Abstract
Infections of the reproductive tract or mammary gland with Gram-negative bacteria perturb ovarian function, follicular growth and fecundity in cattle. We hypothesised that lipopolysaccharide (LPS) from Gram-negative bacteria stimulates an inflammatory response by ovarian granulosa cells that is mediated by TLR4. The present study tested the capacity of bovine ovarian granulosa cells to initiate an inflammatory response to pathogen associated molecular patterns (PAMPs), and determined subsequent effects on the in vitro maturation of oocytes. Granulosa cells elicited an inflammatory response to PAMPs (LPS, lipoteichoic acid, peptidoglycan or Pam3CSK4) with accumulation of the cytokine IL-6, and the chemokine IL-8, in a time- and dose-dependent manner. Granulosa cells responded acutely to LPS with rapid phosphorylation of TLR signaling components, p38 and ERK, and increased expression of IL6 and IL8 mRNA, although nuclear translocation of p65 was not evident. Targeting TLR4 with siRNA, attenuated granulosa cell accumulation of IL-6 in response to LPS. Endocrine function of granulosa cells is regulated by FSH, but here FSH also enhanced responsiveness to LPS, increasing IL-6 and IL-8 accumulation. Furthermore, LPS stimulated IL-6 secretion and expansion by cumulus-oocyte complexes (COCs), and increased rates of meiotic arrest and germinal vesicle breakdown failure. In conclusion, bovine granulosa cells initiate an innate immune response to LPS via the TLR4 pathway leading to inflammation and to perturbation of meiotic competence.
Keywords: Granulosa cell, immunity, inflammation, lipopolysaccharide, oocyte maturation, ovary, Toll-like receptor 4
INTRODUCTION
Bacterial infections of the uterus or mammary gland commonly cause disease in dairy cattle, and these infections are associated with infertility (1-4). Uterine infection following parturition cause metritis in 40% of animals, and the resulting decreased milk yields, delayed ovulation, reduced fecundity and animal culling for failure to conceive cost the EU dairy industry €1.4 billion/year. Infection of the mammary gland causes mastitis in a comparable proportion of animals and these infections reduce conception rates. Metritis or mastitis also retard follicular growth, reduce circulating and intra-follicular estradiol concentrations, extended luteal phases, and disrupt ovarian cyclic activity (1-4). The Gram-negative bacterium Escherichia coli is a main pathogen causing metritis and mastitis, and these animals have reduced fecundity even after resolution of clinical disease (8, 9). Accumulation of lipopolysaccharide (LPS) from Gram-negative bacteria in follicular fluid of animals with metritis may link infection and ovarian dysfunction (2). Estradiol are reduced in granulosa cells cultured with LPS (3), while animals with mastitis have altered granulosa cell gene expression and lower follicular estradiol (4). Bacterial infections of the uterus in women also cause infertility (6, 10). Recently, microbial colonisation and altered cytokine profiles were reported in follicular fluid from IVF patients with low conception rates (11). However, mechanisms linking bacterial infection and perturbation of ovarian function or oocyte quality remain to be determined.
The Toll-like receptors (TLRs) are a family of 10 cellular receptors responsible for detecting and initiating the innate immune defence against bacterial, viral and fungal pathogens (12, 13). These receptors are primarily found on immune cells such as macrophages, and generate the initial inflammatory response to a pathogen by binding pathogen-associated molecular patterns (PAMPs). LPS derived from E. coli is a prototypical PAMP binding TLR4 in complex with co-receptors CD14 and MD-2, resulting in phosphorylation of ERK 1/2 and p38, and nuclear translocation of NFκB components, which leads to production of pro-inflammatory cytokines such as IL-1β, IL-6, TNFα, and chemokines such as IL-8 (12, 13). Bovine and murine granulosa cells also express mRNA for the TLR4 receptor complex (2, 14). It remains unclear whether granulosa cells respond to LPS via TLR4 to generate an inflammatory response akin to cells of the immune system. This is important because, although ovarian stroma contains immune cells for tissue remodelling, healthy follicles are devoid of immune cells (15).
Mammalian oocyte growth and maturation from the primordial follicle until ovulation is dictated by a highly ordered cascade of hormones, growth factors, nutrients and signaling molecules from the surrounding environment (16, 17). Oocytes must undergo nuclear and cytoplasmic maturation for successful fertilisation and embryonic development, progressing from the germinal vesicle stage until pausing at the M-phase of meiosis II (18). Oocytes depend on their surrounding granulosa cells for nutrition and there is bi-directional communication between oocyte and granulosa cells. However, these intimate interactions expose mammalian oocytes to more exogenous factors than invertebrate eggs enclosed in an impermeable shell. So, in the absence of immune cells in the ovarian follicle, perhaps granulosa cells play an active role to protect mammalian oocytes against PAMPs. Although, mice with defective TLR4 signaling have normal fertility (19, 20), TLR2 and TLR4 complexes binding endogenous ligands such as hyaluronic acid in ovulated cumulus-oocyte complexes play a role in sperm capacitation and oocyte fertilisation (21). Ovulation itself is regarded as sterile inflammation involving the innate immune system (22, 23). However, it is not clear whether during disease the activation of TLR4 by LPS could impact oocyte competence during follicle development.
Here we explore the mechanism of ovarian perturbation associated with PAMPs and investigate the possibility that granulosa cells act as immune sensors within the ovarian follicle. We tested the capacity of bovine ovarian granulosa cells to initiate an inflammatory response to PAMPs, and determined subsequent effects on the in vitro maturation (IVM) of oocytes. Here, we show that in vitro exposure of granulosa cells and oocytes to LPS generates a TLR4-dependent inflammatory response and ultimately perturbations in oocyte meiotic competence.
MATERIALS AND METHODS
Tissue collection and cell isolation
Ovaries were collected from cows within 15 min of slaughter and transported to the laboratory on ice in PBS containing 1% penicillin/streptomycin (Sigma-Aldrich, Poole, UK). Ovaries from between 10 and 20 cows were pooled for each experiment. Within 90 min of excision, ovaries were processed for collection of mural granulosa cells and cumulus-oocyte complexes (COCs). Ovaries were rinsed in 70% ethanol followed by a brief rinse in sterile PBS. An endotoxin-free 2 ml syringe and 20 G needle was employed to aspirate 4-8 mm follicles into collection medium (Medium 199; (Invitrogen, Paisley, UK), 0.5% BSA, 25mM HEPES, 50μg/ml heparin, 5U penicillin and 50μg/ml streptomycin; Sigma-Aldrich). The 4-8 mm follicles were chosen because they are a representative homogenous pool of follicles with granulosa cells that are FSH-responsive and LH-unresponsive (24). The COCs were collected and pooled for two 5 min washes in fresh collection medium without heparin and then placed into defined maturation culture medium (see below). Granulosa cells were pooled and washed twice in either serum-free medium as previously defined (25) or granulosa cell culture medium containing 10% FCS. Granulosa cells were then re-suspended and plated at 1.5 × 106 cells/ml in a final volume of 500 μl in 24-well plates (TPP, Trasadingen, Switzerland). Each experiment was carried out at least 4 times using tissue collected on different days. Treatments were performed in single wells with negative and positive controls.
Assessment of immune cells
Independent granulosa and blood cell cultures on three separate occasions were subjected to mRNA extraction for RT-PCR or fixation for immunocytochemistry (see below). Freshly isolated granulosa cells were also subjected to flow cytometry analysis by incubating with mouse anti-ovine MHCII antibody (1:100 AbD Serotec; Kidlington, UK; bovine MHCII cross reactive). Cells were incubated for 1 h on ice in PBS/0.2% BSA (Sigma-Aldrich), washed three times in fresh PBS/0.2% BSA, incubated 30 min with goat anti-mouse Alex-488 (1:500 Invitrogen), washed in PBS/0.2% BSA three times, and 10000 events analysed using FACSAria (BD; San Jose; CA). The proportion of MHCII positive cells was determined by plotting against the unstained population.
