B cell development requires the Zinc-finger protein and ATM substrate ASCIZ to signal through DYNLL1 and Bim.
Abstract
Developing B lymphocytes expressing defective or autoreactive pre-B or B cell receptors (BCRs) are eliminated by programmed cell death, but how the balance between death and survival signals is regulated to prevent immunodeficiency and autoimmunity remains incompletely understood. In this study, we show that absence of the essential ATM (ataxia telangiectasia mutated) substrate Chk2-interacting Zn2+-finger protein (ASCIZ; also known as ATMIN/ZNF822), a protein with dual functions in the DNA damage response and as a transcription factor, leads to progressive cell loss from the pre-B stage onwards and severely diminished splenic B cell numbers in mice. This lymphopenia cannot be suppressed by deletion of p53 or complementation with a prearranged BCR, indicating that it is not caused by impaired DNA damage responses or defective V(D)J recombination. Instead, ASCIZ-deficient B cell precursors contain highly reduced levels of DYNLL1 (dynein light chain 1; LC8), a recently identified transcriptional target of ASCIZ, and normal B cell development can be restored by ectopic Dynll1 expression. Remarkably, the B cell lymphopenia in the absence of ASCIZ can also be fully suppressed by deletion of the proapoptotic DYNLL1 target Bim. Our findings demonstrate a key role for ASCIZ in regulating the survival of developing B cells by activating DYNLL1 expression, which may then modulate Bim-dependent apoptosis.
Developing B cells undergo a series of positive and negative selection steps to allow the generation of a repertoire of peripheral B cells that are able to respond efficiently to foreign antigens but are tolerant to self-antigens (LeBien and Tedder, 2008). One key event during B cell development in the bone marrow is the targeted rearrangement of Ig genes. At the pro-B cell stage, V(D)J recombination of IgH allows production of the pre-BCR, which then sustains proliferation and survival of pre-B cells. V, D, and J coding segments are fused by site-specific recombination, where double-strand breaks generated by RAG1 and RAG2 (Oettinger et al., 1990) are repaired by the NHEJ (nonhomologous end-joining) pathway. Accordingly, RAG-null mice and NHEJ-deficient SCID mice, which have a mutation in DNA-dependent protein kinase, lack mature B lymphocytes (Mombaerts et al., 1992; Shinkai et al., 1992), highlighting the importance of these proteins for early B cell development. At the pre-B cell stage, IgL genes undergo VJ recombination, and upon productive IgL rearrangement, heavy and light chains associate to form the BCR, marking the beginning of the immature B cell stage. Immature and transitional B cells expressing an autoreactive BCR are then eliminated by apoptosis to prevent autoimmunity, a process which depends on the proapoptotic BH3-only protein Bim (Enders et al., 2003). It is widely believed that pre-BCR/BCR signals that are too weak or too strong result in programmed cell death (Strasser, 2005), but how apoptotic signaling thresholds are set during these developmental stages and to what extent Bim regulates cell survival also at the pre-B stage remain unclear.
The initial Ig diversity within the B cell repertoire is refined through class switch recombination and somatic hypermutation, both of which are initiated by activation-induced cytidine deaminase (AID) upon antigen stimulation in splenic or lymph node germinal centers (Muramatsu et al., 2000). During class switch recombination (Stavnezer et al., 2008), AID mutagenesis can lead to the formation of double-strand breaks in switch regions upstream of the IgH constant region exons and recombination-dependent isotype switching. During somatic hypermutation (Peled et al., 2008), processing of AID-dependent base damage in the Ig variable region leads to the formation of abasic sites whose mutagenic repair may improve antibody affinity to the antigen.
The ATM (ataxia telangiectasia mutated) substrate Chk2-interacting Zn2+-finger protein (ASCIZ; also known as ATMIN/ZNF822) was originally identified as a DNA damage response protein (McNees et al., 2005) specifically involved in the BER (base excision repair) pathway, including oxidative stress responses in vivo (Jurado et al., 2010; Kanu et al., 2010). In the chicken DT40 B cell line, in which AID is constitutively active, ASCIZ deficiency leads to increased rates of Ig diversification, suggesting that under normal conditions ASCIZ channels the repair of AID-dependent base damage into the error-free nonmutagenic BER pathway (Oka et al., 2008). In addition, ASCIZ also has DNA damage–independent functions as an essential transcription factor with crucial roles during the earliest stages of lung organogenesis (Jurado et al., 2010; Heierhorst et al., 2011). A major transcriptional target of ASCIZ is DYNLL1 (dynein light chain 1; Jurado et al., 2012), a multifunctional protein originally identified as a component of the dynein motor complex (King and Patel-King, 1995) with emerging roles as a regulator of hundreds of likely targets (Barbar, 2008; King, 2008; Rapali et al., 2011), including Bim (Puthalakath et al., 1999; Day et al., 2004).
We show in this study that absence of ASCIZ leads to severe peripheral B lymphopenia in mice caused by progressively impaired B cell development in the bone marrow from the pre-B cell stage onwards. This defect appears to be DNA damage independent and mainly caused by unrestrained Bim activity, likely as a consequence of substantially lower levels of its negative regulator DYNLL1 in ASCIZ-deficient B cells. The results highlight the importance of ASCIZ and DYNLL1 in setting homeostatic survival levels during B cell development.
RESULTS
Pan-hematopoietic deletion of Asciz leads to B lymphopenia
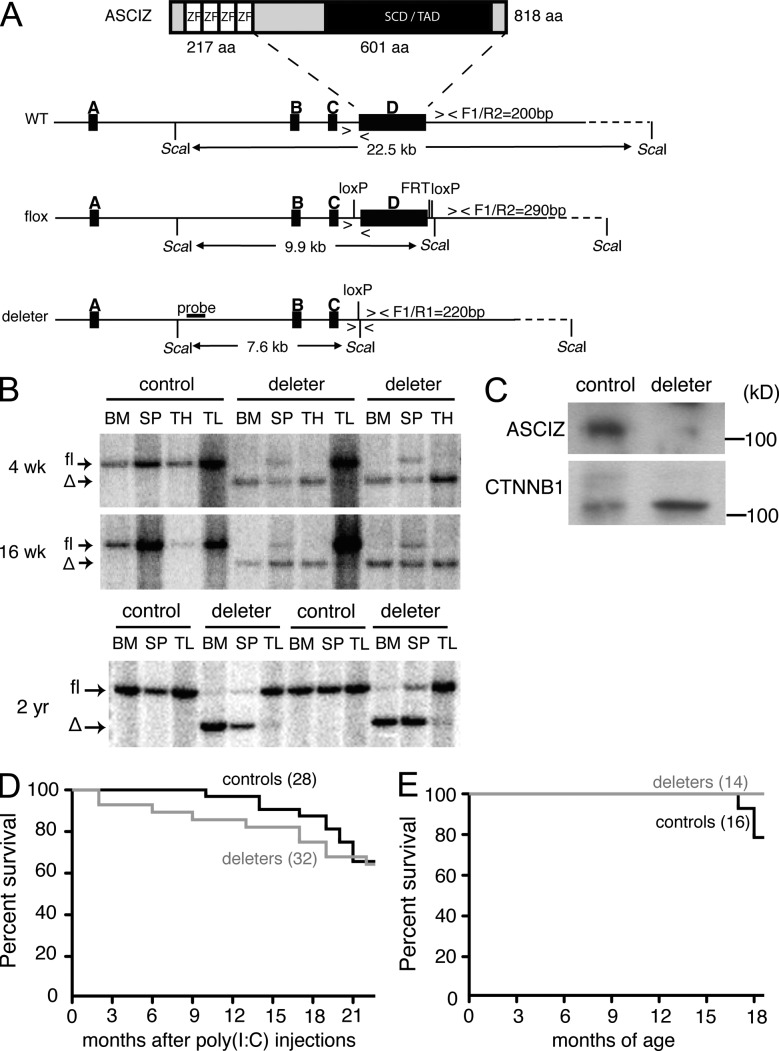
To study ASCIZ function in vivo in a wide range of cell types while avoiding the embryonic lethality resulting from its germline deletion (Jurado et al., 2010), we conditionally deleted Asciz using interferon-inducible Mx1-Cre (Kühn et al., 1995; in the following we refer to this strain as ASCIZ-Mx). In the floxed allele, Cre recombinase deletes the entire exon D, which codes for the vast majority of the protein (Fig. 1 A); this is similar to the strategy used for generation of the germline Asciz KO (Jurado et al., 2010). After Cre induction by polyinosinic:polycytidylic acid (poly(I:C)) injection, Asciz was efficiently deleted in the bone marrow and thymus but not the tail, which was used as a nonresponsive control tissue (Fig. 1 B). Deletion was incomplete in the spleen, which could reflect lack of recombination in interferon nonresponsive stromal cells or reduced Cre activation in the periphery. Recombination patterns in deleters did not change between 4 and 16 wk, and even up to 2 yr, after the last poly(I:C) injection (Fig. 1 B), indicating that ASCIZ-Mx deletion in hematopoietic precursors was efficient and stable without selective growth advantages for nondeleted stem cells.
