Abstract
The objective of this study was to investigate the involvement of the insulin-like growth factor (IGF) system in the developing mandibular condylar cartilage and temporomandibular joint (TMJ). Fetal mice at embryonic day (E) 13.0–18.5 were used for in situ hybridization studies using [35S]-labeled RNA probes for IGF-I, IGF-II, IGF-I receptor (-IR), and IGF binding proteins (-BPs). At E13.0, IGF-I and IGF-II mRNA were expressed in the mesenchyme around the mandibular bone, but IGF-IR mRNA was not expressed within the bone. At E14.0, IGF-I and IGF-I mRNA were expressed in the outer layer of the condylar anlage, and IGF-IR mRNA was first detected within the condylar anlage, suggesting that the presence of IGF-IR mRNA in an IGF-rich environment triggers the initial formation of the condylar cartilage. IGFBP-4 mRNA was expressed in the anlagen of the articular disc and lower joint cavity from E15.0 to 18.5. When the upper joint cavity was formed at E18.5, IGFBP-4 mRNA expression was reduced in the fibrous mesenchymal tissue facing the upper joint cavity. Enhanced IGFBP-2 mRNA expression was first recognized in the anlagen of both the articular disc and lower joint cavity at E16.0 and continued expression in these tissues as well as in the fibrous mesenchymal tissue facing the upper joint cavity was observed at E18.5. IGFBP-5 mRNA was continuously expressed in the outer layer of the perichondrium/fibrous cell layer in the developing mandibular condyle. These findings suggest that the IGF system is involved in the formation of the condylar cartilage as well as in the TMJ.
Key words: IGF, IGF binding protein, mandibular condylar cartilage, temporomandibular joint.
Introduction
The temporomandibular joint (TMJ) is distinguished from other joints because it is involved in growth of the jaw as well as mastication. Structural features of TMJ are well studied in the clinical field of oral surgery.1–3 The mandibular condyle is a member of TMJ and composed of cartilage and fibrous layers covering the surface of the joint.4 The condylar cartilage also works as a growth cartilage in mandible, and development and growth of this cartilage is important in the clinical field of orthodontics.4 Embryologically, the mandibular condylar cartilage is the principal secondary cartilage and differs somewhat from primary cartilage skeleton.5–7 The condylar cartilage derives from alkaline phosphatase (ALP)-positive, type I collagen mRNA-expressing progenitor cells continuous with the ossifying mandible8–12 and progenitor cells of this cartilage (skeletoblasts)13 rapidly differentiate into hypertrophic chondrocytes.8,10–13 The TMJ, comprising the condylar cartilage, articular disc, and upper and lower joint cavities, is morphologically identified by embryonic day (E) 18.5.14
We previously described several transcription factors involved in bone and cartilage formation, including Runx2, Osterix, and Sox9, which regulate the formation of the condylar cartilage.15,16 In addition, several growth factors and their receptors, such as transforming growth factors,17,18 bone morphogenetic proteins,12 fibroblast growth factors,19,20 and insulin-like growth factors (IGFs)20–29 are also involved in the formation/growth of the mandibular condylar cartilage. IGFs are polypeptide growth factors that control pre- and postnatal development and growth in various tissues via the endocrine, paracrine, and autocrine systems. The IGF ligands IGF-I and IGF-II are involved in cell differentiation, proliferation, morphogenesis, growth, and control of metabolic functions. IGF-I is a somatomedin, a growth hormone mediator that stimulates the synthesis of IGF-I by negative feedback mechanisms. IGF-I is synthesized mainly in the liver, but it is also produced locally in various tissues.30–32 Although there is a 76% homology between the IGF-I and IGF-II peptides, IGF-II is thought to have an important role mainly during the fetal period. Furthermore, IGFs bind to specific receptors, but the tyrosine kinase receptor IGF-I-receptor (IGF-IR) mediates most of the actions of IGF.30–32 Studies using immunohistologic and molecular biologic techniques