Granulosa cell PAMP challenge
Following an initial establishment period of 48 h, cultured granulosa cell supernatants were replaced with fresh medium containing the following PAMPs: ultrapure LPS (ligand for TLR4), lipoteichoic acid (LTA; ligand for TLR2), peptidoglycan (PGN; ligand for TLR2) or Pam3CSK4 (PAM; synthetic ligand for TLR1 and 2) at 10-fold increasing doses between 100 pg/ml and 10 μg/ml (all Invivogen, San Diego, CA). Cell-free supernatants were collected 24 or 48 h post-treatment and stored at −20°C for analysis by ELISA. Each experiment was repeated on 4-6 separate occasions.
To determine time-dependent activation of granulosa cells in response to LPS, cells were treated with 1 μg/ml of LPS for 0, 30, 60, 90, 180 min. At the end of each experiment, the cells were collected and total mRNA or protein was isolated (see below). Each experiment was repeated on 4 separate occasions.
Oocyte IVM
COCs were pooled and randomly assigned to undergo IVM in different conditions for 24 h. Meiotic evaluation was performed on 3 independent occasions, IL-6 accumulation on 4 occasions and COC expansion on 8 occasions. Maturation medium (Medium 199, 0.25 mM pyruvate, 10% FCS, 1 μg/ml estradiol, 1% ITS, 5 U penicillin, 50 μg/ml streptomycin and 2 mM L-glutamine) was supplemented with gonadotropins using 2.5 μg/ml FSH and/or 10 μg/ml LH (26, 27), (both A. F. Parlow, National Hormone & Peptide Program, Torrance, CA) and LPS at 1 or 10 μg/ml. The COCs were cultured in 1 ml of defined medium in round bottomed organ culture dishes in groups of 10-15 per experiment. Following IVM, COC expansion was recorded, cell-free supernatants collected and COCs partially denuded in 10 IU hyaluronidases (Irvine Scientific, Wicklow, Ireland) ready for meiotic evaluation by confocal microscopy.
Tissue fixation, immuno-fluorescence and confocal microscopy
Cultured granulosa cells or COCs were fixed in 2% paraformaldehyde for 10 min at 37°C followed by microtubule stabilising buffer (100 mM PIPES, 5 mM MgCl2, 2.5 mM EGTA, 2% formaldehyde, 0.1% Triton-X-100, 1 mM taxol and 10 U/ml aprotinin; Sigma-Aldrich) for 45 min at 37°C and stored in wash solution, as described previously (28, 29). Samples were incubated overnight in the presence of an antibody cocktail of mouse anti-α-tubulin and mouse anti-β-tubulin to assess meiotic progression (1:100; Sigma-Aldrich) or ovine anti-MHCII to evaluate presence of immune cells (1:50; AbD Serotec) at 4°C. Samples were washed three times in wash solution for a total of 30 min and antibody detection was performed using goat anti-mouse-Alexa-488 secondary antibody (1:800, Molecular Probes, Carlsbad CA), in combination with Phalloidin-Alexa-555 (1:100, Molecular Probes) for the detection of F-actin, and 1 μg/ml of Hoechst 33342 (Molecular Probes) at 37°C with gentle agitation. Samples were washed three times in wash solution for a total of 30 min before mounting in 50% glycerol/PBS using wax cushions to avoid compression of samples (28, 29). Ovary or spleen were fixed in 4% paraformaldehyde overnight, wax embedded and subjected to immunohistochemistry to identify presence of immune cells by MHCII immunoreactivity.
Samples were analysed on a Zeiss LSM 710 confocal microscope using a 40x Plan-Apochromat objective (na=1.3), KrArg (405,488 nm) and HeNe (543nm) lasers to collect three channel z-stacks through the entire spindle of each oocyte using Zen software (Zeiss, Jena, Germany). Oocytes were categorized by evaluating chromatin condensation, cortical actin arrangement, spindle bipolarity and presence of a polar body; only oocytes with a polar body and bipolar spindle with condensed chromatids were considered as normal MII oocytes (29). Oocytes were evaluated by an experienced observer without reference to treatment group.
RNA isolation and real time RT-PCR
Total RNA was isolated from cell samples after two washes in PBS. Samples were resuspended in RLT buffer before being passed through an RNAse-free 20 G needle 10 times to disrupt cells. Total mRNA extraction was performed using the RNA Easy Mini-kit (QIAGEN, Crawley, UK), according to the manufacturer’s instructions. Total mRNA was measured using the NanoDrop spectrophotometer and 2 μg of mRNA was subjected to reverse transcription using the QuantiTect Reverse Transcription Kit (QIAGEN) according to the manufacturer’s instruction. Primers were designed using the NCBI database and initial specificity verified by BLAST to assure no cross-reactivity with other loci (Supplemental Table 1). Real time PCR was performed in 25 μl reactions containing 1 μM of each forward and reverse primer (Sigma Genosys). An iQ5 light cycler (Biorad, Hemel Hempstead, UK) was employed to perform quantitative PCR. The starting quantity of mRNA from each sample was determined using standard curves generated from reference RNA with Quantifast SYBR green (Qiagen) and expression levels of genes of interest were then normalized to the reference gene ACTB after verification of stable expression.(30) To examine cell purity, PCR products for MHCII and AMH were electrophoresed on a 2% agarose gel containing ethidium bromide and visualized under UV illumination.
Protein isolation and Western blotting
Total protein was isolated from cells after two washes in PBS. A total of 10 μg of protein was electrophoresed on a 10% SDS-PAGE gel. Protein was transferred onto PVDF membranes and blocked overnight in 5% BSA in TBS/T at 4°C. Protein blots were probed for diphosphorylated-ERK1/2 (M8159; Sigma-Aldrich) and phosphorylated-p38 (Thr180/Tyr182; Acris Antibodies, Herford, Germany). Membranes were incubated for 2 h at room temperature with the appropriate primary antibody diluted 1:1000 in block solution. After three 15 min washes in TBS/T, membranes were incubated with an appropriate secondary antibody conjugated to horseradish peroxidise diluted 1:1000 in block solution for 2 h (Cell Signaling, Danvers, MA). After three washes, protein reactivity was visualised using enhanced chemiluminescence (Western C, Bio-Rad). Protein loading was normalized to tubulin immunoreactivity (Invitrogen) performed on the same blot.
siRNA
Inhibition of TLR4 mRNA translation was performed using siRNA targeted to TLR4 mRNA (Supplemental Table 1; Thermo Scientific, UK). Cells were incubated with Lipofectamine-RNAiMAX (Invitrogen) and 10 pmol TLR4-siRNA or scramble-siRNA (Thermo Scientific) in the absence of antibiotics for a period of 24 h. Following washing in PBS, cells were challenged with 1 μg/ml LPS for an additional 24 h. Cell-free supernatant and total mRNA were collected for analysis. Experiments were performed on 4 separate occasions.
IκBα-EGFP and p65-dsRed cell transfection for time-lapse confocal microscopy
The transcription factor p65 remains inactive when sequestered by IκBα in the cytoplasm, but upon IκBα ubiquitination and degradation, p65 is free to translocate to the nucleus (31, 32). To determine IκBα degradation and p65 translocation, granulosa cells were cultured on four chamber glass bottom dishes at 104 cells/ml for 24 h under normal culture conditions and then transfected with Lipofectamine 2000 (Invitrogen) and a plasmid containing IκBα-EGFP and a p65-dsRed plasmid, both under control of the hCMV-IE promoter (gift from Dr Violaine Sée, University of Liverpool) (31, 32). Transfection of plasmids was initially performed in complete granulosa cell medium in the absence of antibiotics. Following 6 h of culture, cells were washed in PBS and cultured for a further 18 h in serum free granulosa cell medium to facilitate p65 localization to the cytoplasm before imaging. All microscopy was carried out in complete granulosa cell culture medium containing serum, and cells were placed in control medium or media containing 1 μg/ml LPS, or 20 ng/ml TNFα as a positive control. Cells were visualized using multiplexed, time lapse confocal microscopy with an automated stage in a live cell chamber (Zeiss 710 LSM) maintained at 37°C with 5% CO2 in air allowing visualization of control and treated cells contemporaneously. Experiments were carried out on 3 separate occasions with a minimum of 5 cells visualized per treatment for each experiment.
ELISA
IL-6 and IL-8 were measured in cell-free supernatants using commercially available ELISAs (Thermo Scientific and R&D, respectively) according to the manufacturer’s instructions. While the IL-6 ELISA is bovine specific, the IL-8 ELISA is cross-reactive for bovine IL-8 (33).
