Abstract
Thalia democratica is a cosmopolitan tunicate belonging to the Thaliacea class. To further investigate the anatomy of this species, immunohistochemical labelling was performed using anti-tubulin and anti-serotonin antibodies on specimens collected in the Mediterranean Sea. The anti-tubulin antibody stained the cilia of the endostyle, the pericoronal bands and of the gill bar, enabling a detailed description of these structures. Moreover, immunolabelling of the nervous system showed the presence of eight pairs of nerve fibres emerging from the neural ganglion. Serotonergic cells were observed in the distal tract of the intestine, along the pericoronal bands, and in the placenta of gravid blastozooids, as well as in the neural ganglion. The presence of serotonin in the central nervous system has also been reported in the larvae of ascidians and may be linked to the planktonic life of these animals, a condition shared by adult thaliaceans and ascidian larvae. This work improves our knowledge of the anatomy of T. democratica and demonstrates the presence of a complex serotonergic system.
Key words: salp, serotonin, cerebral ganglion, protochordate.
Introduction
Thaliaceans are tunicates known for their complex life cycles and their ecological relevance. Nevertheless, the morphology of members of this class is not as well studied as that of other tunicates, such as ascidians, which are among the favourite organisms of developmental biologists.1,2
Thalia democratica is a cosmopolitan thaliacean that is found in all oceans, with the exclusion of the Polar Regions. It swims by rhythmic contractions of the muscle bands, which normally project water from the anterior to the posterior opening, providing oxygen and food to the transverse gill bar. Like other thaliaceans, this species has a complex metagenetic life cycle that consists of an alternating succession of sexually produced forms, the oozooids, to blastogenetically produced forms, the blastozooids. The oozoid is a barrel-shaped solitary form, carrying five muscular bands. It generates a ventral stolon, producing a chain of 25–30 small blastozooids. A single blastozooid, endowed with four muscular bands, breaks off from the stolon and swims free. As in other salps, the blastozooids are protogynous hermaphrodites: a single oocyte develops from the simple ovary joined to the atrial wall by a solid rod called the fertilization duct,3 through which only selected sperm reaches the egg.4 The zygote gives rise to the oozoid, which develops very close to the layers of syncythial maternal tissues in order to build a placenta.5 The salps are the only tunicates that lack a real larval stage. After the oozoid is released into the seawater along with the placenta, the testis ripens and sperm cells are released.5
The embryonic development of the central nervous system of T. democratica has been described from electron microscope reconstructions.6 During development, the central nervous system passes through a neural tube stage, very similar to that of larvae of other tunicates. Briefly, an early dorsal mass of neurons with an open central canal (tube phase) becomes enriched with a thick mantle of neuroblasts. Afterwards, the neural tube shortens and the central canal disappears because it appears to be filled by the neurites originating from the surrounding neurons (ganglion phase). The nerves coming from the ganglion towards the periphery appear to originate from three paired clusters of cells with large cell bodies (C1, C2 and C3 from the anterior to the posterior). These clusters are located in the equatorial plane of the ganglion and three pairs of nerves directed anteriorly from C1 in addition to two nerves directed laterally and one posteriorly from C2 have been identified. The fibres emerging from the more posterior dorsal cluster C3 are directed posteriorly. Similar to other thaliacea, the salps have ciliarich organs that are the main components of the filter-feeding apparatus, such as the transverse gill bar and the endostyle. Bone6,7 accurately described the different regions of the T. democratica endostyle: at the bottom, ciliated zones and ciliary fences are located between glandular cells and produce an obliquely forward current; in a more external position, the columnar cilia beat upwards to the top of the endostyle.
In this work, the morphology of the nervous system and ciliary apparatus of T. democratica was further investigated by immunolabelling techniques using anti-tubulin and anti-serotonin antibodies to show the three-dimensional pathway of the peripheral nerves emerging from the cell clusters of the ganglion and to compare it with patterns of brain organization in other chordates.
Material and Methods
Animals
Blastozooids and oozoids of T. democratica were collected near the coast of Talamone (Italy) in September 2008 with a plankton net (mesh size of 500 µm) during the reproductive period of this species. The samples were sorted under a stereo microscope by means of a glass pipette, rinsed in Millipore-filtered sea-water (MFSW), fixed in 4% paraformaldehyde in 0.1 M PBS pH 7.4 for 1 h at room temperature and stored in 70% ethanol at −20 °C.