Figure 1.
Conditional deletion of Asciz. (A) ASCIZ domain organization and conditional deletion strategy. The probe and fragment sizes for Southern blot analysis and primer locations (F1, R1, and R2) and product sizes for PCR genotyping are indicated. SCD, SQ/TQ cluster domain; TAD, transcription activation domain; ZF, Zinc finger. (B) Southern blot analysis of ASCIZ-Mx control and deleter tissues 4 wk, 16 wk, and 2 yr after poly(I:C) injection. Samples from four different controls (Mx1-Cre−Ascizfl/fl) and six different deleters (Mx1-Cre+Ascizfl/fl) are shown. SP, spleen; TH, thymus; TL, tail. (C) Western blot analysis of MACS-purified splenic B cells from single ASCIZ-Mb control and deleter mice. β-Catenin (CTNNB1) is used as loading control. Molecular mass standards are indicated on the right. (D) Kaplan-Meyer survival curve of ASCIZ-Mx deleters and controls for the indicated times after poly(I:C) injection. n = 28 for controls, which included a mixture of Mx1-Cre+Ascizfl/+ and Mx1-Cre−Ascizfl/fl mice, and n = 32 for Mx1-Cre+Ascizfl/fl deleters. Note that there was no statistical difference between the curves and that all nonsurviving mice were sacrificed because of general well-being issues unrelated to neoplasia (seizures, malocclusions, eye infections, etc.). (E) Kaplan-Meyer survival curve of ASCIZ-Mb deleters (n = 14) and controls (n = 16; 10 Mb1/Mb1-Cre Asciz+/+ plus 6 Mb1-Cre−Ascizfl/fl).
Overall, ASCIZ-Mx deletion was well tolerated with a modest (albeit significant) reduction in red blood cell numbers (Fig. 2 A, left) and similar long-term survival rates compared with littermate controls (Fig. 1 D). However, ASCIZ-Mx deletion led to a persistent greater than twofold reduction in the number of circulating lymphocytes compared with poly(I:C)-injected Mx1-Cre+Ascizfl/+ and flox-only controls (P < 0.0001; Fig. 2 A, right). Cell surface marker analysis revealed that this lymphopenia was specific for B cells (Fig. 2 B), with no gross effect on circulating T cell levels (Fig. 3 B). The B cell defect in ASCIZ-Mx deleters was also present in the spleen, where the absolute numbers of B220+IgM+ positive mature B cells were reduced more than twofold compared with controls (P < 0.0001; Fig. 2 C) and already evident from the pre-B cell stage (B220+IgM−CD43−CD19+) onwards in the bone marrow (P < 0.001; Fig. 2 D). In contrast, despite the complete absence of ASCIZ protein in the thymus (Fig. 3 A), comparable stages of T cell development appeared unimpaired (Fig. 3 C). Altogether, these results demonstrate that ASCIZ is required for normal B cell development.
Figure 2.
Mx1-Cre deletion of Asciz leads to B lymphopenia. (A) Blood cell content in ASCIZ-Mx controls and deleters, showing red blood cells (left) and lymphocytes (right) from 4 wk (w) to 12 mo (m) after poly(I:C) injection. Animal numbers: 4 wk, 31 controls/26 deleters; 8 wk, 31/26; 12 wk, 29/24; 16 wk, 30/22; 6 mo, 30/21; 9 mo, 28/23; and 12 mo, 27/22. Animals are from the same cohorts shown in Fig. 1 D with the control group comprised of Mx1-Cre+Ascizfl/+ and Mx1-Cre−Ascizfl/fl mice, with no differences between subgroups. Note that at all time points, differences between controls and deleters are P < 0.0001, except for red blood cells at 12 mo (P < 0.01). (B) Total blood B lymphocyte numbers (right) of ASCIZ-Mx controls and deleters and representative FACS plots (left) using B220 as a B cell marker and costaining for CD4 and CD8 conjugated to the same fluorophore as T cell markers. Results shown here were obtained at 4 wk after poly(I:C) injection. n = 13 for Mx1-Cre+Ascizfl/fl deleters, and n = 14 for pooled controls (n = 4 Mx1-Cre+Ascizfl/+ and n = 10 Mx1-Cre−Ascizfl/fl) with no difference between subgroups. (C) Representative FACS profiles for splenic B220+IgM+ B cell populations (boxed gates; left) and absolute numbers (right) of splenic B220+IgM+ cells in ASCIZ-Mx controls (n = 14; n = 8 Mx1-Cre+Ascizfl/+ and n = 6 Mx1-Cre Ascizfl/fl) and Mx1-Cre+Ascizfl/fl deleters (n = 5) at 4 wk after poly(I:C) injection. (D) Gating strategy (left) and total numbers of bone marrow B cell populations (right). The boxed B220+IgM+ population includes immature, transitional, and recirculating B cells, and the boxed B220+IgM− population is further gated for CD43 and CD19 to distinguish pro-B cells (CD43+CD19+) and pre-B cells (CD43−CD19+). Total numbers represent B cell populations in one hind limb of ASCIZ-Mx controls (n = 15; n = 9 Mx1-Cre+Ascizfl/+ and n = 6 Mx1-Cre−Ascizfl/fl) and Mx1-Cre+Ascizfl/fl deleters (n = 7) at 4 wk after poly(I:C) injection. (A–D) All data represent mean ± SEM. Each symbol in B and C represents one animal. Closed bars and circles indicate controls; open bars and circles indicate deleters. Data in B–D were obtained in at least two independent experiments. Similar results were obtained in mice sacrificed at 16 wk after poly(I:C) injection (not depicted).
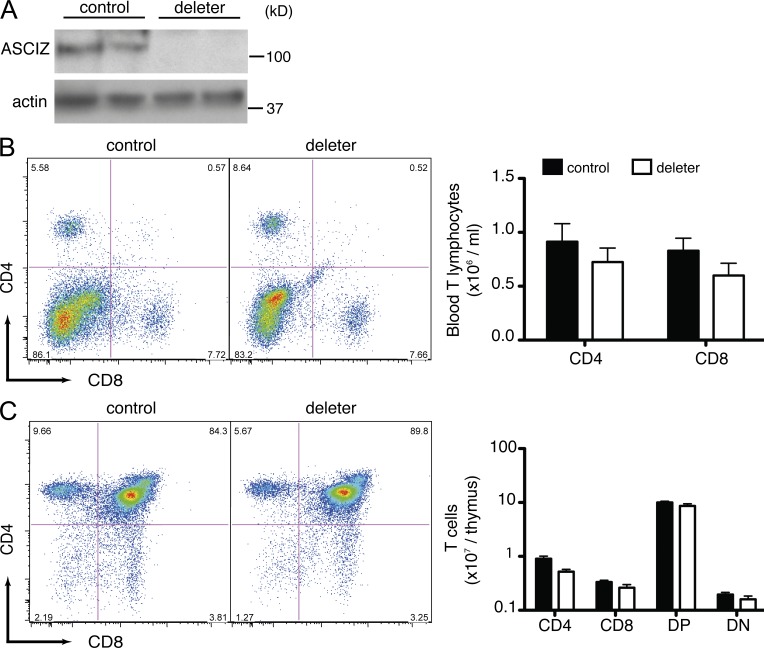
Figure 3.