confirmed that IGF-I and IGF-II are expressed in the upper layer of the condylar cartilage in young rat21,22 and in aged mice.23 The IGF-I gene is highly expressed in the perichondrium fraction of the condylar cartilage in neonatal mice.24 In organ cultures of the mandibular condyle, exogenous IGF-I significantly increases the uptake of 3H-thymidine and 35S-sulfate by the condylar cartilage.25,26 In vivo experiments revealed that local administration of IGF-I into the articular cavity of the TMJ accelerates endochondral bone growth of the mandibular condyle,27 and IGF-I is also involved in chondrocyte apoptosis in the condylar cartilage.28 In the condylar cartilage of young rats fitted with a mandibular propulsive appliance, IGF-I mRNA expression is increased,21 indicating that IGF-I mediates the effects of mechanical stimulation to promote local growth of the mandibular condyle. Furthermore, IGF-IR immunostaining is also observed in the upper layer of condylar cartilage in young rat20,29 and in aged mice.23 The IGF-IR gene is highly expressed in the cartilage fraction in neonatal mice.24 Together, these findings indicate that both exogenous and endogenous IGFs affect the growth and development of the condylar cartilage during the postnatal period. The involvement of IGFs and the IGF-IR in the initial formation of the condylar cartilage, however, has not been investigated. Meanwhile, IGF-I and IGF-II in the serum and other extracellular environments are bound to IGF binding proteins (IGFBPs), which modulate the endocrine actions of IGFs by inhibiting or potentiating the bioavailability of IGFs for binding their receptors. Additionally, some IGFBPs have IGF-independent actions.33 In the cartilage tissue, although IGFBP expression is well studied in the growth plates of long bones,34 only a few studies have been performed in the mandibular condylar cartilage of aged mice,23 or in young rats,35 and none have been performed in fetuses. These results made us hypothesize that the IGF system including IGFs, IGF-IR, and IGFBPs is also involved in the initial formation of the condylar cartilage. Since we have established the experimental system analyzing initial formation process of mouse condylar cartilage,8,10,11,15 we performed the present in situ hybridization study using this system.
Materials and Methods
All animals were housed in facilities approved by the Health Sciences University of Hokkaido. Our animal-use protocol conformed to the Institutional Administrator's Manual for Laboratory Animal Care and Use (NIH publication no. 88–2959) and was reviewed and approved by the Screening Committee for Animal Research of the Health Sciences University of Hokkaido.
Tissue preparation
A total of 10 pregnant ICR mice, at E13.0–18.5 (08:00 am on the day of the vaginal plug was designated as E0), were used for this study. At each time point, the pregnant mice were killed by cervical dislocation under ether anesthesia, after which each fetal mouse was killed by cervical dislocation. The heads were then removed and immersed in 4% paraformaldehyde (0.1 M phosphate buffer, pH 7.4) for 1 d at 4°C. The specimens were embedded in paraffin using standard procedures. Sections (5 µm) were cut in the coronal plane, perpendicular to the sagittal plane, and parallel to the long axis of the condylar or angular process of the mandible. Sections were stained with 0.1% toluidine blue (0.1 M phosphate buffer, pH 7.4) for histologic observations.
In situ hybridization
RNA probes for IGF-I, IGF-II, and IGF-IR, and IGFBP-2, IGFBP-3, IGFBP-4, IGFBP-5, and IGFBP-6, were used in previous studies.36,37 IGBP-1 mRNA is expressed only in the liver,36 therefore it was excluded in the present study. Probes were labeled with [35S]-UTP, as previously described.15,16 Sections were dipped in emulsion (NTB, Kodak, Rochester, NY, USA) after hybridization and RNAase treatment, and then exposed for 1 wk at 4°C for autoradiography. Sense probes were used as negative controls. Although we did not accept some quantitative analyzes, we examined three different samples for each embryonic day to confirm the consistency of results obtained.