Immunohistochemistry
After rehydration, fixed samples were rinsed with 0.1 M PBS and were processed for immunolocalization experiments according to the method described by Pennati et al.22 with a few modifications. Whole-mount individuals were washed in PBT (PBS plus 0.25% Triton - X and 0.1% Tween 20) twice for 10 min and incubated for 2 h with 50% heat-inactivated normal goat serum (NGS) in PBT. Then, they were incubated at 4°C overnight with rabbit anti-5-hydroxytryptamine antibody (Medak), diluted 1:400 in PBS/ NGS (1:1), with monoclonal anti-β-tubulin antibody (clone 2-28-33; Sigma, Milano, Italy) diluted 1:200 in PBS/NGS (1:1) or with monoclonal anti-acetylated α-tubulin antibody (clone 6-11B-1, Sigma). The samples were rinsed several times in PBT (PBS plus 0.2% Tween-20), incubated for 2 h in 1% BSA in PBT and then incubated overnight with a solution containing the secondary antibody diluted at 1:500 in PBT and TRITC-conjugated Phalloidin. The anti-5-HT antibody was detected by Alexa Fluor 488 anti-rabbit IgG secondary antibody, and anti-β-tubulin and anti-acetylated tubulin antibodies were detected by the Alexa Fluor 488 anti-mouse IgG secondary antibody. Specimens were mounted in 1,4-diazabicyclo [2,2,2] octane (DABCO, Sigma) plus MOWIOL (Sigma) on microscope slides. The negative control samples were processed without incubation in primary antibodies. No detectable fluorescence was exhibited by the specimens in the control experiments. The samples were examined using a Leica TCS-NT confocal laser scanning microscope (Leica Microsystems, Heidelberg, Germany) equipped with an argon/krypton (75 mW) multiline laser.
Results
Morphology
Both oozooids and blastozooids of T. democratica were present in the plankton collected. This species is easily recognizable by the violet colour of the tunic and the visceral nucleus, which is characteristic of living animals and disappears after fixation. Barrel-like oozoid specimens presented a ventral stolon with small immature blastozooids (Figure 1A). Actin staining by TRITC-conjugated phalloidin revealed five muscular bands (Figure 1B). Free blastozooids were identifiable by four muscular bands (Figure 1 C,D). They carried embryos at different developmental stages (Figure 1 E,F).
Figure 1.
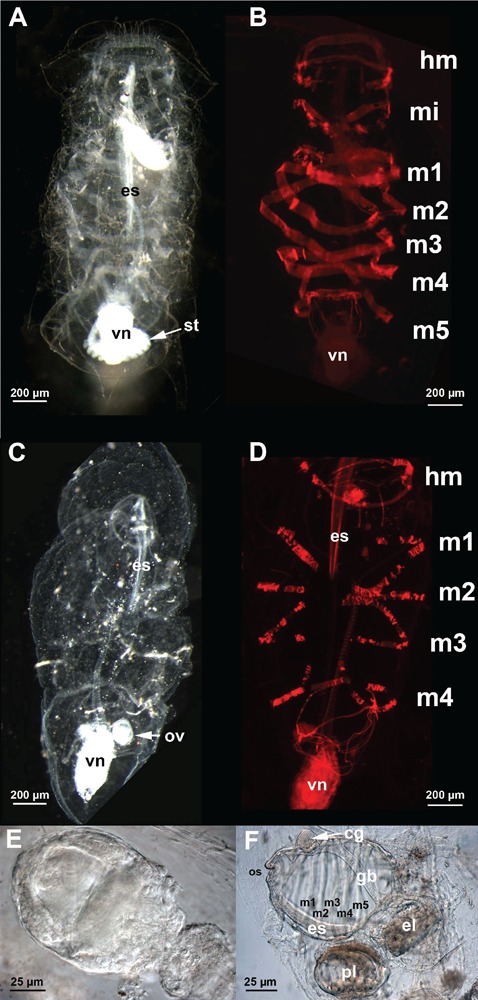
Whole-mount specimens of Thalia democratica. A,B) oozoids; C,D) blastozooids; E,F) embryos; A,C) dark-field light microscope images; B,D) fluorescent microscope images of specimens labelled with TRITC-conjugated phalloidin; E,F) light microscope images. cg, cerebral ganglion; el, eleoblast; es, endostyle; m1–m5, muscle bands; ov, ovary; pl, placenta; st, stolon; vn, visceral nucleus.