Normal T cell development in Mx1-Cre Asciz deleters. (A) Western blot of thymic protein levels of two different ASCIZ-Mx deleters and two controls probed for ASCIZ and actin as a loading control at 4 wk after poly(I:C) injection. Molecular mass standards are indicated on the right. (B) Representative FACS plots of blood isolated from ASCIZ-Mx controls and deleters (left) stained for CD4 and CD8 and absolute blood T cell numbers at 4 wk after poly(I:C) injection (right). n = 11 for controls (n = 5 Mx1-Cre+Ascizfl/+ and n = 6 Mx1-Cre−Ascizfl/fl), and n = 8 for Mx1-Cre+Ascizfl/fl deleters. (C) Representative FACS plots of a thymus isolated from an ASCIZ-Mx control and a deleter thymus (left) and absolute thymic T cell numbers (right) at 4 wk after poly(I:C) injection. n = 15 for controls (n = 9 Mx1-Cre+Ascizfl/+ and n = 6 Mx1-Cre−Ascizfl/fl), and n = 8 for Mx1-Cre+Ascizfl/fl deleters. (B and C) All grouped data represent mean ± SEM. Similar results were obtained at 16 wk after poly(I:C) injection.
B cell lineage–specific Asciz deletion leads to severe B cell deficiency
To examine whether the ASCIZ-Mx phenotype was indeed caused by a defect in B cell precursors, we used the B lineage–specific Mb1-Cre knockin, which efficiently deletes floxed alleles from the pro-B cell stage onward (Hobeika et al., 2006). Efficient Asciz deletion by Mb1-Cre (in the following simplified as ASCIZ-Mb) was confirmed by Western blot analysis of enriched splenic B cells (Fig. 1 C). Strikingly, spleens of ASCIZ-Mb deleters were approximately twofold smaller than those of Cre-only and flox-only littermate controls (P < 0.0001; Fig. 4 A), concomitant with a more than sevenfold decrease in B220+IgM+ positive mature splenic B cell numbers (P < 0.0001; Fig. 4 B). Bone marrow phenotyping of ASCIZ-Mb deleters revealed a similar greater than sevenfold reduction of B220lowIgM+-gated immature B cell numbers compared with controls (P < 0.0001; Fig. 4 C), as well as an approximately threefold decrease (P < 0.0001) in the pre-B cell compartment (B220+IgM−CD43−CD19+) but no difference at the pro-B cell stage (Fig. 4 D). Similar results were obtained in reconstitution experiments of lethally irradiated Ly5.1-expressing recipient mice with Ly5.2-positive ASCIZ-Mb donor bone marrow. In these experiments, the percentage of donor-derived B220+IgM+ B cells in recipient spleens was 4.6-fold lower for deleters compared with Mb1-Cre–only or Cre-negative Ascizfl/fl control donors (P < 0.0001; Fig. 5 A) and >10-fold lower for B220lowIgM+ immature B cells (P < 0.0001; Fig. 5 B) and 3.3-fold lower for B220+IgM−CD43−CD19+ pre-B cells (P < 0.0001; Fig. 5 C) in the bone marrow. Altogether, these data demonstrate that ASCIZ regulates B cell development in a cell-intrinsic manner.
Figure 4.
Mb1-Cre Asciz deletion leads to progressive B cell loss during B cell development. (A) Photograph of representative spleens from Mb1-Cre−Ascizfl/fl, Mb1/Mb1-Cre Asciz+/+ controls, and an Mb1/Mb1-Cre Ascizfl/fl deleter (left) and relative spleen weights of controls (n = 28) and deleters (n = 36; right). (B) Representative FACS plots of spleens isolated from an ASCIZ-Mb control and a deleter (left) and absolute numbers of splenic B220+IgM+ B cells in controls (n = 20) and Mb1/Mb1-Cre Ascizfl/fl deleters (n = 28; right). The control group contains both Mb1-Cre−Ascizfl/fl and Mb1/Mb1-Cre Asciz+/+ animals. (C) Representative FACS plots of bone marrows from an ASCIZ-Mb control and an Mb1/Mb1-Cre Ascizfl/fl deleter (left) and absolute cell numbers (right) of B220lowIgM+ immature B cells (gated as indicated on the FACS plots) in a single hind leg of controls (n = 9) and deleters (n = 14). The control group contains both Mb1-Cre−Ascizfl/fl and Mb1/Mb1-Cre Asciz+/+ animals. (D) Representative FACS plots of B220+IgM−-gated bone marrow cells from an ASCIZ-Mb control and an Mb1/Mb1-Cre Ascizfl/fl deleter stained for CD43 and CD19 (left). The right panel shows grouped data for pro-B cells (B220+IgM−CD43+CD19+) and pre-B cells (B220+IgM−CD43−CD19+) in controls (n = 9) and deleters (n = 9). The control group contains both Mb1-Cre−Ascizfl/fl and Mb1/Mb1-Cre Asciz+/+ animals. (E) Representative FACS plots of cell death (AnnexinV+7-AAD+) in immature and transitional B cells (B220+IgM+IgD−/low) in an ASCIZ-Mb control and a deleter (left) and grouped data for Mb1/Mb1-Cre Asciz+/+ controls (n = 5) and Mb1/Mb1-Cre Ascizfl/fl deleters (n = 4). (A–E) Each data point represents one mouse. All grouped data represent mean ± SEM. Data were obtained in at least two independent experiments. Closed circles indicate controls; open circles indicate deleters.
Figure 5.
Cell-intrinsic requirement for ASCIZ during B cell development. (A) FACS analysis of spleen cells of lethally irradiated Ly5.1 recipients 8 wk after transplantation with Ly5.2 control or Mb1/Mb1-Cre Ascizfl/fl deleter bone marrow and percentage of B220+IgM+ B cells among donor-derived spleen cells (control transplants, n = 8; deleter transplants, n = 8). Half of the control transplants were performed with bone marrow from a single Mb1/Mb1-Cre Asciz+/+ donor, the other half with bone marrow from a single Mb1-Cre−Ascizfl/fl donor. Two different deleter bone marrows were used for four different recipients each. (B and C) Percentages of donor-derived pre-B and immature B cells in bone marrows from the same recipients shown in A. (A–C) Error bars represent mean ± SEM. Each symbol represents one animal. All data were obtained in one experiment.
To identify possible causes of this B lymphopenia, we monitored cell death levels in the bone marrow. Interestingly, in B220+IgM+IgD−/low-gated immature and transitional B cells, there was an approximately threefold increase in AnnexinV/7-AAD double-positive cells (Fig. 4 E) in ASCIZ-Mb deleters compared with controls. These data indicate that the main reason for the peripheral B lymphopenia in ASCIZ-Mb deleters may be reduced cell survival during B cell development before exiting the bone marrow.