Statistics
SPSS version 13.0 was used for statistical analysis. Data are presented as the mean + standard error of the mean (SEM). Real time RT-PCR and gonadotropin experiments were analysed using a non-parametric Mann-Whitney U test. The PAMP challenge experiments were analysed using ANOVA followed by Dunnett’s pair-wise post-hoc test. The IL-6 data for COCs was first log transformed to normalise data distribution before ANOVA followed by Dunnett’s pair-wise post-hoc test. Meiotic failure of oocytes was compared using the χ2 test. P < 0.05 was assumed to be statistically significant.
RESULTS
Granulosa cells are free of immune cells
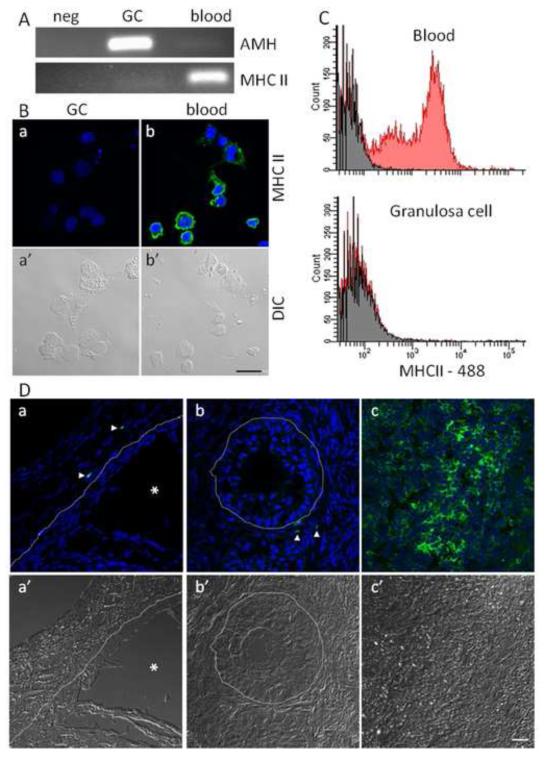
Contamination of cultured granulosa cells with immune cells was assessed to ensure inflammatory responses were due to non-immune cells. Previous work showed granulosa cell cultures do not express the CD45 immune cell marker (2). In the present study, the MHCII marker of antigen presenting cells was not expressed by granulosa cell cultures (Fig 1A). Also, cultured granulosa and blood cells had 0% and 71.4% MHCII positive cells, respectively (Fig 1B). Flow cytometry analysis of freshly isolated granulosa and blood cells had 0% and 44% MHCII positive cells, respectively (Fig 1C). Furthermore, MHCII immunoreactive cells were absent within the basement membrane of intact 4-8mm follicles (Fig 1D).
Figure 1. Granulosa cell cultures are free of professional cells.
Cultured granulosa cells were assessed for contamination with professional immune cells. (A) PCR amplification of MHCII and AMH in cultured granulosa and blood cells. (B) Confocal micrographs of cultured granulosa (a) and blood (b) cells showing MHCII immunoreactivity (green), DNA (blue) and DIC (a’, b’). (C) flow cytometry histograms of freshly isolated blood and granulosa cells showing MHC reactivity in red and unstained cells in grey. (D) Confocal micrographs representing a cross section of a 4-8 mm follicle (a), a <4 mm follicle (b) and spleen (c) showing MHCII immunoreactivity (green), DNA (blue) and DIC (a’, b’, c’). Arrow heads indicate MHCII positive cells, the asterix represents the antral cavity and the dashed line represents the basement membrane extrapolated from the DIC image. Scale bar represents 20 μm.
Granulosa cells produce an inflammatory response to PAMPs
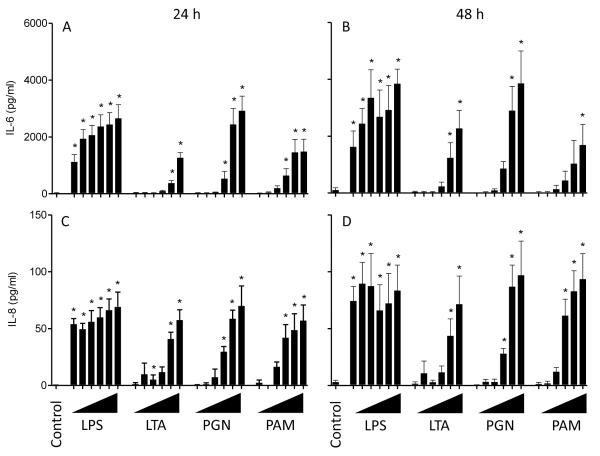
Granulosa cells were challenged with a range of bacterial PAMPs to determine their ability to signal through TLRs and initiate an inflammatory response, as determined by the accumulation of the pro-inflammatory cytokine IL-6 and the chemokine IL-8. Initially, granulosa cells were cultured in either serum-free medium (25) or medium containing 10% FCS. Granulosa cells had little or no response to PAMPs in serum-free conditions (Supplemental Fig. 1). All further cultures were performed with serum and granulosa cells incubated for 24 h in the presence of LPS (TLR4 ligand), LTA (TLR2 ligand), PGN (TLR2 ligand) or PAM (TLR1 and 2 synthetic ligand) accumulated IL-6 and IL-8 in supernatants in a dose-dependent manner (Fig. 2A, C. P < 0.05). The accumulation of IL-6 and IL-8 was further increased following 48 h of culture with each PAMP compared to 24 h (Fig. 2B, D; P < 0.05). The remaining experiments used 1 μg/ml LPS because granulosa cells had the greatest sensitivity to LPS (Fig. 2); the follicular fluid of cattle contains LPS (2); and, similar concentrations of LPS are used to study immune cell activation (20).
Figure 2. Granulosa cells produced IL-6 and IL-8 in response to LPS, LTA, PGN and PAM in a dose-dependent manner.
Accumulation of IL-6 (A, B) and IL-8 (C, D) in the supernatants of granulosa cells was measured by ELISA following 24 h (A, C) or 48 h (B, D) in culture with the PAMPs ultrapure lipopolysaccharide (LPS), lipoteichoic acid (LTA), peptidoglycan (PGN) or Pam3CSK4 (PAM). Granulosa cells were treated with 10-fold increasing doses of each PAMP from 100 pg/ml up to 10 μg/ml of each PAMP (ranging left to right and represented by triangles). Data are presented as mean + SEM from 4 independent experiments. * P < 0.05 compared to untreated controls; analysis by ANOVA followed by Dunnett’s pair-wise post-hoc tests.
Granulosa cells respond acutely to LPS
To determine the acute response of granulosa cells to LPS we used western blot, real time RT-PCR and live cell imaging to evaluate initiation of the signalling cascade, IL6 and IL8 mRNA expression, and p65-NFκB nuclear translocation, respectively.
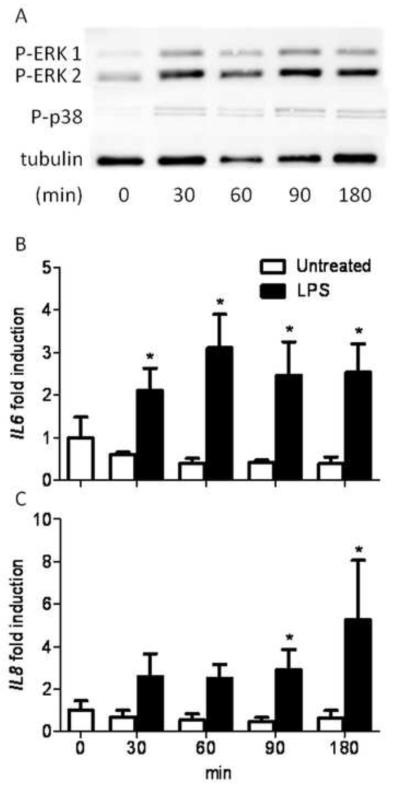
Granulosa cells before LPS exposure showed little or no initial phosphorylation of p38 or ERK 1/2, but following a 30 min treatment with 1 μg/ml of LPS there was phosphorylation of both ERK 1/2 and p38, which was maintained up to 180 min (Fig. 3A).