Results
In situ hybridization for IGF-I, IGF-II and IGF-IR mRNA in developing condylar cartilage
At E13.0, mandibular bone (MB) was clearly formed lateral to the Meckel's cartilage (MC), and the developing masseter muscle (MM) was recognized lateral to the mandibular bone (Figure 1a). IGF-I mRNA was expressed in the mesenchymal tissue around mandibular bone (Figure 1b, arrows), in the MM (Figure 1b), and in the perichondrium of Meckel's cartilage (Figure 1b, arrowheads), but not in the mandibular bone and Meckel's cartilage. IGF-II mRNA showed a similar expression pattern to that of IGF-I (Figure 1c). IGF-IR mRNA was strongly expressed in the masseter muscle (MM), but not in the other areas at this stage (Figure 1d). At E14.0, the anlage of the future condylar process (termed the condylar anlage), consisting of a mesenchymal cell condensation, was clearly identifiable in the posterior position of the ossifying mandible (Figure 1e, arrow), as previously descri bed.8,10,11,15 Matrix metachromasia, indicative of cartilage formation, was not observed in the anlage at this stage (Figure 1e). IGF-I mRNA was expressed in the MM (Figure 1f), in the periosteum of mandibular bone (Figure 1f, arrowhead), and in the outer region of the condylar anlage continuous with the periosteum (Figure 1f, arrow). The expression pattern of IGF-II mRNA was similar to that of IGF-I, but the IGF-II mRNA-positive area was broader (Figure 1g). IGF-IR mRNA was expressed in the MM (Figure 1h) and in the center of the condylar anlage (Figure 1h, arrow). At E15.0, a metachromatically-stained matrix was first detected in the condylar anlage, indicating the initial formation of the condylar cartilage (Figure 1i, arrow). The region posterior to the newly formed cartilage, consisting of a mesenchymal cell condensation, was named the embryonic zone (E) (Figure 1i), as previously described.10,15 The outer layer continuous with the periosteum was identified as the perichondrium of newly formed condylar cartilage (PC) (Figure 1i). IGF-I and IGF-II mRNA were expressed in the perichondrium (Figure 1j,k, arrows), but the IGF-II positive area was broader. IGF-IR mRNA was expressed in the embryonic zone and in the upper part of the newly formed cartilage (Figure 1l, arrow), but not in the lower part (Figure 1l, arrowhead).
Figure 1.
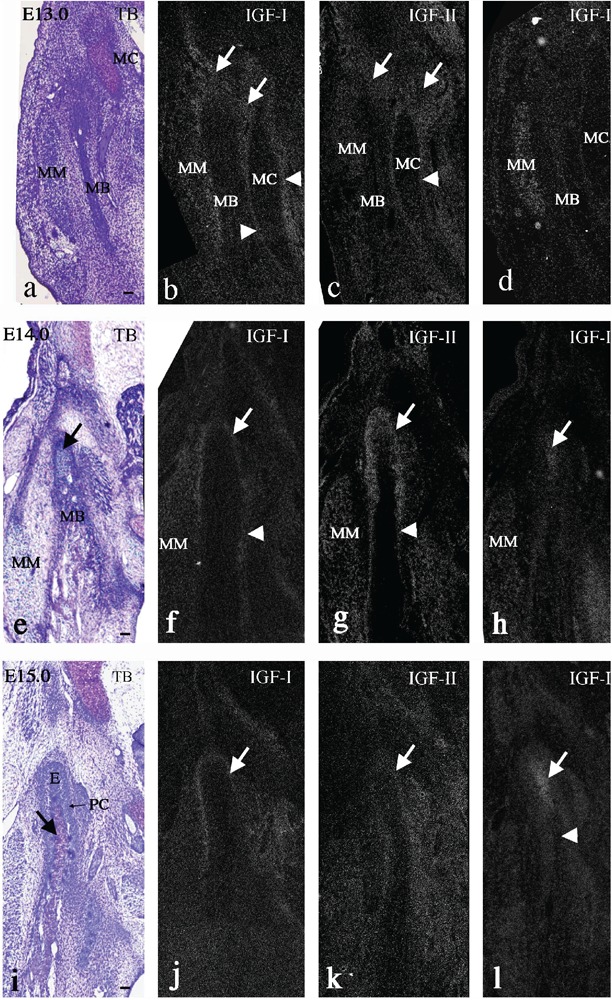
Mandible cut in coronal plane at embryonic day (E) 13.0 (a–d), 14.0 (e–h), and 15.0 (i–l). Toluidine blue staining (a, e, i), and in situ hybridization for IGF-I (b, f, j), -II (c, g, k), and IGF-IR (d, h, l) mRNA. a–d, IGF-I and -II mRNA were expressed in mesenchymal tissue (arrows) around mandibular bone (MB), and IGF-IR mRNA was expressed in the masseter muscle (MM). e–h, IGF-I and -II mRNA were expressed in the periosteum of mandibular bone (f-g, arrowheads), and in the outer region of the condylar anlage (f-g, arrows); IGF-II mRNA-positive area was broader; IGF-IR mRNA was expressed in the center of the condylar anlage (h, arrow). i–l, IGF-I and -II mRNA were expressed in the perichondrium (j–k, arrows), and IGF-IR mRNA was expressed in the embryonic zone and in the upper part of the newly formed cartilage (l, arrow). MC, Meckel's cartilage. Scale bar: 100 µm.