Loss of ASCIZ-deficient B cells is largely independent of its DNA damage response functions
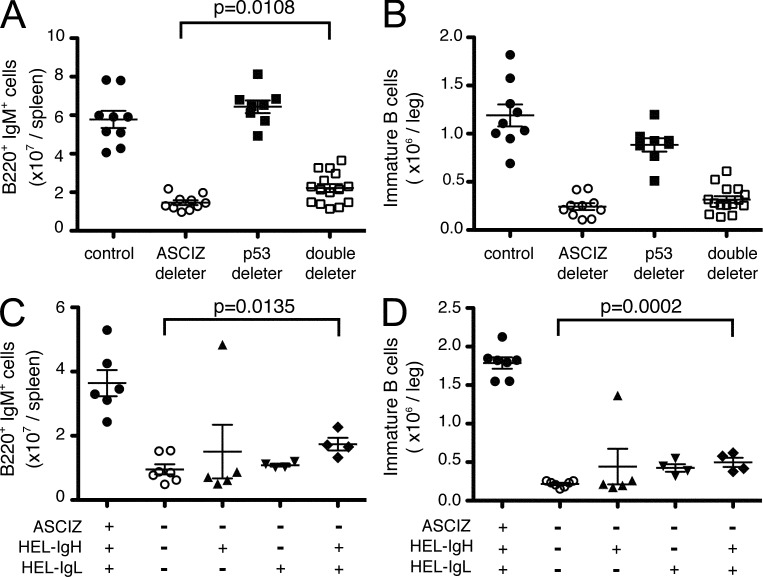
As ASCIZ was originally identified as a DNA damage response protein, we examined whether the B lymphopenia could be caused by impaired DNA damage responses. DNA base damage–induced apoptosis is typically p53 dependent, and related B lymphopoiesis defects can be prevented by p53 deletion, for example in case of apurinic endonuclease deficiency (Guikema et al., 2011). We therefore crossed ASCIZ-Mb mice with p53 floxed mice (Jonkers et al., 2001); however, additional p53 deletion only marginally increased splenic B cell levels (albeit in a statistically significant manner; Fig. 6 A) or bone marrow immature B cell levels (Fig. 6 B) in ASCIZ-Mb deleters.
Figure 6.
Loss of B cells in the absence of ASCIZ is not rescued by p53 deletion or complementation with the prearranged SWHEL BCR. (A and B) Absolute splenic B220+IgM+ B cell (A) and bone marrow B220lowIgM+ immature B cell numbers (B) in Mb1/Mb1-Cre Ascizfl/fl deleters (n = 10), Mb1/Mb1-Cre p53fl/fl deleters (n = 8), Mb1/Mb1-Cre Ascizfl/flp53fl/fl double deleters (n = 15), and controls (n = 8). The control group contains four Mb1-Cre−Ascizfl/flp53fl/fl and four Mb1/Mb1-Cre Asciz+/+p53+/+ animals. The data were obtained in six independent experiments. (C and D) Absolute splenic B220+IgM+ B cell (C) and bone marrow B220lowIgM+ immature B cell numbers (D) in Mb1/Mb1-Cre Ascizfl/fl deleters (n = 7) and Mb1/Mb1-Cre Ascizfl/fl deleters expressing a prearranged SWHEL IgH (HEL-IgH; n = 5), SWHEL IgL (HEL-IgL; n = 4), or both SWHEL IgH and IgL (n = 4), and Mb1/Mb1-Cre Asciz+/+ controls expressing both SWHEL IgH and IgL (spleen n = 6; bone marrow n = 7). The data were obtained in three independent experiments. (A–D) Absolute numbers were determined by flow cytometry using gates as shown in Fig. 4. All grouped data represent mean ± SEM. Each symbol represents one animal.
Another possible explanation for the gradual decline of cells at both the pre-B and immature B cell stages could be inefficient V(D)J recombination of the IgH and IgL loci to generate functional pre-BCRs and BCRs, respectively, defects which would be suppressible by complementation with prearranged BCR genes (Spanopoulou et al., 1994; Young et al., 1994; Cook et al., 2003). We therefore crossed ASCIZ-Mb deleters with SWHEL mice, which contain a hen egg lysozyme (HEL)–specific prearranged BCR caused by a VDJ-recombined IgH locus knockin and an IgL transgene that can restore normal B cell development even in RAG KO or SCID mice (Phan et al., 2003). However, neither complementation with the complete HEL-BCR nor its individual HEL-IgH or HEL-IgL components meaningfully increased the numbers of splenic B cells or bone marrow immature B cells in ASCIZ-Mb deleters (Fig. 6, C and D). Collectively, the findings that neither provision of a prearranged BCR nor p53 deletion was able to rescue the B lymphopenia in ASCIZ-Mb deleters demonstrate that this defect must be largely unrelated to the DNA damage response functions of ASCIZ.
ASCIZ-deficient lymphopenia is caused by reduced Dynll1 expression and increased Bim activity
As these data showed that the lymphopenia observed in ASCIZ-deficient mice is most likely DNA damage independent, we explored the possibility that it could be related to the role of ASCIZ as a transcription factor. We recently identified Dynll1 as the main transcriptional target for ASCIZ in primary mouse fibroblasts (Jurado et al., 2012). Consistently, DYNLL1 protein levels were also dramatically reduced in sorted ASCIZ-Mb deleter bone marrow B cell precursors compared with controls (Fig. 7 A). To test whether impaired Dynll1 expression was a cause of the ASCIZ-Mb phenotype, Asciz deleter bone marrow was transduced with empty vector or Dynll1-containing retroviruses and transplanted together with nontransduced deleter bone marrow carrier cells into lethally irradiated recipients, followed by FACS identification of Ly5.2+ donor-derived cells in recipient spleens 8 wk after transplantation (Fig. 7 B). IRES-GFP tracking of transduced cells revealed a significant enrichment of Dynll1-complemented deleter-derived cells in the splenic B220+IgM+ compartment compared with the empty vector control (Fig. 7 B, top), as well as compared with nontransduced deleter-derived cells as an internal control (Fig. 7 B, bottom). Thus, this finding that ectopic DYNLL1 can compensate for loss of ASCIZ demonstrates that ASCIZ regulates B lymphopoiesis as a transcriptional activator of Dynll1 expression.
Figure 7.
ASCIZ regulates B cell development via DYNLL1. (A) Western blot analysis of DYNLL1 levels in FACS-isolated bone marrow B220+IgM− cells of two controls and two Mb1/Mb1-Cre Ascizfl/fl deleters. GAPDH is used as loading control. Molecular mass standards are indicated on the right. (B) Reconstitution of spleens of lethally irradiated recipients with Mb1/Mb1-Cre Ascizfl/fl deleter–derived cells that were retrovirally transduced with MigR1 empty vector (control; n = 2) or MigR1-Dynll1 (n = 3). The graph shows the percentage of B220+IgM+ spleen cells among transduced GFP-positive donor-derived cells. FACS plots (below) indicate the gating strategy. Ly5.2-positive donor-derived cells were gated for IRES-GFP expression to detect vector (left)- or Dynll1 (right)-transduced cells. Error bars represent mean ± SEM. Each symbol represents one animal. All data were obtained in one experiment.