Tubulin immunolocalization
Whole-mount specimens of T. democratica were labelled with an anti-β-tubulin antibody; subsequent observations by confocal laser microscopy showed a strong signal present in the cilia in many structures of the filter feeding apparatus (Figure 2 A,B). In particular, the gill bar, endostyle and pericoronal bands, which are two lateral structures that run anteriorly from the ventral endostyle to the dorsal gill bar, were intensely marked cilia-rich organs (Figure 2 B–E). The anti-acetylated tubulin antibody marked the microtubules present in long projections more specifically, which started from the epidermis and passed through the tunic. These projections were very abundant, regularly distributed along the entire body and were up to 500 µm long (Figure 2 F,G). Acetylated tubulin staining appeared discontinue, probably indicating that microtubules were in course of stabilization when the samples were fixed. In fact, acetylation is generally considered to occur on stable microtuble assemblies.8
Figure 2.
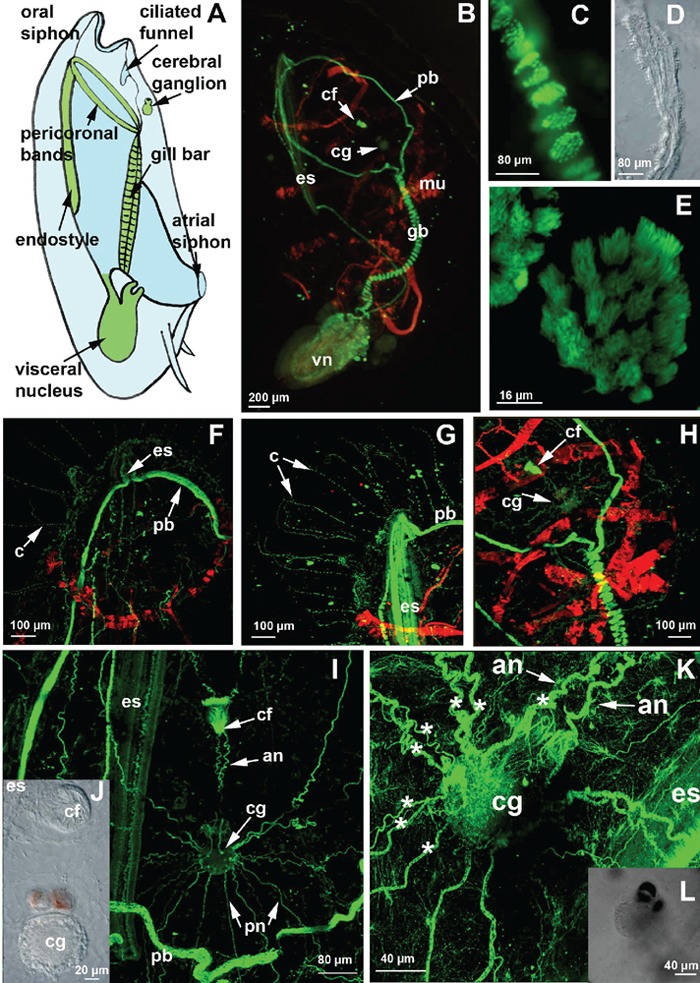
Whole-mount labelling by FITC-conjugated anti-tubulin antibodies and by TRITC-conjugated phalloidin. A) schematic drawing of the lateral view of a blastozooid (redrawn after 4); B) whole-mount specimen observed using a fluorescent microscope; C,E) confocal laser microscope (CLM) images of the ciliary apparatus of the gill bar at different magnifications, which were obtained by the sum of 30 and 20 optical sections, step size of 1 µm. D) transmission microscope image of the gill bar; F,G) CLM images of the anterior end of a specimen immunolabelled with an anti-acetylated tubulin antibody, showing the long cilia passing through the tunic; H,I,K) CLM images at different magnifications of the cerebral ganglion labelled with anti-β-tubulin antibody; K) CLM image of the cerebral ganglion in which the 8 main fibres of the left side are indicated by asterisks; J) details using light microscopy with Normasky optics of the cerebral ganglion and of the ciliated funnel; L) transmission microscope image of the samples observed in K at a lower magnification. an, anterior nerves; cf, ciliated funnel; cg, cerebral ganglion; c, cilia; cf, ciliated funnel; es, endostyle; gb, gill bar; mu, muscle band; pb, pericoronal band; pn, posterior nerves; vn, visceral nucleus.
The cerebral ganglion was intensely marked by the anti-β-tubulin antibody. It is located in the anterior part of the body, dorsal to the pericoronal arches (Figure 2H). This pear-shaped organ is characterized by a narrowing in its anterior portion that divides it into two unequal regions: a small anterior region carrying the pigmented spots and a larger posterior region (Figure 2 I,J).