At E16.0, the zones usually present in the growing condyle38 had become distinct: the fibrous cell (articulation) zone, polymorphic cell zone, flattened cell zone, and hypertrophic cell zone. The condylar cartilage had increased in length, especially the hypertrophic cell zone (Figure 2a). IGF-I mRNA was expressed in the perichondrium and in the fibrous cell zone continuous with the perichondrium (Figure 2b, arrows). IGF-II mRNA was expressed in the perichondrium, and in the fibrous cell zone (Figure 2c, arrows) as well as in the polymorphic cell zone (Figure 2c, arrowhead). IGF-IR mRNA was expressed in the zones from the fibrous to flattened cell zone (Figure 2d, arrow), but not in the hypertrophic cell zone (Figure 2d, arrowhead).
Figure 2.
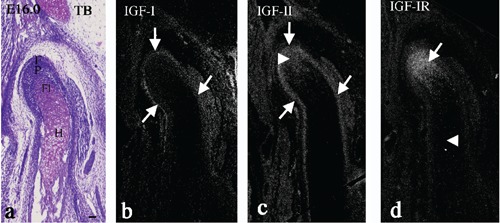
Condylar cartilage in coronal plane at embryonic day (E) 16.0. Toluidine blue staining (a), and in situ hybridization for IGF-I (b), -II (c), and IGF-IR (d) mRNA. The fibrous cell zone (F), polymorphic cell zone (P), flattened cell zone (Fl), and hypertrophic cell zone (H) were established. IGF-I and -II mRNA were expressed in the perichondrium and in the fibrous cell zone (b–c, arrows), and IGF-II mRNA was further expressed in the polymorphic cell zone (c, arrowhead). IGF-IR mRNA was expressed in zones from the fibrous to flattened cell zone (d, arrow). Scale bar: 100 µm.
Negative controls using sense probes showed no positive reactions at any stage examined (data not shown). We examined three different samples for each embryonic day and the same results were obtained each time.
In situ hybridization for IGFBPs mRNA in developing condylar cartilage and temporomandibular joint
Among IGFBPs, IGFBP-6 mRNA was not significantly expressed in condylar cartilage/TMJ throughout the experimental period, and IGFBP-3 mRNA was mainly expressed in invading capillaries, as previously described in fetal long bone.36 Thus, we showed the expression of these molecules at E16.0 as representative (Figure 3m,n) and mainly described the expression of IGFBP-2, IGFBP-4, and IGFBP-5 mRNA in the present study.
Figure 3.