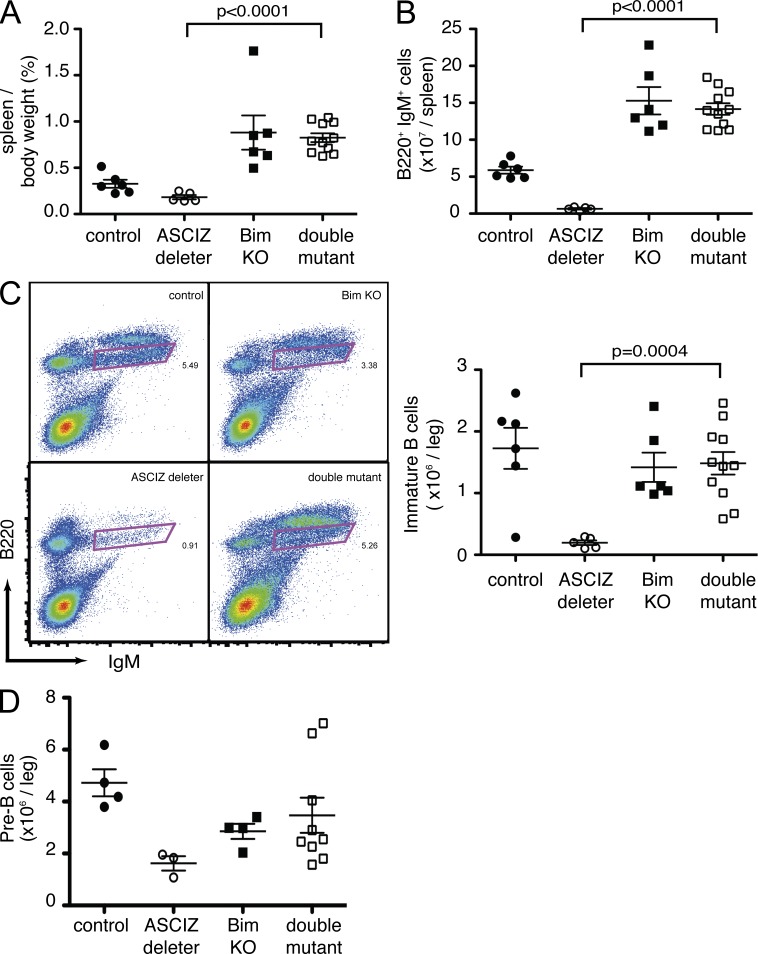
As DYNLL1 has been shown to attenuate the proapoptotic activity of the BH3-only protein Bim (Puthalakath et al., 1999) and as Bim is responsible for the elimination of developing B cells (Enders et al., 2003), we hypothesized that the B lymphopenia in Asciz deleters may be caused by increased Bim activity. To test this hypothesis, we crossed ASCIZ-Mb mice to Bim KO mice (Bouillet et al., 1999). Again, ASCIZ-Mb deleters had smaller spleens and approximately sevenfold lower splenic B220+IgM+ B cell levels compared with littermate controls (Fig. 8, A and B), and as expected from a previous study (Bouillet et al., 1999), Bim KO mice exhibited splenomegaly with highly elevated B cell numbers (Fig. 8, A and B). Remarkably, when combined with the Bim KO, the ASCIZ-Mb phenotype was completely rescued with highly elevated splenic B cell levels and even splenomegaly similar to Bim single mutants (P < 0.0001; Fig. 8, A and B). Loss of Bim also increased the numbers of B220lowIgM+-gated immature B cells (Fig. 8 C) and B220+IgM−CD43−CD19+ pre-B cells (Fig. 8 D) in the bone marrow of ASCIZ-Mb deleters to levels similar to the Bim KO alone. These results that the Bim KO phenotype is dominant over the ASCIZ-Mb deleter phenotype place Asciz and Bim in a common genetic pathway and, in light of highly reduced DYNLL1 levels, suggest that the ASCIZ-Mb phenotype may be caused by excessive Bim activity as the result of impaired Dynll1 transcription.
Figure 8.
Impaired B cell development in the absence of ASCIZ is rescued by deletion of Bim. (A and B) Relative spleen weights (A) and total numbers of splenic B220+IgM+ B cells (B) in Mb1/Mb1-Cre Ascizfl/fl deleters (n = 5), Bim KO (n = 6), double mutants (n = 11), and controls (n = 6). All controls and all Bim KO mice are Mb1/Mb1-Cre Asciz+/+. Data were obtained in five independent experiments. (C) Bone marrow phenotyping (left) and total cell numbers (right) for B220lowIgM+-gated immature B cells in one hind limb of Mb1/Mb1-Cre Ascizfl/fl deleters (n = 5), Bim KO mice (n = 6), double mutants (n = 11), and controls (n = 6). The animals are the same as in A. (D) Pre-B cell numbers (B220+IgM−CD43−CD19+) in Mb1/Mb1-Cre Ascizfl/fl deleters (n = 3), Bim KO mice (n = 4), double mutants (n = 9), and controls (n = 4). All controls and all Bim KO mice are Mb1/Mb1-Cre Asciz+/+. The data were obtained in three independent experiments. (A–D) Each symbol represents one mouse. Error bars represent mean ± SEM.
DISCUSSION
In this study, we have shown with two independent conditional KO models that ASCIZ is essential for normal B cell development. The resulting B lymphopenia was more severe in the Mb1-Cre model, conceivably because in this line Asciz is constitutively deleted in every single B cell past the pro-B stage, whereas in the Mx1-Cre model large numbers of mature B cells would already have been established in the periphery by the time (4–5 wk of age) of the first poly(I:C) injections. The genetic rescue of this defect by complementation with ectopic DYNLL1 (Fig. 7) but not by deletion of p53 or complementation with a prearranged BCR (Fig. 6) demonstrates that this phenotype is predominantly caused by loss of ASCIZ’s transcriptional function in regulating Dynll1 expression rather than its role in the DNA damage response. This notion is further supported by the rescue of the Asciz deleter phenotype by the absence of Bim, which predominantly functions in DNA damage–independent cell death pathways (Strasser, 2005). It is important to note that the phenotype of ASCIZ-Mb Bim double mutants represents to our knowledge the first reported case where reduced peripheral B cell numbers as a result of a B cell developmental defect in the bone marrow are completely rescued all the way to the abnormally elevated B cell levels characteristic of the Bim KO alone. For example, in the case of Mb1-Cre Dicer deletion, Bim KO only partially restored splenic B cell numbers to ∼20% of WT levels (Koralov et al., 2008), and in the case of an Atp11c mutation, the Bim KO had no beneficial effect at all on peripheral or bone marrow B cell numbers (Siggs et al., 2011). Likewise, even broader inhibition of all intrinsic cell death pathways by transgenic overexpression of the antiapoptotic protein Bcl2 lead to no or only partial improvement of peripheral B cell numbers relative to WT, but much less so relative to the elevated B cell levels in the transgene control, in the case of Dicer (Koralov et al., 2008), Atp11c (Siggs et al., 2011; Yabas et al., 2011), or Stat5 (Malin et al., 2010) mutations. These considerations indicate that absence of Bim rescues the Asciz deleter phenotype not just by generally elevating B cell numbers, but in a highly specific manner suggestive of functions in a common genetic pathway.
As cell death during B cell development, which is known to be at least partially regulated by Bim (Enders et al., 2003), is elevated in Asciz deleters (Fig. 4 E) and as Bim’s proapoptotic function has been reported to be held in check by DYNLL1 (Puthalakath et al., 1999), the simplest explanation for our findings is a model in which ASCIZ positively regulates DYNLL1 expression, which may in turn prevent excessive Bim-dependent apoptosis during B cell development. However, given that DYNLL1 has been proposed to regulate >100 diverse proteins (Rapali et al., 2011), it is remarkable that deletion of Bim alone was sufficient to rescue the ASCIZ-Mb deleter phenotype. In this sense, it is formally possible that other defects that indirectly lead to increased Bim-dependent apoptosis could contribute to the phenotype described here in addition to the direct effects lower DYNLL1 levels may have on Bim activity. For example, DYNLL1 was originally identified as a component of the dynein motor complex (King and Patel-King, 1995) that has recently been shown to be involved in the regulation of BCR clustering (Schnyder et al., 2011), a feature which could conceivably affect Bim activation by BCR signaling pathways. Nevertheless, the restoration of normal B cell development in ASCIZ-Mb deleters by ectopic Dynll1 expression (Fig. 7), to our knowledge, represents the first in vivo evidence in any vertebrate species that reduced DYNLL1 levels are a direct cause of a severe developmental defect.
A unique feature of ASCIZ as a regulator of Dynll1 expression is that this activity is inhibited by high DYNLL1 levels as a result of >10 DYNLL1-binding sites in its transcription activation domain (Jurado et al., 2012), which allows for an efficient feedback mechanism to maintain free DYNLL1 at a homeostatic level. This feedback mechanism may serve to set a default threshold of apoptosis in developing B cells by providing sufficient DYNLL1 to prevent Bim from unnecessarily killing normal cells, yet also ensures that DYNLL1 levels are not too high to preclude legitimate Bim-dependent elimination of dysfunctional or autoreactive B cells. In this way, ASCIZ-dependent regulation of DYNLL1 levels could tone the strength of death signals in the B cell lineage in a manner analogous to the recently described role of schnurri-2 during T cell development (Staton et al., 2011).