Eight pairs of fibres and several minor ones were identified (Figure 2K). The two most anterior fibres connected the visceral ganglion to the ciliated funnel, which is a cilia-rich organ with an as yet unknown function (Figure 2 I,K).
Serotonin (5-HT) immunolocalization
In the whole mount oozooids incubated with an anti-serotonin antibody, a positive signal was present in the cells located in the pericoronal bands, in the proximal tract of the intestine and in the cerebral ganglion (Figure 3A). Serotonin-positive cells were numerous (more than 50) in the pericoronal bands and were regularly positioned to form two almost uninterrupted rows (Figure 3 B,C).
Figure 3.
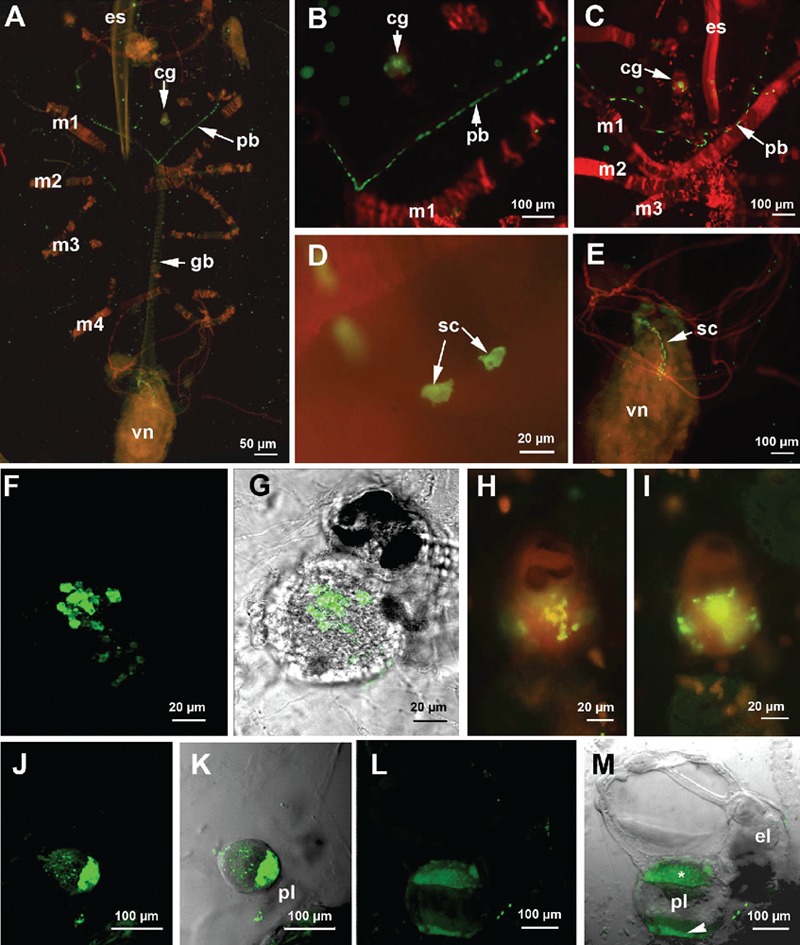
Immunolocalizaton of serotonin revealed by the FITC-conjugated antibody. A–E and H,I) samples were counterstained with TRITC-conjugated phalloidin; A) whole-mount specimen observed by fluorescent microscopy. Serotonin is present in the cerebral ganglion, pericoronal bands and visceral nucleus; B–D) details of the serotonin-positive cells in the pericoronal bands; C,D) Confocal laser microscope (CLM) images; E) magnification of a detail in A showing the arrangement of serotonin-positive cells in the first tract of the digestive apparatus; F–I) serotonin localization in the cerebral ganglion; G) CLM image obtained by the sum of 54 optical sections, step size of 1 µm; F) superimposition of G with a transmission microscope image. H,I) fluorescent microscope images at different focal depths showing the presence of serotonin-positive cells mainly in the core region of the cerebral ganglion; J) CLM image of an early embryo obtained by the sum of 38 optical sections (step size of 2 µm) showing an intense signal in the region adjacent to the placenta; K) superimposition of J with a transmission microscope image; L) CLM image of a late embryo obtained by the sum of 44 optical sections, step size of 2 µm. m, superimposition of L with a transmission microscope image. Serotonin is present in the region contacting the placenta (asterisk) and in the placenta itself (arrowhead). cg, cerebral ganglion; el, eleoblast; es, endostyle; gb, gill bar; m1–m5, muscle bands; pb, pericoronal bands; pl, placenta; sc, serotonergic cells; vn, visceral nucleus.