Figure 3. LPS induced phosphorylation of ERK and p38, and IL6 and IL8 gene expression in granulosa cells in a time-dependent manner.
(A) Granulosa cells were treated with 1 μg/ml of ultrapure LPS and cultured for 30, 60, 90 or 180 min before Western blot analysis. Bands are shown corresponding to di-phosphorylated ERK 1/2, phosphorylated p38 (Thr180/Tyr182) and tubulin as a loading control. Image is representative of 3 independent experiments. Granulosa cells were cultured in control medium (□) or medium containing 1 μg/ml of ultrapure LPS (■) for 30, 60, 90 or 180 min and the IL6 (B) and IL8 (C) gene expression was measured using real time RT-PCR. Data are represented as mean + SEM fold induction compared with control cells at time 0 from 4 independent experiments. * P < 0.05 compared to untreated control within time points; analysis by non-parametric Mann-Whitney U test.
Granulosa cells were challenged with 1 μg/ml LPS for 0, 30, 60, 90 or 180 min to determine IL6 and IL8 mRNA expression. The IL6 mRNA was increased by 2.1-fold in cells following only 30 min treatment compared to untreated controls, and remained 2.5-fold elevated at 180 min (Fig. 3B; P < 0.05); IL8 mRNA was elevated 2.6-fold after 30 min compared to untreated controls and remained elevated 5.3-fold at 180 min (Fig. 3C; P < 0.05).
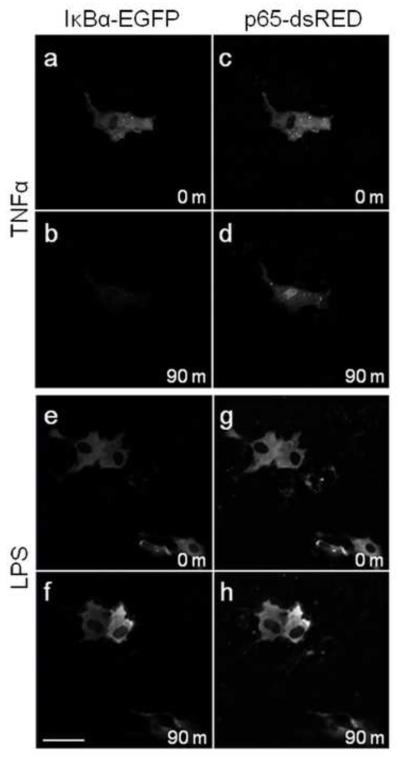
As nuclear translocation of NFκB is part of the TLR signalling pathway (12, 13), cells transfected with IκBα-EGFP and p65-dsRed were monitored for 3 h using live cell confocal microscopy (32). Treatment of cells with 20 ng/ml of TNFα as a positive control showed both degradation of IκBα-EGFP (Fig. 4 a, b) and nuclear translocation of p65-dsRed (Fig. 4 c, d) following 90 min in approximately 20% of analyzed cells. Untreated and LPS treated cells showed no breakdown of IκBα-EGFP (Fig. 4 e, f) or nuclear translocation of p65-dsRed (Fig. 4 g, h) over 90 min, and there was no change when visualized for a further 90 min.
Figure 4. Nuclear translocation of p65 or IκBα degradation in granulosa cells was induced by TNFα but not LPS.
Granulosa cells were cultured in glass bottom petri dishes and transfected with IκBα-EGFP (a-b, e-f) and p65-dsRed (c-d, e-h). Live cell confocal microscopy under culture conditions was performed to track degradation of IκBα-EGFP and nuclear translocation of p65-dsRed. Cells were treated with 20 ng/ml TNFα (a-d) as a positive control, or 1 μg/ml ultrapure LPS (e-h). Scale bar represents 50μm.
LPS induced inflammatory response of granulosa cells is attenuated by siRNA-TLR4
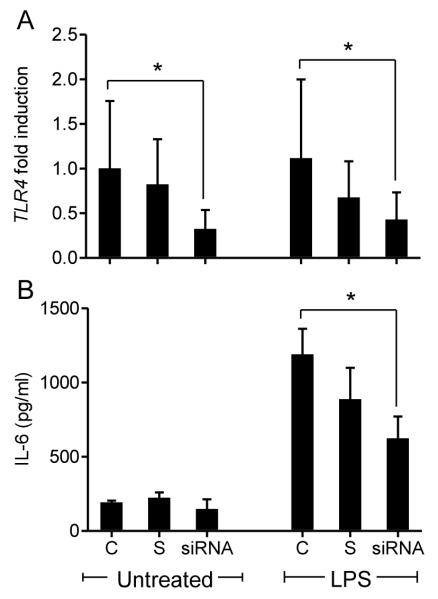
The recognition of LPS by cells of the immune system is mediated through TLR4 (19, 20), so TLR4 targeted siRNA was used to determine if LPS induced inflammatory responses by bovine granulosa cells are also dependent on TLR4. The siRNA reduced TLR4 expression in granulosa cells either untreated or treated with 1 μg/ml LPS (Fig. 5A; P < 0.05). Furthermore, siRNA targeting of TLR4 reduced the accumulation of IL-6 in response to LPS (Fig. 5B; P < 0.05)
Figure 5. siRNA targeting of TLR4 in granulosa cells reduced TLR4 gene expression and the accumulation of IL-6 in response to LPS.
Granulosa cells were cultured for 24 h with lipofectamine (control, C), scramble-siRNA (S) or a specific TLR4-targeted siRNA (siRNA) prior to treatment with 1 μg/ml of ultrapure LPS for an additional 24 h. (A) Real time RT-PCR was used to evaluate the knock down of TLR4 mRNA with expression presented as fold-change relative to untreated controls. (B) IL-6 accumulation was measured in cell-free supernatants by ELSIA following 24 h treatment with 1 μg/ml of ultrapure LPS. Data are presented as mean + SEM from 4 independent experiments. * P < 0.05 compared to controls within treatment group. Real time RT-PCR data were analyzed using a non-parametric Mann-Whitney U test, ELISA data were analysed using ANOVA followed by a Bonferonni post-hoc test.
Exogenous gonadotropins alter innate immune responses to LPS
Granulosa cells from the growing pool of follicles (4-8mm) are FSH responsive and LH receptor negative (24). Previously, Tlr4, Cd14 and Myd88 mRNA expression was shown to increase in mouse COCs due to FSH exposure in vitro (14). Here, exogenous recombinant bovine-FSH was used to assess its role in mediating the inflammatory response in granulosa cells. To determine if FSH per se had an effect on innate immunity, as in lower organisms (34), we investigated whether FSH alone induced changes in phosphorylation of p38 and ERK 1/2, IL6 and IL8 mRNA, and accumulation of IL-6 and IL-8. In the absence of LPS, FSH alone did not increase phosphorylation of p38 or ERK 1/2 (data not shown), or IL6 or IL8 mRNA after 180 min (71% and 21% of control, respectively). Additionally, the accumulation of IL-6 or IL-8 was unchanged when treated with FSH alone (1.0- and 1.8-fold, respectively).
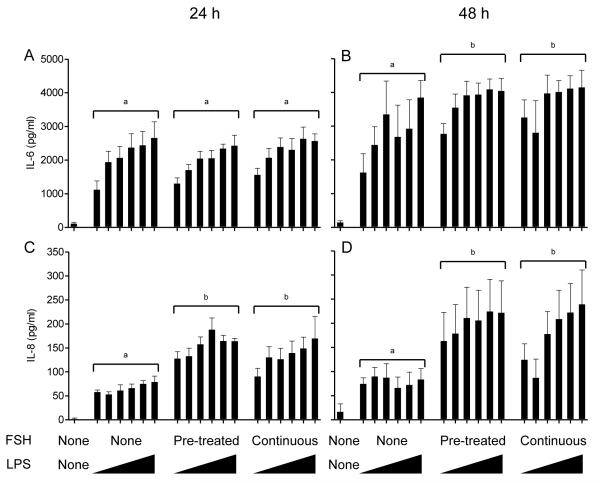
To explore interactions between gonadotropins and LPS, granulosa cells were either pre-treated with FSH for 6 h prior to LPS exposure or treated with FSH for the duration of the LPS exposure. In the absence of gonadotropins, as expected there was a dose-dependent accumulation of IL-6 and IL-8 following treatment of granulosa cells with LPS for 24 h (Fig. 6A, C; P < 0.05) or 48 h (Fig. 6B, D; P < 0.05). Pre-treatment or continuous treatment of granulosa cells with FSH had no further effect on the IL-6 accumulation following 24 h of LPS exposure (Fig. 6A). However, after 48 h exposure to LPS, either pre-treatment or continuous treatment with FSH increased accumulation of IL-6 compared to cells with no FSH treatment (Fig. 6B; P < 0.05). The accumulation of IL-8 after 24 or 48 h exposure to LPS was significantly increased when granulosa cells were pre-treated or continuously treated with FSH compared to those with no FSH treatment (Fig. 6C, D; P < 0.05). There was no significant interaction between FSH treatment and LPS dose in each experiment.