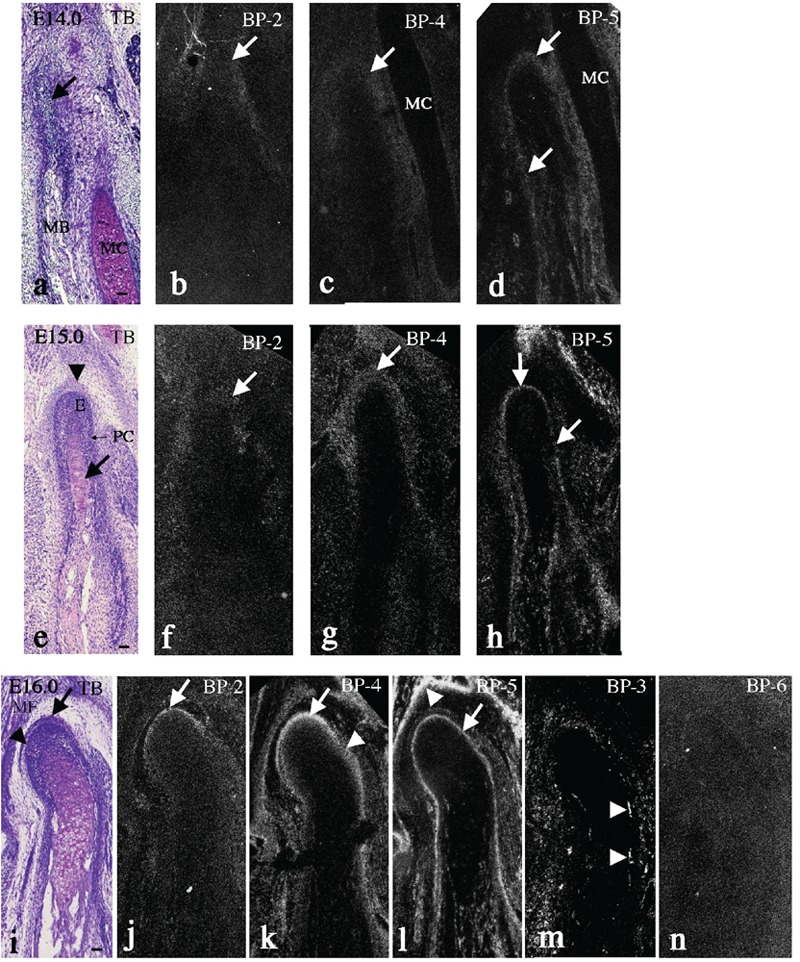
Mandible cut in coronal plane at embryonic day (E) 14.0 (panels a–d), 15.0 (panels e–h), and 16.0 (panels i–n). Toluidine blue staining (a, e, i), and in situ hybridization for IGFBP-2 (b, f, j), -4 (c, g, k), -5 (d, h, l), -3 (m), and -6 (n) mRNA. a–d, IGFBP-2 and -4 mRNA was only slightly expressed in the mesenchyme around condylar anlage (b-c, arrows); IGFBP-5 mRNA was expressed in the periosteum of the mandibular bone and in the outer region of the condylar anlage (d, arrows). e–h, IGFBP-2 mRNA was only slightly expressed in the anlage of the future articular disc (f, arrow); IGFBP-4 mRNA was strongly expressed in this anlage (g, arrow). IGFBP-5 mRNA was expressed in the outer layer of the perichondrium (h, arrows). i–n, Enhanced IGFBP-2 mRNA was recognized in the anlagen of the articular disc and lower joint cavity (j, arrow); IGFBP-4 mRNA was strongly expressed in the anlagen of the articular disc and lower joint cavity (k, arrow), as well as in the continuous mesenchymal tissue including the outer layer of the perichondrium (k, arrowhead); IGFBP-5 mRNA was expressed in the outer layer of the perichondrium/fibrous cell zone (l, arrow), IGFBP-3 mRNA was mainly expressed in the invading capillaries (m, arrowheads), and IGFBP-6 mRNA was not significantly expressed (n). MB, mandibular bone; MC, Meckel's cartilage; E, embryonic zone; PC, perichondrium; MF, mandibular fossa. Scale bar: 100 µm.
At E14.0, the condylar anlage was recognized posterior to the ossifying mandible, as described above (Figure 3a, arrow). IGFBP-2 and IGFBP-4 mRNA was only slightly expressed in the mesenchyme around the condylar anlage (Figure 3b,c, arrows). IGFBP-5 mRNA was clearly expressed in the periosteum of the mandibular bone, and in the outer region of the condylar anlage continuous with the periosteum (Figure 3d, arrows).