In addition, 5-HT immunoreactivity was localized in the first tract of the intestine in several scattered cells with an irregular shape (Figure 3 D,E). Serotonin was present in numerous pericaria localized in the posterior region of the cerebral ganglion (Figure 3 F–I). Optical sections taken by confocal laser microscopy indicated that serotonin-positive cellular bodies were particularly abundant in the deeper layer of the ganglion. Moreover, a 5-HT signal was present in the placenta of blastozooids that were carrying embryos (Figure 3 J–M). We analysed two developmental stages: an early stage corresponding to a segmentation stage (Figure 3 J,K) and a late stage in which the organs of the embryos were completely differentiated (Figure 3 L,M). The placenta showed strong immunoreactivity against serotonin in both cases. In the latter embryo, the anti-serotonin antibody signal appeared to be localized in two distinct regions: one closer to the developing embryo and the other closer to the maternal tissues (Figure 3M).
Discussion
An antibody against acetylated α-tubulin was used to stain the ciliary apparatus of oozoides of T. democratica. This type of antibody has been widely used in ascidian larvae, mainly to stain the cilia.9,10 We observed very long and numerous projections crossing the tunic of the animals all around the body. Although TEM observations are necessary to define the structure of these projections, they were reminiscent of the long cilia of epidermal sensory cells of the trunk and tail of ascidian larvae.
In Ciona intestinalis larvae, cell bodies of epidermal sensory neurons are found in pairs in the mid-dorsal and mid-ventral tail epithelium, and extend regularly spaced cilia into the tunic fin. Similar cilia are found in other ascidian species, including Diplosoma macdonaldi and H. roretzi.9–13 It has been proposed that the cilia of epidermal sensory neurons play a role in the perception of mechanical or chemical stimuli.9,12,14,15
In resemblance with ascidian larvae, it is possible that the tubulin-rich projections observed in T. democratica are cilia of mechanosensory cells. They could be an adaptation to the planktonic lifestyle shared by adult thaliaceans and ascidian larvae. The tunic of thaliaceans is different from that of ascidians; it has a smaller number of tunic cells and is covered by a cuticle layer decorated with minute cuticular protrusions.16 The presence of this cuticle may be the reason for why sensory cells are so numerous in these animals. In fact, it is of fundamental importance for a planktonic animal encased in a cuticular envelop to be able to sense the environment by means of sensory cilia. Cilia were clearly seen in the ciliated funnel by both interference microscopy and anti-β-tubulin immunostaining. Moreover, the anti-β-tubulin antibody stained several nerves joining the cerebral ganglion to the funnel. According to Lacalli and Holland,6 the cells of the duct combine cells of both pharyngeal and neural tube origin, resembling the situation of the ascidian neural gland. Bassam and Postlethwait17 showed that the homologs of the Pitx and Six 3/6 genes, which are important for vertebrate pituitary placodes, are expressed in the primordial ciliary funnel of the larvacean Oikopleura dioica. Therefore, the ciliated funnel of T. democratica could be considered a chemosensory structure important for collecting olfactory information from the environment, eliciting specific behavioural responses in the planktonic organisms.
The distribution of the serotonergic cells has been reported in adults and larvae of several solitary ascidian species18–23 and in colonial ascidians.24 In all of the swimming larvae examined, serotonin was present in a few cells of the central nervous system, in the primary neurons of adhesive papillae and in some epidermal neurons of the tail. It has been suggested that serotonin is essential in ascidian larvae during the signalling cascade that triggers metamorphosis,25 and serotonin is also known to stimulate metamorphosis in the larvae of many animal phyla.26–28 Serotonin is not present in the nervous ganglion in juveniles or adult ascidians, in which larval sensory organs are completely lost after metamorphosis, but it is abundant in the digestive system. In the thaliacean Doliolum nationalis, serotonin was found to immunolocalize in the dorsal ganglion, the ciliated funnel and the intestinal tract.29 The presence of serotonin in the ganglion of adult thaliaceans is similar to that in ascidian larvae. Interestingly, both adult thaliaceans and ascidian larvae have a common pelagic life. T. democratica oozooids can be considered as directly developing juveniles that lead a planktonic life without the need to choose a substratum on which to adhere and begin metamorphosis. Serotonin is known to modulate circadian rhythms in different organisms.30 In particular, many planktonic species accomplish vertical and circadian migrations that are directly controlled by light stimuli. Therefore, the presence of serotonin in the central nervous system may be required for orientation and muscle coordination in directional locomotion. It will be of interest to investigate whether or not the contraction of circular muscle bands in thaliacea is controlled by serotonergic neurons. In fact, serotonergic neurons project towards motor neurons and have functions in the coordination and modulation of locomotion in a variety of taxa, such as mollusks31,32 and hirudinean annelids.33
Notably, strong 5-HT immunoreactivity was shown in T. democratica by the placental cells. The mature placenta of the pelagic tunicate Salpa fusiformis is formed by two syncytial layers, intimately connected by interdigitating microvilli, that separate maternal and embryonic circulations.34 The placenta shows two distinct regions of serotonin localization in late embryos of T. democratica: one area is close to the embryo whilst the other is closer to the maternal tissue, and these two regions are separated by a central area in which no signal has been found. The presence of serotonin and serotonin receptors has been reported in the human placenta, in which they are thought to play a role not only in placental development and pregnancy maintenance, but also in regulating foetal development.35 It is well known that among diverse physiological functions, serotonin can act as a regulator of cell growth in a variety of cell types, including mammalian placental cells.36 We suggest that 5-HT has a conserved role in the development of chordates.