Figure 6. Exogenous FSH increases accumulation of IL-6 and IL-8 in response to LPS by granulosa cells.
Accumulation of IL-6 (A, B) and IL-8 (C, D) measured by ELISA following 24 h (A, C) or 48 h (B, D) in culture after challenge of granulosa cells with ultrapure LPS. Granulosa cells were either pre-incubated with 2.5 μg/ml FSH for 6 h prior to LPS exposure or treated with 2.5 μg/ml FSH for the duration of the LPS exposure. Granulosa cells were challenged with 10-fold increasing doses of ultrapure LPS between 100 pg/ml and 10 μg/ml (ranging left to right and represented by triangles). Data are presented as mean + SEM from 4 independent experiments. Different superscripts represent statistically significant differences between FSH treatment groups (P < 0.05); analysis by ANOVA followed by Bonferonni post-hoc test.
Cumulus-oocyte complex IVM is perturbed by LPS exposure
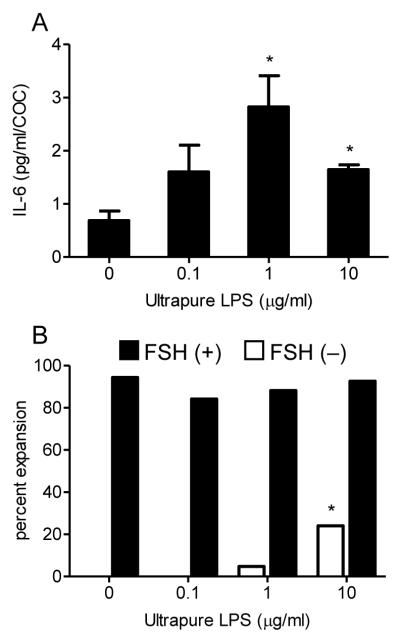
As LPS stimulated an inflammatory response in granulosa cells, and in the mouse IL-6 is critical for COC expansion and can bypass amphiregulin-dependent expansion of the COC (35), we posited that this could change the follicular environment for developing oocytes. So, we performed IVM of COCs using defined medium with the addition of exogenous LPS for 24 h to determine perturbations in IL-6 production (216 COC used in 4 independent experiments), COC expansion (422 COCs used across 8 independent experiments) and meiotic maturation (290 COCs used across 3 independent experiments). Treatment of COCs with 1 or 10 μg/ml of LPS induced 76% and 58% increase in IL-6 accumulation compared to untreated controls, respectively (Fig. 7A; P < 0.05). Examination of the expansion of COCs revealed that in the absence of FSH, 10 μg/ml of LPS significantly increased the rates of cumulus expansion compared to untreated controls (0.0% vs 24.0%, P < 0.05; Fig. 7B).
Figure 7. LPS induces IL-6 accumulation and expansion in in vitro matured COCs.
(A) IL-6 accumulation in cell-free supernatants was measured from groups of 10 – 20 COCs treated with 0, 0.1, 1 or 10 μg/ml LPS in the presence of 2.5 μg/ml FSH. IL-6 concentrations are presented as mean pg/ml/COC + SEM from a total of 216 across 4 independent experiments. * P < 0.05 compared to untreated controls; analysis by ANOVA followed by Dunnett’s post-hoc test. (B) COC expansion rates were recorded from groups of 10 – 20 COCs,, following treatment with 0, 0.1, 1 or 10 μg/ml LPS in the absence (□) or presence (■) of 2.5 μg/ml FSH. COC expansion data are presented as percentages from a total of 422 COCs across 8 independent experiment. * P < 0.05 compared to untreated COCs within FSH treatment group using a χ2 test.
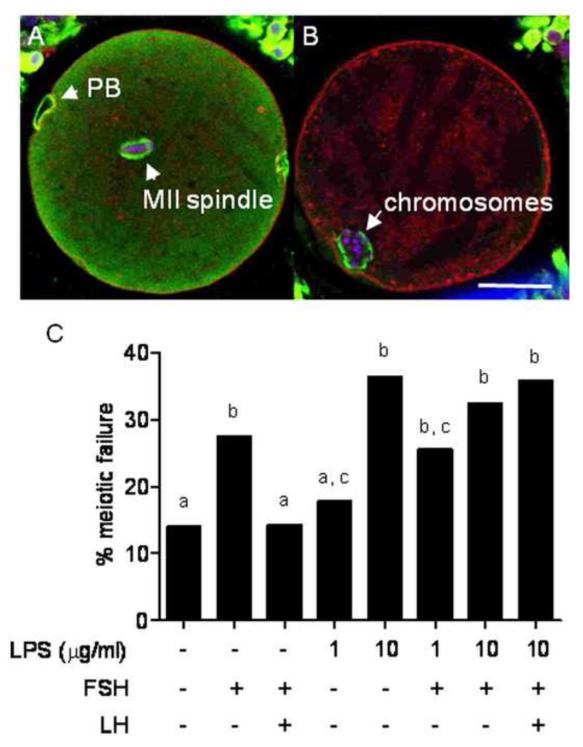
Following 24 h IVM, oocytes were subjected to confocal microscopy to determine their meiotic status. Morphologically normal oocytes containing bipolar spindles and aligned chromosomes along the meta-phase plate were deemed normal MII oocytes (Fig. 8A). Oocytes that failed to reach the M-phase of meiosis II, or those with perturbed meiotic structures (aberrant spindles, chromosomal ejection or parthenogenic activation) were deemed to have failed meiosis (Fig. 8B). In all, 290 COCs were assessed in 8 treatment groups. Control IVM consisting of basal medium with no exogenous gonadotropins resulted in a meiotic failure rate of 14.0%, with the addition of 10 μg/ml of LPS the meiotic failure rate significantly increased to 34.4% (P < 0.05). IVM in the presence of both FSH and LH resulted in a 14.3% meiotic failure rate, but the addition of 10 μg/ml of LPS again increased the meiotic failure rate to 35.7% (Fig. 8C; P < 0.05).
Figure 8. LPS induces meiotic failure in in vitro matured COCs.
Meiotic progression was assessed in 290 COCs by confocal microscopy. Oocytes at the M-phase of meiosis with a normal bipolar spindle and aligned chromosomes were deemed to have completed meiosis (A). Any oocyte not to reach the M-phase of meiosis II or those which had significantly perturbed meiotic structures such as aberrant spindles, chromosomal ejection, parthenogenic activation or germinal vesicle breakdown failure were deemed to have failed meiosis (B). Representative confocal micrographs of COCs show DNA (blue), tubulin (green) and F-actin (red). (C) Meiotic failure rates of COCs cultured in the defined IVM media containing LPS, FSH and or LH (as indicated) for 24 h are presented as percentages. Different superscripts represent statistical significance following a χ2 test; P < 0.05. PB, polar body; scale bar represents 50 μm.
