–88. doi: 10.1016/S0074-7696(05)47002-3. [DOI] [PubMed] [Google Scholar]
- 37.Carmena M, Earnshaw WC. INCENP at the kinase crossroads. Nature cell biology. 2006;8:110–111. doi: 10.1038/ncb0206-110. [DOI] [PubMed] [Google Scholar]
- 38.Cao L, Yan X, Wu Y, Hu H, Li Q, Zhou T, Jiang S, Yu L. Survivin mutant (Surv-DD70, 71AA) disrupts the interaction of Survivin with Aurora B and causes multinucleation in HeLa cells. Biochem Biophys Res Commun. 2006;346:400–407. doi: 10.1016/j.bbrc.2006.05.131. [DOI] [PubMed] [Google Scholar]
- 39.Giodini A, Kallio MJ, Wall NR, Gorbsky GJ, Tognin S, Marchisio PC, Symons M, Altieri DC. Regulation of microtubule stability and mitotic progression by survivin. Cancer Res. 2002;62:2462–2467. [PubMed] [Google Scholar]
- 40.Okada H, Bakal C, Shahinian A, Elia A, Wakeham A, Suh WK, Duncan GS, Ciofani M, Rottapel R, Zuniga-Pflucker JC, Mak TW. Survivin loss in thymocytes triggers p53-mediated growth arrest and p53-independent cell death. J Exp Med. 2004;199:399–410. doi: 10.1084/jem.20032092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosa J, Canovas P, Islam A, Altieri DC, Doxsey SJ. Survivin modulates microtubule dynamics and nucleation throughout the cell cycle. Molecular biology of the cell. 2006;17:1483–1493. doi: 10.1091/mbc.E05-08-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li F, Ackermann EJ, Bennett CF, Rothermel AL, Plescia J, Tognin S, Villa A, Marchisio PC, Altieri DC. Pleiotropic cell-division defects and apoptosis induced by interference with survivin function. Nature cell biology. 1999;1:461–466. doi: 10.1038/70242. [DOI] [PubMed] [Google Scholar]
- 43.Pennati M, Binda M, Colella G, Zoppe M, Folini M, Vignati S, Valentini A, Citti L, De Cesare M, Pratesi G, Giacca M, Daidone MG, Zaffaroni N. Ribozyme-mediated inhibition of survivin expression increases spontaneous and drug-induced apoptosis and decreases the tumorigenic potential of human prostate cancer cells. Oncogene. 2004;23:386–394. doi: 10.1038/sj.onc.1207071. [DOI] [PubMed] [Google Scholar]
- 44.Wang Z, Sampath J, Fukuda S, Pelus LM. Disruption of the inhibitor of apoptosis protein survivin sensitizes Bcr-abl-positive cells to STI571-induced apoptosis. Cancer Res. 2005;65:8224–8232. doi: 10.1158/0008-5472.CAN-05-0303. [DOI] [PubMed] [Google Scholar]
- 45.Ambrosini G, Adida C, Sirugo G, Altieri DC. Induction of apoptosis and inhibition of cell proliferation by survivin gene targeting. The Journal of biological chemistry. 1998;273:11177–11182. doi: 10.1074/jbc.273.18.11177. [DOI] [PubMed] [Google Scholar]
- 46.Song Z, Liu S, He H, Hoti N, Wang Y, Feng S, Wu M. A single amino acid change (Asp 53 --> Ala53) converts Survivin from anti-apoptotic to pro-apoptotic. Molecular biology of the cell. 2004;15:1287–1296. doi: 10.1091/mbc.E03-07-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Samejima K, Ogawa H, Cooke CA, Hudson DF, Macisaac F, Ribeiro SA, Vagnarelli P, Cardinale S, Kerr A, Lai F, Ruchaud S, Yue Z, Earnshaw WC. A promoter-hijack strategy for conditional shutdown of multiply spliced essential cell cycle genes. Proc Natl Acad Sci U S A. 2008;105:2457–2462. doi: 10.1073/pnas.0712083105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mackay AM, Eckley DM, Chue C, Earnshaw WC. Molecular analysis of the INCENPs (inner centromere proteins): separate domains are required for association with microtubules during interphase and with the central spindle during anaphase. The Journal of cell biology. 1993;123:373–385. doi: 10.1083/jcb.123.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Emanuele MJ, Lan W, Jwa M, Miller SA, Chan CS, Stukenberg PT. Aurora B kinase and protein phosphatase 1 have opposing roles in modulating kinetochore assembly. The Journal of cell biology. 2008;181:241–254. doi: 10.1083/jcb.200710019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang Y, Wu F, Ward T, Yan F, Wu Q, Wang Z, McGlothen T, Peng W, You T, Sun M, Cui T, Hu R, Dou Z, Zhu J, Xie W, Rao Z, Ding X, Yao X. Phosphorylation of HsMis13 by Aurora B kinase is essential for assembly of functional kinetochore. The Journal of biological chemistry. 2008;283:26726–26736. doi: 10.1074/jbc.M804207200. [DOI] [PMC free article] [PubMed] [Google Scholar]