It is interesting to compare the organization of the cerebral ganglion of the salps with the central nervous system (CNS) of other chordates. The common ground plan of the vertebrate embryonic CSN is characterized by a tripartite organization, consisting of the anterior forebrain, central midbrain and posterior hind-brain expressing the Otx, Pax2/5/8 and Hox genes, respectively. Comparative studies in ascidian larvae showed a homologue tripartite organization. In the larvae of Ciona intestinalis, one of the most studied ascidian species, the anterior part of the CNS, where the main sensory organs are located, consists of a sensory vesicle expressing Otx. This region is considered to be homologous to the forebrain of vertebrates. Posterior to this region, the visceral ganglion expressing Hox and hosting the motor neurons that innervate the tail is considered to be homologous to the vertebrate hindbrain. These two regions are separated by an intervening gap, in which Pax2/5/8 is expressed.37 This is the so-called neck region, which is considered the mid-hindbrain boundary equivalent.38
Based on our results, we developed a hypothetical structural relationship of the T. demo-cratica ganglion in comparison to that of the ascidian larva. The anterior zone containing the photoreceptor organ is comparable to the sensory vesicle of ascidian larvae in which an ocellus is usually present and, in turn, to the vertebrate forebrain. There is no evidence of a neck region in T. democratica, but the middle zone containing the two motor neuron clusters C1 and C2 could be equivalent to the visceral ganglion of the ascidian larvae in which the motor neurons are located, which, in turn, has the vertebrate hindbrain as a counterpart. This homology is further supported by the presence of serotonergic neurons in this region. In ascidian larvae, the serotonergic neurons are located in the ventral part of the anterior visceral ganglion, as evidenced by the expression of the gene Ci-Tph, the rate limiting synthetic enzyme of serotonin.22 Finding the presence of serotonin in the middle zone of the T. democratica cerebral ganglion is one of the main results of this paper, while it is known that the main serotonergic neurons in the vertebrate CNS are the raphe nuclei of the hindbrain.
Finally, the posterior region around the C3 cluster of the T. democratica cerebral ganglion that contains fibres directed towards the posterior muscular bands, could tentatively be considered equivalent to the ascidian posterior nerve cord. Future gene expression analysis would be required to corroborate these homologies.
Acknowledgements:
the confocal microscopy images were obtained using the facilities of C.I.M.A. (Advanced Microscopy Center of the University of Milano). The authors would thank Dr. Umberto Fascio for the confocal microscopy images and Mr. Tommaso Cattaneo for collecting and sorting the samples. This work was supported by grants from the MIUR (PRIN 2006058952 to F.D.) and by the University of Milano.