DISCUSSION
Reproductive tract infection in dairy cattle is common after parturition and leads to endocrine dysfunction and ultimately infertility (5, 6). The Gram-negative bacteria, E. coli is the most prevalent pathogen detected in the uterus post-partum (2, 9). Although infection of the ovary itself is rare, animals with post-partum uterine infection have concentrated LPS in the follicular fluid of growing follicles (2). Previously it has been demonstrated that in the absence of LPS, TLR4 may play a role in ovulation in the mouse by using endogenous ligands (21). In contrast, here we explore the role of granulosa cells in a pathological context and hypothesize that LPS from Gram-negative bacteria stimulates an inflammatory response by ovarian granulosa cells that is mediated by TLR4. Absence of immune cells within the ovarian follicle or granulosa cell cultures in the present study further highlight potential roles for granulosa cells in innate immunity. In the present study, the bacterial PAMPs LPS, LTA, PGN and PAM initiated an inflammatory response by granulosa cells in a dose-dependent manner with accumulation of the inflammatory cytokine IL-6 and the chemokine IL-8. Using LPS as the prototypical PAMP, granulosa cells initiated phosphorylation of the downstream mediators ERK and p38 and acute up-regulation of IL6 and IL8 mRNA. Accumulation of IL-6 and IL-8 was evident with low doses of LPS, akin to the responsiveness of immune cells (36). This response to LPS was reduced by siRNA targeting TLR4 of granulosa cells. Interestingly, the addition of FSH into the culture system increased granulosa cell sensitivity and responsiveness to LPS. Furthermore COCs accumulated IL-6 in response to LPS and underwent aberrant expansion in the absence of exogenous gonadotropins. Finally, IVM of oocytes was perturbed by the presence of exogenous LPS, resulting in their failure to complete meiosis. Taken together these data support the concept that granulosa cells have roles in innate immunity and provide mechanisms for perturbation of fertility by bacterial infections.
Bovine granulosa cells express the molecular machinery (TLR4, CD14 and MD2) important for initiating the innate immune response to Gram-negative pathogens (2). LPS accumulates in follicular fluid of animals with metritis, and microbial colonization of human follicular fluid occurs in IVF patients (11). Taken together with the absence of immune cells from ovarian follicles (37) these data present the intriguing possibility that granulosa cells could act as immune sensors. Here we demonstrated granulosa cell functional responsiveness to PAMPs for TLR1, TLR2 and TLR4. Functional TLR1 and TLR2 are relevant because Gram-positive bacteria, which their ligands, commonly causes metritis (1).
The pro-inflammatory cytokine IL-6 is a potent regulator of early inflammation and is commonly increased in response to LPS (36). However, IL-6 also has a critical role in the ovary. Temporal regulation of IL-6 is important in coordinating meiotic maturation, COC expansion, ovulation and corpus luteum formation (35, 38, 39). The chemokine IL-8 is critical for the recruitment of leukocytes into the site of infection and similar to IL-6, is up regulated in response to LPS via the TLR4 pathway (36). The role of IL-8 in the ovary is less well characterised, with a suggested role in the recruitment of leukocytes following ovulation, which then aid in the formation and function of the corpus luteum (40, 41). We surmise that in granulosa cells, altered expression of IL-8 in response to LPS is involved in aberrant inflammation in the ovary, while altered IL-6 expression may have a direct impact on the oocyte, negatively effecting meiotic maturation.
Binding of LPS to TLR4 on immune cells activates the MAPK and NFκB pathways to increase expression of inflammatory mediators (13, 42). In the present study initial activation of both p38 and ERK were visualised in response to LPS after 30 min of treatment. Also, silencing of TLR4 by specific siRNA targeting reduced LPS initiated inflammation, providing further evidence that TLR4 signalling is important in a pathophysiological response to LPS in bovine granulosa cells. It is also interesting to note that bovine granulosa cell mRNA expression of TLR4 was not increased in response to LPS (43).
Gonadotropins are an essential element in successful development and function of the ovarian follicle (16, 17). Granulosa cells used in the present studies were selected from the 4-8 mm pool of growing follicles, which provided a homogenous population of granulosa cells with limited apoptotic cells, similar to the environment of the developing oocyte. These granulosa cells are known to be FSH receptor positive and LH receptor negative (24). In mice, the treatment of COCs with FSH increased mRNA of Tlr4, Myd88 and Cd14, but not in granulosa cells (14). In Caenorhabditis elegans the FSH receptor plays an integral role in the innate immune response to bacterial PAMPs by eliciting functional inflammatory responses to pathogens (34). To examine the impact of FSH in bovine granulosa cell immunity in here, cells were treated with FSH before or during challenge with LPS.
The addition of FSH to granulosa cells increased the accumulation of IL-6 and IL-8 in response to LPS and in addition increased their sensitivity to LPS. In the present study, in the absence of LPS the addition of FSH alone to granulosa cell cultures did not increase ERK 1/2 phosphorylation, IL6 or IL8 mRNA production, or IL-6 or IL-8 accumulation in supernatants. This varies from studies in the mouse where TLR4 and FSHR both act in part via ERK 1/2 signaling pathways (44), increasing IL-6 production in response to FSH (14). Alternatively, in bovine granulosa cells FSH may signal via the AKT or PI3K pathways to initiate endocrine changes important in their function (45, 46).
The process of COC expansion is critical for ovulation and fertilisation and is indicative of oocyte quality (47). Expansion of the COC requires intrinsic signaling molecules expressed in a temporal manner in response to the LH surge, and their downstream products such as IL-6 (35). We investigated the ability of LPS to induce COC expansion and IL-6 production in the absence of gonadotropins. Although LPS induced IL-6 accumulation during IVM of bovine COCs, high doses of LPS were required to induce gonadotropin independent expansion, above the levels detected in follicular fluid of infected cattle. We suggest that in the cow, LPS may induce the increased expression of cumulus derived factors needed for COC expansion, including IL-6 (35).
Cattle suffering uterine or mammary infection have delayed conception, suggesting failure to conceive as a result of uterine and/or ovarian dysfunction (6, 48). Meiotic maturation is an essential process in readying both the nuclear and cytoplasmic compartments of the oocyte for ovulation, fertilisation and the first cleavage events of the new zygote. In cattle, meiotic maturation from the germinal vesicle stage to MII takes approximately 60 days, while in vitro the process can be accelerated with the use of exogenous gonadotropins and completed in 24 h. Here, maturation to the MII stage of meiosis was perturbed by the presence of LPS with no protective effect of exogenous gonadotropins. Production of IL-6 within the ovarian follicle seems to be temporally modulated with elevated IL-6 in follicles containing mature oocytes compared to those containing immature oocytes (38). In the rat, LPS administration leads to increased follicular atresia and granulosa cell apoptosis (49), processes closely linked to increased IL-8 expression (50). While oocytes collected from dogs suffering uterine infection have shown very low IVM rates (51). In addition, women suffering endometriosis have poor oocyte quality and elevated IL-6 in the circulation (52) and increased IL-6 production by their granulosa cells (53). It would appear while IL-6 expression within the ovary is important for oocyte development, appropriate temporal expression is critical. So, altered oocyte competence due to LPS exposure, increased by FSH, may be a protective mechanism developed by the ovary to prevent the maturation and fertilisation of sub-optimal quality oocytes, while elevated IL-8 may increase follicle atresia.
As bacterial infection of the ovary itself is a rare event, inhibition of key downstream regulators of the TLR4 signalling pathway may provide suitable targets for blocking the aberrant inflammatory response to LPS within follicles. Here, p65 nuclear translocation and IκB degradation required for classical NFκB signalling (54) was absent. Surprisingly the kinetics of p65 nuclear translocation and IκB degradation in TNFα treated granulosa cells was reduced by an order of magnitude compared to immune cells (32). It is important to note the number of components within the NFκB pathway, some of which can increase NFκB responsive genes in the absence of p65 and may explain the ability of granulosa cells to initiate LPS-induced inflammatory responses in granulosa cells without p65 nuclear translocation (55).
In conclusion, bovine granulosa cells initiated an inflammatory response to PAMPs and the response to LPS was via the TLR4 pathway. Intriguingly there may be interactions between endocrine and immune pathways as the granulosa cells were more sensitive to LPS in the presence of FSH. The impact of LPS is not limited to granulosa cells as COCs underwent aberrant cumulus expansion and increased meiotic failure in vitro. The present studies support the concept that bovine granulosa cells have roles in innate immunity that are linked to fertility.
Supplementary Material
ACKNOWLEGMENTS
The authors wish to thank Dr James Cronin and Professors Venkateswarlu Kanamarlapudi and David Albertini for invaluable advice.
This work was supported in part by BBSRC (Grant N° F005121/1).