While the work reported here was in progress, another group reported that a similarly targeted conditional Asciz deletion using CD19-Cre leads to increased rates of peripheral B cell lymphoma but only very modest B cell developmental defects with normal splenic B cell numbers (Loizou et al., 2011). The simplest explanation for the differing phenotypes is that CD19-Cre is known to be much less efficient at recombining loxP-flanked genes than Mb1-Cre during early B cell developmental stages (Rickert et al., 1997; Hobeika et al., 2006; Kwon et al., 2008), which would have attenuated the severity of the pre-B and immature B cell defects and obscured the splenic defect. Conversely, the absence of B cell lymphomas in both our Mb1-Cre and Mx1-Cre Asciz deleters up to 2 yr of age (Fig. 1, D and E) could simply be caused by much lower peripheral B cell numbers or alternatively could potentially be affected by a different genetic background, which was not specified in the other study. Thus, although it is well possible that DNA base damage–related functions of ASCIZ affect the onset of germinal center–derived B cell lymphomas, our work has uncovered a major unexpected function of ASCIZ as a novel regulator of DNA damage–independent cell death signaling pathways during early B cell development in the bone marrow.
A notable result from the ASCIZ-Mx deleters was that despite efficient deletion of ASCIZ in the T cell lineage (Figs. 1 B and 3 A), there were no noticeable differences in circulating T cell levels or thymic T cell developmental stages (Fig. 3, B and C), which is consistent with findings that basal Bim activity in T cells is regulated via DYNLL1-independent mechanisms (Zhu et al., 2004). Thus, in contrast to V(D)J recombination defects that lead to a SCID phenotype affecting both lymphocyte lineages (Mombaerts et al., 1992; Shinkai et al., 1992; Blunt et al., 1995), the phenotype of ASCIZ-Mx deleters more closely resembles CVID (common variable immunodeficiencies), a heterogeneous group of hereditary B cell dysfunctions in which most of the underlying mutations remain to be identified. This raises the possibility that mutations in Asciz, and by extension mutations in Dynll1, could be involved in a subset of human CVID cases of unexplained etiology.
MATERIALS AND METHODS
Mice.
All experimental procedures were approved by the St. Vincent’s Hospital Melbourne Animal Ethics Committee. Mice were maintained on a C57BL/6 background and housed in specific pathogen–free microisolators to minimize pathogen exposure at the St. Vincent’s Hospital Melbourne Bioresources Centre.
The Asciz gene was targeted in C57BL/6 embryonic stem cells by integrating loxP sites on both sides of exon D (Fig. 1 A) as described previously (Jurado et al., 2010), followed by removal of the FRT-flanked PGK-neo cassette by crossing with ACTB-FLPe transgenic mice on a C57BL/6 background as a contracted service by Ozgene Pty Ltd. After removal of the PGK-neo cassette, the floxed Asciz allele was backcrossed twice to WT C57BL/6 mice to remove the FLPe transgene and for embryo transfer into our mouse facility.
Mx1-cre (Kühn et al., 1995), Mb1-Cre (Hobeika et al., 2006; provided by M. Reth, Max Planck Institute of Immunobiology, Freiburg, Germany), p53 floxed (Jonkers et al., 2001), SWHEL (Phan et al., 2003; provided by R. Brink, Garvan Institute, Darlinghurst, New South Wales, Australia), and Bim KO (Bouillet et al., 1999) mice have been described previously. All of these lines had previously been backcrossed to C57BL/6 for at least 10 generations. As Mb1-Cre is a knockin and homozygotes lack B cells, only Mb1/Mb1-Cre heterozygotes were used for phenotypic analyses. SWHEL mice were heterozygous for the HEL-IgH knockin and transgenic for HEL-IgL.
Unless otherwise specified, mice were used at 6–14 wk of age. The ASCIZ-Mx control group contained Mx1-Cre+Ascizfl/+ and Mx1-Cre−Ascizfl/fl mice. Control groups for other experiments contained Mb1/Mb1-Cre–only mice and in some cases also Ascizfl/fl or p53fl/fl mice without Cre expression as indicated in the figure legends. Genotyping of mice was performed by PCR using the primer sets shown in Table S1.
Mx-Cre induced ASCIZ deletion.
4–5-wk-old ASCIZ-Mx controls and deleters were injected every other day with 25 mg/kg poly(I:C) (Poly(I:C)-LMW, tlrl-picw; InvivoGen) to induce Cre expression. Mice were bled at 4, 8, 12, and 16 wk and 6, 9, and 12 mo after injection for blood cell analyses and observed for long-term survival for up to 24 mo of age. Additional mice were sacrificed at 4 and 16 wk after poly(I:C) injection for bone marrow and spleen cell analyses.
Bone marrow transplantation and retroviral DYNLL1 complementation.
For retroviral complementation experiments, the human Dynll1 cDNA (Puthalakath et al., 1999) was cloned into the MigR1 vector (Pear et al., 1993), and virus was produced using the Phoenix packaging cell line. Donor cells were isolated from Ly5.2 ASCIZ-Mb deleters and controls (Ascizfl/fl without Cre expression or Mb1/Mb1-Cre–only mice). Hematopoietic stem cell–enriched fractions (Lin−ckit+Sca+) were FACS isolated from whole bone marrow of ASCIZ-Mb deleters as described previously (Walkley et al., 2005) and cultured overnight in StemPro-34 medium (Invitrogen) with 10 ng/ml mSCF, 20 ng/ml mTPO, 20 ng/ml mIGF2, and 10 ng/ml hFGF1 (Zhang and Lodish, 2004) before two rounds of infection with MIGR1 control or DYNLL1 retroviruses. Equal numbers (4,000–5,000) of cells were then transplanted by tail vein injection into lethally irradiated Ly5.1 recipient mice together with 200,000 nontransduced bone marrow cells of the same donor genotype. Mice were sacrificed 8 wk after transplant for bone marrow and spleen cell analyses. Ly5.2-stained donor-derived cells were further gated for IRES-GFP expression to distinguish between carrier cells and retrovirally transduced cells for the analyses shown in Figs. 5 and 7.
Cytometry and FACS analyses.
Spleens were weighed and passed through a 70-µm cell strainer to allow preparation of a homogeneous cell suspension. Cells were collected in PBS 2% FBS. Bone marrow cells were isolated from hind limbs. Typically, one leg (femur plus tibia) was cleaned of soft tissues, and bones were flushed with PBS 2% FBS with a syringe connected to a 23G needle. Blood, spleen, and bone marrow cellularities were determined using an automated KX-21N cell counter (Sysmex). Cells were stained with appropriate antibodies in PBS 2% FBS on ice. AnnexinV staining was performed in its own buffer (eBioscience) at room temperature for 15 min according to the manufacturer’s recommendation. Stained cells were analyzed on an LSRFortessa (BD). Compensation for multicolor stains and data acquisition were performed using the FACSDiva software (BD), and data were analyzed using FlowJo (Tree Star).
Antibodies used were as follows: B220-APC (eBioscience), B220-FITC (BD), IgM-FITC (eBioscience), IgM-eFluor650 (eBioscience), IgM-biotin (eBioscience), IgD-eFluor450 (eBioscience), IgD–Alexa Fluor 647 (eBioscience), CD19-FITC (eBioscience), CD19-PerCP-Cy5.5 (eBioscience), CD43-PE (BD), CD4-PE (BD), CD4-eFluor450 (eBioscience), CD8a-FITC (eBioscience), CD8a-eFluor450 (eBioscience), CD44-APC (eBioscience), CD25-PE (eBioscience), Ly5.1-PECy7 (eBioscience), Ly5.2-biotin (eBioscience), streptavidin-Qdot605 (Invitrogen), and AnnexinV-eFluor450 detection kit (eBioscience). Bone marrow B cell populations were defined according to the following surface marker profiles: pro-B cells, B220+IgM−CD43+CD19+; pre-B cells, B220+IgM−CD43−CD19+; and immature B cells, B220lowIgM+.
Blot analyses.