References
- 1.Satoh N. The ascidian tadpole larva: comparative molecular development and genomics. Nature Rev Genetics. 2003;4:285–95. doi: 10.1038/nrg1042. [DOI] [PubMed] [Google Scholar]
- 2.Lemaire P, Smith WC, Nishida H. Ascidians and the plasticity of the chordate developmental program. Curr Biol. 2008;18:R620–31. doi: 10.1016/j.cub.2008.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holland LZ, Miller RL. Mechanism of internal fertilization in Pegea socia (Tunicata Thaliacea), a salp with a solid oviduct. J Morphol. 1994;219:257–67. doi: 10.1002/jmor.1052190305. [DOI] [PubMed] [Google Scholar]
- 4.Boldrin F, Martinucci G, Holland LZ, Miller RL, Burighel P. Internal fertilization in the salp Thalia democratica. Can J Zool. 2009;87:928–40. [Google Scholar]
- 5.Bone Q, Carre C, Chang P. Tunicate feeding filters. J Mar Biol Assoc UK. 2003;83:907–19. [Google Scholar]
- 6.Lacalli TC, Holland LZ. The developing dorsal ganglion of the salp Thalia democratica, and the nature of the ancestral chordate brain. Phil Trans R Soc Lond B. 1998;353:1943–67. [Google Scholar]
- 7.Bone Q, Carre C, Ryan KP. The endostyle and the feeding filter in salps (Tunicata) J Mar Biol Assoc UK. 2000;80:523–34. [Google Scholar]
- 8.Janke C, Kneussel M. Tubulin post-translational modifications: encoding functions on the neuronal microtubule cytoskeleton. Trends Neurosci. 2010;33:362–372. doi: 10.1016/j.tins.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Pasini A, Amiel A, Rothbacher U, Roure A, Lemaire P, Darra S. Formation of the ascidian epidermal sensory neurons: insights into the origin of the chordate peripheral nervous system. PLoS Biol. 2006;4:e225–e225. doi: 10.1371/journal.pbio.0040225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konno A, Kaizu M, Hotta K, Horie T, Sasakura Y, Ikeo K, et al. Distribution and structural diversity of cilia in tadpole larvae of the ascidian Ciona intestinalis. Dev Biol. 2010;337:42–62. doi: 10.1016/j.ydbio.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 11.Terakubo HQ, Nakajima Y, Sasakura Y, Horie T, Konno A, Takahashi H, et al. Network structure of projections extending from peripheral neurons in the tunic of ascidian larva. Dev Dynam. 2010;239:2278–87. doi: 10.1002/dvdy.22361. [DOI] [PubMed] [Google Scholar]
- 12.Torrence SA, Cloney RA. Nervous system of ascidian larvae: caudal primari sensory neurons. Zoomorphology. 1982;99:103–15. [Google Scholar]
- 13.Crowther RJ, Whittaker JR. Serial repetition of cilia pairs along the tail surface of an ascidian larva. J Exp Zool. 1994;268:9–16. doi: 10.1002/jez.1402680103. [DOI] [PubMed] [Google Scholar]
- 14.Imai JH, Meinertzhagen IA. Neurons of the ascidian larval nervous system in Ciona intestinalis: II. Peripheral nervous system. J Comp Neurol. 2007;501:335–52. doi: 10.1002/cne.21247. [DOI] [PubMed] [Google Scholar]
- 15.Caicci F, Zaniolo G, Burighel P, Degasperi V, Gasparini F, Manni L. Differentiation of papillae and rostral sensory neurons in the larva of the ascidian Botryllus schlosseri (Tunicata) J Comp Neurol. 2009;518:547–66. doi: 10.1002/cne.22222. [DOI] [PubMed] [Google Scholar]
- 16.Hirose E, Kimura S, Itoh T. Tunic morphology and cellulosic components of pyrosomas, doliolids, and salps (Thaliacea, Urochordata) Biol Bull. 1999;196:113–20. doi: 10.2307/1543173. [DOI] [PubMed] [Google Scholar]
- 17.Bassham S, Postlethwait JH. The evolutionary history of placodes: a molecular genetic investigation of the larvacean urochordate Oikopleura dioica. Development. 2005;132:4259–72. doi: 10.1242/dev.01973. [DOI] [PubMed] [Google Scholar]
- 18.Pestarino M. Occurrence of different secretin-like cells in the digestive tract of the ascidian Styela plicata (Urochordata, Ascidiacea) Cell Tissue Res. 1982;226:231–5. doi: 10.1007/BF00217097. [DOI] [PubMed] [Google Scholar]
- 19.Georges D. Presence of cells resembling serotonergic elements in four species of tunicates. Cell Tissue Res. 1985;242:341–8. [Google Scholar]
- 20.Buznikov GA, Lambert HW, Lauder JJ. Serotonin and serotonin-like substances as regulators of early embryogenesis and morphogenesis. Cell Tissue Res. 2001;305:177–86. doi: 10.1007/s004410100408. [DOI] [PubMed] [Google Scholar]