Footnotes
Disclosure Summary: J.J.B. and I.M.S have nothing to disclose.
REFERENCES
- 1.Sheldon IM, Noakes DE, Rycroft AN, Pfeiffer DU, Dobson H. Influence of uterine bacterial contamination after parturition on ovarian dominant follicle selection and follicle growth and function in cattle. Reproduction. 2002;123:837–845. [PubMed] [Google Scholar]
- 2.Herath S, Williams EJ, Lilly ST, Gilbert RO, Dobson H, Bryant CE, Sheldon IM. Ovarian follicular cells have innate immune capabilities that modulate their endocrine function. Reproduction. 2007;134:683–693. doi: 10.1530/REP-07-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams EJ, Sibley K, Miller AN, Lane EA, Fishwick J, Nash DM, Herath S, England GC, Dobson H, Sheldon IM. The effect of Escherichia coli lipopolysaccharide and tumour necrosis factor alpha on ovarian function. Am J Reprod Immunol. 2008;60:462–473. doi: 10.1111/j.1600-0897.2008.00645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lavon Y, Leitner G, Klipper E, Moallem U, Meidan R, Wolfenson D. Subclinical, chronic intramammary infection lowers steroid concentrations and gene expression in bovine preovulatory follicles. Domest Anim Endocrinol. 2011;40:98–109. doi: 10.1016/j.domaniend.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Dohmen MJ, Joop K, Sturk A, Bols PE, Lohuis JA. Relationship between intrauterine bacterial contamination, endotoxin levels and the development of endometritis in postpartum cows with dystocia or retained placenta. Theriogenology. 2000;54:1019–1032. doi: 10.1016/s0093-691x(00)00410-6. [DOI] [PubMed] [Google Scholar]
- 6.Sheldon IM, Cronin J, Goetze L, Donofrio G, Schuberth HJ. Defining postpartum uterine disease and the mechanisms of infection and immunity in the female reproductive tract in cattle. Biol Reprod. 2009;81:1025–1032. doi: 10.1095/biolreprod.109.077370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lavon Y, Ezra E, Leitner G, Wolfenson D. Association of conception rate with pattern and level of somatic cell count elevation relative to time of insemination in dairy cows. J Dairy Sci. 2011;94:4538–4545. doi: 10.3168/jds.2011-4293. [DOI] [PubMed] [Google Scholar]
- 8.Hertl JA, Grohn YT, Leach JD, Bar D, Bennett GJ, Gonzalez RN, Rauch BJ, Welcome FL, Tauer LW, Schukken YH. Effects of clinical mastitis caused by gram-positive and gram-negative bacteria and other organisms on the probability of conception in New York State Holstein dairy cows. J Dairy Sci. 2010;93:1551–1560. doi: 10.3168/jds.2009-2599. [DOI] [PubMed] [Google Scholar]
- 9.Sheldon IM, Rycroft AN, Dogan B, Craven M, Bromfield JJ, Chandler A, Roberts MH, Price SB, Gilbert RO, Simpson KW. Specific strains of Escherichia coli are pathogenic for the endometrium of cattle and cause pelvic inflammatory disease in cattle and mice. PLoS One. 2010;5:e9192. doi: 10.1371/journal.pone.0009192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross JD. An update on pelvic inflammatory disease. Sex Transm Infect. 2002;78:18–19. doi: 10.1136/sti.78.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pelzer ES, Allan JA, Cunningham K, Mengersen K, Allan JM, Launchbury T, Beagley K, Knox CL. Microbial colonization of follicular fluid: alterations in cytokine expression and adverse assisted reproduction technology outcomes. Hum Reprod. 2011;26:1799–1812. doi: 10.1093/humrep/der108. [DOI] [PubMed] [Google Scholar]
- 12.Beutler B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature. 2004;430:257–263. doi: 10.1038/nature02761. [DOI] [PubMed] [Google Scholar]
- 13.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 14.Shimada M, Hernandez-Gonzalez I, Gonzalez-Robanya I, Richards JS. Induced expression of pattern recognition receptors in cumulus oocyte complexes: novel evidence for innate immune-like functions during ovulation. Mol Endocrinol. 2006;20:3228–3239. doi: 10.1210/me.2006-0194. [DOI] [PubMed] [Google Scholar]
- 15.Spanel-Borowski K, Rahner P, Ricken AM. Immunolocalization of CD18-positive cells in the bovine ovary. J Reprod Fertil. 1997;111:197–205. doi: 10.1530/jrf.0.1110197. [DOI] [PubMed] [Google Scholar]
- 16.Albertini DF, Combelles CM, Benecchi E, Carabatsos MJ. Cellular basis for paracrine regulation of ovarian follicle development. Reproduction. 2001;121:647–653. doi: 10.1530/rep.0.1210647. [DOI] [PubMed] [Google Scholar]
- 17.Matzuk MM, Burns KH, Viveiros MM, Eppig JJ. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science. 2002;296:2178–2180. doi: 10.1126/science.1071965. [DOI] [PubMed] [Google Scholar]
- 18.Eppig JJ. Coordination of nuclear and cytoplasmic oocyte maturation in eutherian mammals. Reprod Fertil Dev. 1996;8:485–489. doi: 10.1071/rd9960485. [DOI] [PubMed] [Google Scholar]
- 19.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 20.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 21.Shimada M, Yanai Y, Okazaki T, Noma N, Kawashima I, Mori T, Richards JS. Hyaluronan fragments generated by sperm-secreted hyaluronidase stimulate cytokine/chemokine production via the TLR2 and TLR4 pathway in cumulus cells of ovulated COCs, which may enhance fertilization. Development. 2008;135:2001–2011. doi: 10.1242/dev.020461. [DOI] [PubMed] [Google Scholar]
- 22.Spanel-Borowski K. Ovulation as danger signaling event of innate immunity. Mol Cell Endocrinol. 2011;333:1–7. doi: 10.1016/j.mce.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Espey LL. Ovulation as an inflammatory reaction--a hypothesis. Biol Reprod. 1980;22:73–106. doi: 10.1095/biolreprod22.1.73. [DOI] [PubMed] [Google Scholar]
- 24.Xu Z, Garverick HA, Smith GW, Smith MF, Hamilton SA, Youngquist RS. Expression of follicle-stimulating hormone and luteinizing hormone receptor messenger ribonucleic acids in bovine follicles during the first follicular wave. Biol Reprod. 1995;53:951–957. doi: 10.1095/biolreprod53.4.951. [DOI] [PubMed] [Google Scholar]
- 25.Gutierrez CG, Campbell BK, Webb R. Development of a long-term bovine granulosa cell culture system: induction and maintenance of estradiol production, response to follicle-stimulating hormone, and morphological characteristics. Biol Reprod. 1997;56:608–616. doi: 10.1095/biolreprod56.3.608. [DOI] [PubMed] [Google Scholar]
- 26.Fukui Y, Ono H. Effects of sera, hormones and granulosa cells added to culture medium for in-vitro maturation, fertilization, cleavage and development of bovine oocytes. J Reprod Fertil. 1989;86:501–506. doi: 10.1530/jrf.0.0860501. [DOI] [PubMed] [Google Scholar]
- 27.Rose TA, Bavister BD. Effect of oocyte maturation medium on in vitro development of in vitro fertilized bovine embryos. Mol Reprod Dev. 1992;31:72–77. doi: 10.1002/mrd.1080310113. [DOI] [PubMed] [Google Scholar]
- 28.Combelles CM, Cekleniak NA, Racowsky C, Albertini DF. Assessment of nuclear and cytoplasmic maturation in in-vitro matured human oocytes. Hum Reprod. 2002;17:1006–1016. doi: 10.1093/humrep/17.4.1006. [DOI] [PubMed] [Google Scholar]
- 29.Bromfield JJ, Coticchio G, Hutt K, Sciajno R, Borini A, Albertini DF. Meiotic spindle dynamics in human oocytes following slow-cooling cryopreservation. Hum Reprod. 2009;24:2114–2123. doi: 10.1093/humrep/dep182. [DOI] [PubMed] [Google Scholar]