For Southern blots, DNA was isolated from tissues or cell suspensions by overnight digestion with 0.5 mg/ml proteinase K in 1% SDS at 55°C followed by isopropanol precipitation and ScaI endonuclease treatment. Membranes were incubated overnight with a radioactive Asciz probe and exposed to phosphorimager screens as described previously (Jurado et al., 2010, 2012). For Western blots, splenic and bone marrow B cells were isolated from total cell suspensions using B220 (CD45R)-MACS beads (Miltenyi Biotec) and an auto-MACS Pro Separator (Miltenyi Biotec) according to the manufacturer’s instructions. Bone marrow B cell precursors were isolated using a FACSAria (BD) at the St. Vincent’s Institute FACS facility. Cells were lysed in SDS gel loading buffer before electrophoresis and transfer to polyvinyldifluoride membranes. Antibodies used were as follows: actin (EMD Millipore/Merck), ASCIZ (McNees et al., 2005; available from EMD Millipore/Merck), CTNNB1 (Sigma-Aldrich), DYNLL1/2 (Santa Cruz Biotechnology, Inc.), and GAPDH-HRP (Cell Signaling Technology).
Statistical analyses.
P-values were calculated by two-tailed unpaired Student’s t test. Grouped data represent the mean ± SEM. Numbers of mice per analysis are indicated in the figure legends.
Online supplemental material.
Table S1 contains oligonucleotide primers for PCR genotyping. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20120785/DC1.
Supplementary Material
Acknowledgments
We thank Bryce van Denderen for help with initial poly(I:C) injections, Monique Smeets for help with retroviral transductions, Sita Dewamitta for help with tail vein injections, Natalie Sanders for help with cell sorting, Nora Tenis for help with Southern blots, the St. Vincent’s Hospital Melbourne BioResources Centre for animal care, Michael Reth for Mb1-Cre mice, and Robert Brink for SWHEL mice.
This work was supported by grants and fellowships from the National Health and Medical Research Council of Australia (NHMRC) to J. Heierhorst, D.J. Izon, C.R. Walkley, D.M. Tarlinton, and A. Strasser, a Melbourne University International Fee Remission Scholarship and a partial PhD scholarship from the Cooperative Research Centre Cancer Therapeutics to S. Jurado, and in part by the Victorian Government’s Operational Infrastructure Support Program and Australian Government NHMRC IRIIS. C.R. Walkley is the Phillip Desbrow Senior Research Fellow of the Leukemia Foundation.
The authors declare that they have no competing financial interests.
Author contributions: S. Jurado designed, performed, and analyzed experiments and wrote the paper; K. Gleeson and K. O’Donnell performed experiments and provided assistance; D.J. Izon, C.R. Walkley, and D.M. Tarlinton designed, discussed, and analyzed experiments; A. Strasser discussed experiments and provided reagents; and J. Heierhorst designed, discussed, and analyzed experiments and wrote the paper.
Footnotes
Abbreviations used:
- AID
- activation-induced cytidine deaminase
- ASCIZ
- ATM substrate Chk2-interacting Zn2+-finger protein
- HEL
- hen egg lysozyme
- poly(I:C)
- polyinosinic:polycytidylic acid
References
- Barbar E. 2008. Dynein light chain LC8 is a dimerization hub essential in diverse protein networks. Biochemistry. 47:503–508 10.1021/bi701995m [DOI] [PubMed] [Google Scholar]
- Blunt T., Finnie N.J., Taccioli G.E., Smith G.C., Demengeot J., Gottlieb T.M., Mizuta R., Varghese A.J., Alt F.W., Jeggo P.A., et al. 1995. Defective DNA-dependent protein kinase activity is linked to V(D)J recombination and DNA repair defects associated with the murine scid mutation. Cell. 80:813–823 10.1016/0092-8674(95)90360-7 [DOI] [PubMed] [Google Scholar]
- Bouillet P., Metcalf D., Huang D.C., Tarlinton D.M., Kay T.W., Köntgen F., Adams J.M., Strasser A. 1999. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 286:1735–1738 10.1126/science.286.5445.1735 [DOI] [PubMed] [Google Scholar]
- Cook A.J., Oganesian L., Harumal P., Basten A., Brink R., Jolly C.J. 2003. Reduced switching in SCID B cells is associated with altered somatic mutation of recombined S regions. J. Immunol. 171:6556–6564 [DOI] [PubMed] [Google Scholar]
- Day C.L., Puthalakath H., Skea G., Strasser A., Barsukov I., Lian L.Y., Huang D.C., Hinds M.G. 2004. Localization of dynein light chains 1 and 2 and their pro-apoptotic ligands. Biochem. J. 377:597–605 10.1042/BJ20031251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders A., Bouillet P., Puthalakath H., Xu Y., Tarlinton D.M., Strasser A. 2003. Loss of the pro-apoptotic BH3-only Bcl-2 family member Bim inhibits BCR stimulation–induced apoptosis and deletion of autoreactive B cells. J. Exp. Med. 198:1119–1126 10.1084/jem.20030411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guikema J.E., Gerstein R.M., Linehan E.K., Cloherty E.K., Evan-Browning E., Tsuchimoto D., Nakabeppu Y., Schrader C.E. 2011. Apurinic/apyrimidinic endonuclease 2 is necessary for normal B cell development and recovery of lymphoid progenitors after chemotherapeutic challenge. J. Immunol. 186:1943–1950 10.4049/jimmunol.1002422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heierhorst J., Smyth I., Jurado S. 2011. A breathtaking phenotype: unexpected roles of the DNA base damage response protein ASCIZ as a key regulator of early lung development. Cell Cycle. 10:1222–1224 10.4161/cc.10.8.15336 [DOI] [PubMed] [Google Scholar]
- Hobeika E., Thiemann S., Storch B., Jumaa H., Nielsen P.J., Pelanda R., Reth M. 2006. Testing gene function early in the B cell lineage in mb1-cre mice. Proc. Natl. Acad. Sci. USA. 103:13789–13794 10.1073/pnas.0605944103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonkers J., Meuwissen R., van der Gulden H., Peterse H., van der Valk M., Berns A. 2001. Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat. Genet. 29:418–425 10.1038/ng747 [DOI] [PubMed] [Google Scholar]
- Jurado S., Smyth I., van Denderen B., Tenis N., Hammet A., Hewitt K., Ng J.L., McNees C.J., Kozlov S.V., Oka H., et al. 2010. Dual functions of ASCIZ in the DNA base damage response and pulmonary organogenesis. PLoS Genet. 6:e1001170 10.1371/journal.pgen.1001170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurado S., Conlan L.A., Baker E.K., Ng J.L., Tenis N., Hoch N.C., Gleeson K., Smeets M., Izon D., Heierhorst J. 2012. ATM substrate Chk2-interacting Zn2+ finger (ASCIZ) Is a bi-functional transcriptional activator and feedback sensor in the regulation of dynein light chain (DYNLL1) expression. J. Biol. Chem. 287:3156–3164 10.1074/jbc.M111.306019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanu N., Penicud K., Hristova M., Wong B., Irvine E., Plattner F., Raivich G., Behrens A. 2010. The ATM cofactor ATMIN protects against oxidative stress and accumulation of DNA damage in the aging brain. J. Biol. Chem. 285:38534–38542 10.1074/jbc.M110.145896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King S.M. 2008. Dynein-independent functions of DYNLL1/LC8: redox state sensing and transcriptional control. Sci. Signal. 1:pe51 10.1126/scisignal.147pe51 [DOI] [PubMed] [Google Scholar]
- King S.M., Patel-King R.S. 1995. The M(r) = 8,000 and 11,000 outer arm dynein light chains from Chlamydomonas flagella have cytoplasmic homologues. J. Biol. Chem. 270:11445–11452 10.1074/jbc.270.19.11445 [DOI] [PubMed] [Google Scholar]
- Koralov S.B., Muljo S.A., Galler G.R., Krek A., Chakraborty T., Kanellopoulou C., Jensen K., Cobb B.S., Merkenschlager M., Rajewsky N., Rajewsky K. 2008. Dicer ablation affects antibody diversity and cell survival in the B lymphocyte lineage. Cell. 132:860–874 10.1016/j.cell.2008.02.020 [DOI] [PubMed] [Google Scholar]