- 21.Pennati R, Groppelli S, Sotgia C, Candiani S, Pestarino M, De Bernardi F. Serotonin localization in Phallusia mammillata larvae and effect of 5-HT antagonists during larval development. Develop Growth Differ. 2001;43:647–56. doi: 10.1046/j.1440-169x.2001.00608.x. [DOI] [PubMed] [Google Scholar]
- 22.Pennati R, Zega G, Groppelli S, De Bernardi F. Immunohistochemical analysis of the adhesive papillae of Botrylloides leachi (Chordata: Tunicata Ascidiacea): implications for their sensory function. Ital J Zool. 2007;74:1–5. [Google Scholar]
- 23.Pennati R, Groppelli S, Mastrototaro F, De Bernardi F, Zega G. Immunohistochemical analysis of the adhesive papillae of Clavelina lepadiformis (Müller, 1776) and Clavelina phlegraea (Salfi, 1929) (Tunicata, Ascidiacea) Eur J Histochem. 2009;53:25–34. doi: 10.4081/ejh.2009.25. [DOI] [PubMed] [Google Scholar]
- 24.Tiozzo S, Murray M, Degnan BM, De Tomaso AW, Croll RP. Development of the neuromuscular system during asexual propagation in an invertebrate Chordate. Devel Dyn. 2009;238:2081–94. doi: 10.1002/dvdy.22023. [DOI] [PubMed] [Google Scholar]
- 25.Zega G, Pennati R, Groppelli S, Sotgia C, De Bernardi F. Dopamine and serotonin modulate the onset of metamorphosis in the ascidian Phallusia mammillata. Dev Biol. 2005;282:246–56. doi: 10.1016/j.ydbio.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Couper JM, Leise EM. Serotonin injections induce metamorphosis in larvae of the gastropod mollusc Ilyassana obsoleta. Biol Bull. 1996;191:178–86. doi: 10.2307/1542921. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto H, Shimizu K, Tachibana A, Fusetani N. Roles of dopamine and serotonin in larval attachment of the barnacle Balanus amphitrite. J Exp Zool. 1999;284:746–58. doi: 10.1002/(sici)1097-010x(19991201)284:7<746::aid-jez4>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 28.Zega G, Pennati R, Fanzago A, De Bernardi F. Serotonin involvement in the metamorphosis of the hydroid Eudendrium racemosum. Int J Dev Biol. 2007;51:307–13. doi: 10.1387/ijdb.062195gz. [DOI] [PubMed] [Google Scholar]
- 29.Stach T. Comparison of the serotonergic nervous system among Tunicata: implications for its evolution within Chordata. Org Divers Evol. 2005;5:15–24. [Google Scholar]
- 30.Yuan Q, Lin FJ, Zheng XZ. Serotonin modulates circadian entrainment in Drosophila. Neuron. 2005;47:115–27. doi: 10.1016/j.neuron.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 31.Norekian TP, Satterlie RA. Serotonergic neural system not only activates swimming but also inhibits competing neural centres in a pteropod mollusc. Am Zool. 2001;41:993–1000. [Google Scholar]
- 32.Yu B, Gamkrelidze GN, Laurienti PJ. Serotonin directly increases a calcium current in swim motoneurons of Aplysia brasiliana. Am Zool. 2001;41:1009–25. [Google Scholar]
- 33.Nusbaum MP, Kristan WB. Swim initiation in the leech by serotonin-containing interneurons, cell-21 and cell-61. J Exp Biol. 1986;122:277–302. doi: 10.1242/jeb.122.1.277. [DOI] [PubMed] [Google Scholar]
- 34.Bone Q, Pulsford AL, Amoroso EC. The placenta of the salp. Placenta. 1985;6:53–64. doi: 10.1016/s0143-4004(85)80032-1. [DOI] [PubMed] [Google Scholar]
- 35.Huang WQ, Zhang CL, Di XY, Zhang RQ. Studies on the localization of 5-hydroxytryptamine and its receptors in human placenta. Placenta. 1998;19:655–61. doi: 10.1016/s0143-4004(98)90027-3. [DOI] [PubMed] [Google Scholar]
- 36.Sonier BA, Lavigne CA, Arseneault M, Ouellette R, Vaillancourt C. Expression of the 5-HT2A serotoninergic receptor in human placenta and choriocarcinoma cells: mitogenic implications of serotonin. Placenta. 2005;6:484–90. doi: 10.1016/j.placenta.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 37.Wada H, Saiga H, Satoh N, Holland PWH. Tripartite organization of the ancestral chordate brain and the antiquity of placodes: insights from ascidian Pax-2/5/8, Hox and Otx genes. Development. 1998;125:1113–22. doi: 10.1242/dev.125.6.1113. [DOI] [PubMed] [Google Scholar]
- 38.Imai KS, Satoh N, Satou Y. Region specific gene expressions in the central nervous system of the ascidian embryo. Mech Dev. 2002;119:S275–7. doi: 10.1016/s0925-4773(03)00128-x. [DOI] [PubMed] [Google Scholar]