- 30.Rico C, Medigue C, Fabre S, Jarrier P, Bontoux M, Clement F, Monniaux D. Regulation of anti-Mullerian hormone production in the cow: a multiscale study at endocrine, ovarian, follicular, and granulosa cell levels. Biol Reprod. 2011;84:560–571. doi: 10.1095/biolreprod.110.088187. [DOI] [PubMed] [Google Scholar]
- 31.Nelson G, Paraoan L, Spiller DG, Wilde GJ, Browne MA, Djali PK, Unitt JF, Sullivan E, Floettmann E, White MR. Multi-parameter analysis of the kinetics of NF-kappaB signalling and transcription in single living cells. J Cell Sci. 2002;115:1137–1148. doi: 10.1242/jcs.115.6.1137. [DOI] [PubMed] [Google Scholar]
- 32.See V, Rajala NK, Spiller DG, White MR. Calcium-dependent regulation of the cell cycle via a novel MAPK--NF-kappaB pathway in Swiss 3T3 cells. J Cell Biol. 2004;166:661–672. doi: 10.1083/jcb.200402136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rinaldi M, Ceciliani F, Lecchi C, Moroni P, Bannerman DD. Differential effects of alpha1-acid glycoprotein on bovine neutrophil respiratory burst activity and IL-8 production. Vet Immunol Immunopathol. 2008;126:199–210. doi: 10.1016/j.vetimm.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Powell JR, Kim DH, Ausubel FM. The G protein-coupled receptor FSHR-1 is required for the Caenorhabditis elegans innate immune response. Proc Natl Acad Sci U S A. 2009;106:2782–2787. doi: 10.1073/pnas.0813048106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Z, de Matos DG, Fan HY, Shimada M, Palmer S, Richards JS. Interleukin-6: an autocrine regulator of the mouse cumulus cell-oocyte complex expansion process. Endocrinology. 2009;150:3360–3368. doi: 10.1210/en.2008-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dentener MA, Bazil V, Von Asmuth EJ, Ceska M, Buurman WA. Involvement of CD14 in lipopolysaccharide-induced tumor necrosis factor-alpha, IL-6 and IL-8 release by human monocytes and alveolar macrophages. J Immunol. 1993;150:2885–2891. [PubMed] [Google Scholar]
- 37.Brannstrom M, Mayrhofer G, Robertson SA. Localization of leukocyte subsets in the rat ovary during the periovulatory period. Biol Reprod. 1993;48:277–286. doi: 10.1095/biolreprod48.2.277. [DOI] [PubMed] [Google Scholar]
- 38.Kawasaki F, Kawano Y, Kosay Hasan Z, Narahara H, Miyakawa I. The clinical role of interleukin-6 and interleukin-6 soluble receptor in human follicular fluids. Clin Exp Med. 2003;3:27–31. doi: 10.1007/s102380300012. [DOI] [PubMed] [Google Scholar]
- 39.Richards JS, Liu Z, Shimada M. Immune-like mechanisms in ovulation. Trends Endocrinol Metab. 2008;19:191–196. doi: 10.1016/j.tem.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 40.Arici A, Oral E, Bukulmez O, Buradagunta S, Engin O, Olive DL. Interleukin-8 expression and modulation in human preovulatory follicles and ovarian cells. Endocrinology. 1996;137:3762–3769. doi: 10.1210/endo.137.9.8756544. [DOI] [PubMed] [Google Scholar]
- 41.Murayama C, Kaji A, Miyauchi K, Matsui M, Miyamoto A, Shimizu T. Effect of VEGF (vascular endothelial growth factor) on expression of IL-8 (interleukin-8), IL-1beta and their receptors in bovine theca cells. Cell Biol Int. 2010;34:531–536. doi: 10.1042/CBI20090498. [DOI] [PubMed] [Google Scholar]
- 42.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 43.Muzio M, Bosisio D, Polentarutti N, D’Amico G, Stoppacciaro A, Mancinelli R, van’t Veer C, Penton-Rol G, Ruco LP, Allavena P, Mantovani A. Differential expression and regulation of toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells. J Immunol. 2000;164:5998–6004. doi: 10.4049/jimmunol.164.11.5998. [DOI] [PubMed] [Google Scholar]
- 44.Wayne CM, Fan HY, Cheng X, Richards JS. Follicle-stimulating hormone induces multiple signaling cascades: evidence that activation of Rous sarcoma oncogene, RAS, and the epidermal growth factor receptor are critical for granulosa cell differentiation. Mol Endocrinol. 2007;21:1940–1957. doi: 10.1210/me.2007-0020. [DOI] [PubMed] [Google Scholar]
- 45.Das S, Maizels ET, DeManno D, St Clair E, Adam SA, Hunzicker-Dunn M. A stimulatory role of cyclic adenosine 3′,5′-monophosphate in follicle-stimulating hormone-activated mitogen-activated protein kinase signaling pathway in rat ovarian granulosa cells. Endocrinology. 1996;137:967–974. doi: 10.1210/endo.137.3.8603610. [DOI] [PubMed] [Google Scholar]
- 46.Mani AM, Fenwick MA, Cheng Z, Sharma MK, Singh D, Wathes DC. IGF1 induces up-regulation of steroidogenic and apoptotic regulatory genes via activation of phosphatidylinositol-dependent kinase/AKT in bovine granulosa cells. Reproduction. 2010;139:139–151. doi: 10.1530/REP-09-0050. [DOI] [PubMed] [Google Scholar]
- 47.Larsen WJ, Chen L, Powers R, Zhang H, Russell PT, Chambers C, Hess K, Flick R. Cumulus expansion initiates physical and developmental autonomy of the oocyte. Zygote. 1996;4:335–341. doi: 10.1017/s096719940000335x. [DOI] [PubMed] [Google Scholar]
- 48.Williams EJ, Fischer DP, Noakes DE, England GC, Rycroft A, Dobson H, Sheldon IM. The relationship between uterine pathogen growth density and ovarian function in the postpartum dairy cow. Theriogenology. 2007;68:549–559. doi: 10.1016/j.theriogenology.2007.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Besnard N, Horne EA, Whitehead SA. Prolactin and lipopolysaccharide treatment increased apoptosis and atresia in rat ovarian follicles. Acta Physiol Scand. 2001;172:17–25. doi: 10.1046/j.1365-201X.2001.00813.x. [DOI] [PubMed] [Google Scholar]
- 50.Polec A, Tanbo T, Fedorcsak P. Cellular interaction regulates interleukin-8 secretion by granulosa-lutein cells and monocytes/macrophages. Am J Reprod Immunol. 2009;61:85–94. doi: 10.1111/j.1600-0897.2008.00668.x. [DOI] [PubMed] [Google Scholar]
- 51.Hishinuma M, Minami S, Okamoto Y, Miyatake K, Sekine J. Recovery, morphological quality, and in vitro maturation of follicular oocytes from bitches with pyometra. Theriogenology. 2004;62:1652–1662. doi: 10.1016/j.theriogenology.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 52.Martinez S, Garrido N, Coperias JL, Pardo F, Desco J, Garcia-Velasco JA, Simon C, Pellicer A. Serum interleukin-6 levels are elevated in women with minimal-mild endometriosis. Hum Reprod. 2007;22:836–842. doi: 10.1093/humrep/del419. [DOI] [PubMed] [Google Scholar]
- 53.Carlberg M, Nejaty J, Froysa B, Guan Y, Soder O, Bergqvist A. Elevated expression of tumour necrosis factor alpha in cultured granulosa cells from women with endometriosis. Hum Reprod. 2000;15:1250–1255. doi: 10.1093/humrep/15.6.1250. [DOI] [PubMed] [Google Scholar]
- 54.Henkel T, Machleidt T, Alkalay I, Kronke M, Ben-Neriah Y, Baeuerle PA. Rapid proteolysis of I kappa B-alpha is necessary for activation of transcription factor NF-kappa B. Nature. 1993;365:182–185. doi: 10.1038/365182a0. [DOI] [PubMed] [Google Scholar]
- 55.Kim JM, Oh YK, Kim YJ, Youn J, Ahn MJ. Escherichia coli up-regulates proinflammatory cytokine expression in granulocyte/macrophage lineages of CD34 stem cells via p50 homodimeric NF-kappaB. Clin Exp Immunol. 2004;137:341–350. doi: 10.1111/j.1365-2249.2004.02542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.