- Kühn R., Schwenk F., Aguet M., Rajewsky K. 1995. Inducible gene targeting in mice. Science. 269:1427–1429 10.1126/science.7660125 [DOI] [PubMed] [Google Scholar]
- Kwon K., Hutter C., Sun Q., Bilic I., Cobaleda C., Malin S., Busslinger M. 2008. Instructive role of the transcription factor E2A in early B lymphopoiesis and germinal center B cell development. Immunity. 28:751–762 10.1016/j.immuni.2008.04.014 [DOI] [PubMed] [Google Scholar]
- LeBien T.W., Tedder T.F. 2008. B lymphocytes: how they develop and function. Blood. 112:1570–1580 10.1182/blood-2008-02-078071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loizou J.I., Sancho R., Kanu N., Bolland D.J., Yang F., Rada C., Corcoran A.E., Behrens A. 2011. ATMIN is required for maintenance of genomic stability and suppression of B cell lymphoma. Cancer Cell. 19:587–600 10.1016/j.ccr.2011.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin S., McManus S., Cobaleda C., Novatchkova M., Delogu A., Bouillet P., Strasser A., Busslinger M. 2010. Role of STAT5 in controlling cell survival and immunoglobulin gene recombination during pro-B cell development. Nat. Immunol. 11:171–179 10.1038/ni.1827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNees C.J., Conlan L.A., Tenis N., Heierhorst J. 2005. ASCIZ regulates lesion-specific Rad51 focus formation and apoptosis after methylating DNA damage. EMBO J. 24:2447–2457 10.1038/sj.emboj.7600704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P., Iacomini J., Johnson R.S., Herrup K., Tonegawa S., Papaioannou V.E. 1992. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 68:869–877 10.1016/0092-8674(92)90030-G [DOI] [PubMed] [Google Scholar]
- Muramatsu M., Kinoshita K., Fagarasan S., Yamada S., Shinkai Y., Honjo T. 2000. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 102:553–563 10.1016/S0092-8674(00)00078-7 [DOI] [PubMed] [Google Scholar]
- Oettinger M.A., Schatz D.G., Gorka C., Baltimore D. 1990. RAG-1 and RAG-2, adjacent genes that synergistically activate V(D)J recombination. Science. 248:1517–1523 10.1126/science.2360047 [DOI] [PubMed] [Google Scholar]
- Oka H., Sakai W., Sonoda E., Nakamura J., Asagoshi K., Wilson S.H., Kobayashi M., Yamamoto K., Heierhorst J., Takeda S., Taniguchi Y. 2008. DNA damage response protein ASCIZ links base excision repair with immunoglobulin gene conversion. Biochem. Biophys. Res. Commun. 371:225–229 10.1016/j.bbrc.2008.04.052 [DOI] [PubMed] [Google Scholar]
- Pear W.S., Nolan G.P., Scott M.L., Baltimore D. 1993. Production of high-titer helper-free retroviruses by transient transfection. Proc. Natl. Acad. Sci. USA. 90:8392–8396 10.1073/pnas.90.18.8392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peled J.U., Kuang F.L., Iglesias-Ussel M.D., Roa S., Kalis S.L., Goodman M.F., Scharff M.D. 2008. The biochemistry of somatic hypermutation. Annu. Rev. Immunol. 26:481–511 10.1146/annurev.immunol.26.021607.090236 [DOI] [PubMed] [Google Scholar]
- Phan T.G., Amesbury M., Gardam S., Crosbie J., Hasbold J., Hodgkin P.D., Basten A., Brink R. 2003. B cell receptor–independent stimuli trigger immunoglobulin (Ig) class switch recombination and production of IgG autoantibodies by anergic self-reactive B cells. J. Exp. Med. 197:845–860 10.1084/jem.20022144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthalakath H., Huang D.C., O’Reilly L.A., King S.M., Strasser A. 1999. The proapoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol. Cell. 3:287–296 10.1016/S1097-2765(00)80456-6 [DOI] [PubMed] [Google Scholar]
- Rapali P., Radnai L., Süveges D., Harmat V., Tölgyesi F., Wahlgren W.Y., Katona G., Nyitray L., Pál G. 2011. Directed evolution reveals the binding motif preference of the LC8/DYNLL hub protein and predicts large numbers of novel binders in the human proteome. PLoS ONE. 6:e18818 10.1371/journal.pone.0018818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickert R.C., Roes J., Rajewsky K. 1997. B lymphocyte-specific, Cre-mediated mutagenesis in mice. Nucleic Acids Res. 25:1317–1318 10.1093/nar/25.6.1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnyder T., Castello A., Feest C., Harwood N.E., Oellerich T., Urlaub H., Engelke M., Wienands J., Bruckbauer A., Batista F.D. 2011. B cell receptor-mediated antigen gathering requires ubiquitin ligase Cbl and adaptors Grb2 and Dok-3 to recruit dynein to the signaling microcluster. Immunity. 34:905–918 10.1016/j.immuni.2011.06.001 [DOI] [PubMed] [Google Scholar]
- Shinkai Y., Rathbun G., Lam K.P., Oltz E.M., Stewart V., Mendelsohn M., Charron J., Datta M., Young F., Stall A.M., et al. 1992. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 68:855–867 10.1016/0092-8674(92)90029-C [DOI] [PubMed] [Google Scholar]
- Siggs O.M., Arnold C.N., Huber C., Pirie E., Xia Y., Lin P., Nemazee D., Beutler B. 2011. The P4-type ATPase ATP11C is essential for B lymphopoiesis in adult bone marrow. Nat. Immunol. 12:434–440 10.1038/ni.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanopoulou E., Roman C.A., Corcoran L.M., Schlissel M.S., Silver D.P., Nemazee D., Nussenzweig M.C., Shinton S.A., Hardy R.R., Baltimore D. 1994. Functional immunoglobulin transgenes guide ordered B-cell differentiation in Rag-1-deficient mice. Genes Dev. 8:1030–1042 10.1101/gad.8.9.1030 [DOI] [PubMed] [Google Scholar]
- Staton T.L., Lazarevic V., Jones D.C., Lanser A.J., Takagi T., Ishii S., Glimcher L.H. 2011. Dampening of death pathways by schnurri-2 is essential for T-cell development. Nature. 472:105–109 10.1038/nature09848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavnezer J., Guikema J.E., Schrader C.E. 2008. Mechanism and regulation of class switch recombination. Annu. Rev. Immunol. 26:261–292 10.1146/annurev.immunol.26.021607.090248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A. 2005. The role of BH3-only proteins in the immune system. Nat. Rev. Immunol. 5:189–200 10.1038/nri1568 [DOI] [PubMed] [Google Scholar]
- Walkley C.R., Fero M.L., Chien W.M., Purton L.E., McArthur G.A. 2005. Negative cell-cycle regulators cooperatively control self-renewal and differentiation of haematopoietic stem cells. Nat. Cell Biol. 7:172–178 10.1038/ncb1214 [DOI] [PubMed] [Google Scholar]
- Yabas M., Teh C.E., Frankenreiter S., Lal D., Roots C.M., Whittle B., Andrews D.T., Zhang Y., Teoh N.C., Sprent J., et al. 2011. ATP11C is critical for the internalization of phosphatidylserine and differentiation of B lymphocytes. Nat. Immunol. 12:441–449 10.1038/ni.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young F., Ardman B., Shinkai Y., Lansford R., Blackwell T.K., Mendelsohn M., Rolink A., Melchers F., Alt F.W. 1994. Influence of immunoglobulin heavy- and light-chain expression on B-cell differentiation. Genes Dev. 8:1043–1057 10.1101/gad.8.9.1043 [DOI] [PubMed] [Google Scholar]
- Zhang C.C., Lodish H.F. 2004. Insulin-like growth factor 2 expressed in a novel fetal liver cell population is a growth factor for hematopoietic stem cells. Blood. 103:2513–2521 10.1182/blood-2003-08-2955 [DOI] [PubMed] [Google Scholar]
- Zhu Y., Swanson B.J., Wang M., Hildeman D.A., Schaefer B.C., Liu X., Suzuki H., Mihara K., Kappler J., Marrack P. 2004. Constitutive association of the proapoptotic protein Bim with Bcl-2-related proteins on mitochondria in T cells. Proc. Natl. Acad. Sci. USA. 101:7681–7686 10.1073/pnas.0402293101 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.