At E15.0, adding to the newly formed condylar cartilage with the perichondrium and the embryonic zone (Figure 3e, arrow), another mesenchymal condensation, corresponding to an anlage of the future articular disc, was observed outside the condylar cartilage (Figure 3e, arrowhead), as previously described.14 IGFBP-2 mRNA was only slightly expressed in this mesenchymal condensation (Figure 3f, arrow), but IGFBP-4 mRNA was strongly expressed within this condensation (Figure 3g, arrow). IGFBP-5 mRNA was expressed in the outer layer of the perichondrium (Figure 3h, arrow).
At E16.0, an anlage of the future articular disc (Figure 3i, arrow) and lower joint cavity (Figure 3i, arrowhead) were clearly discriminated as a dense mesenchymal condensation and loose mesenchymal tissue, respectively. The ossifying mandibular fossa (MF) in the temporal bone was clearly identified (Figure 3i). Enhanced IGFBP-2 mRNA was recognized in the anlagen of the articular disc and lower joint cavity (Figure 3j, arrow). IGFBP-4 mRNA was strongly expressed in the anlagen of the articular disc and lower joint cavity (Figure 3k, arrow), as well as in the continuous mesenchymal tissue, including the outer layer of the perichondrium (Figure 3k, arrowhead). IGFBP-5 mRNA was expressed in the outer layer of the perichondrium/fibrous cell zone (Figure 3l, arrow) as well as in the periosteum of the mandibular fossa (Figure 3l, arrowhead). Compared to IGFBP-4, IGFBP-5 mRNA expression was restricted to the narrow layer. Furthermore, IGFBP-3 mRNA was mainly expressed in invading capillaries (Figure 3m, arrowheads), and IGFBP-6 mRNA was not significantly expressed (Figure 3n). The expression patterns of these two molecules were similar in all samples examined (data not shown).
At E18.5, endochondral ossification had begun in the condylar cartilage (Figure 4a). At higher magnification, the upper joint cavity (UJC) (Figure 4b) was clearly formed and fibrous mesenchymal tissue (Figure 4b, arrow) was observed superior to the upper joint cavity. At the same time, the lower joint cavity (LJC) (Figure 4b) remained as mesenchymal tissue at this stage. IGFBP-2 mRNA was not expressed in the perichondrium (Figure 4c, arrow), but clearly expressed in the fibrous mesenchymal tissue facing the upper joint cavity (Figure 4d, arrow) and in the anlagen of the articular disc and lower joint cavity (Figure 4d, arrowheads). IGFBP-4 mRNA was continuously expressed in the perichondrium (Figure 4e, arrow), in the anlagen of the articular disc and lower joint cavity (Figure 4f, arrowhead), as well as in the continuous mesenchymal tissue, including the outer layer of the perichondrium (Figure 4f, asterisk), but reduced in the fibrous mesenchymal tissue facing the upper joint cavity (Figure 4f, arrow). IGFBP-5 mRNA was expressed in the outer layer of the perichondrium/fibrous cell zone (Figure 4h, arrow) and in the periosteum of the mandibular fossa (Figure 4-h, arrowhead).
Figure 4.

Condylar cartilage (a–h) and angular cartilage (i–l) in coronal plane at embryonic day (E) 18.5. b, d, f, h, enlargements of rectangular areas in a, c, e, g, respectively. Toluidine blue staining (a, b, i), and in situ hybridization for IGFBP-2 (c, d, j), -4 (e, f, k), -5 (g, h, l). a–h, IGFBP-2 mRNA was expressed in the fibrous mesenchymal tissue facing the upper joint cavity (d, arrow), and in the anlagen of the articular disc and lower joint cavity (d, arrowhead); IGFBP-4 mRNA was expressed in the perichondrium (e, arrow), in the anlagen of the articular disc and lower joint cavity (f, arrowhead), as well as in the continuous mesenchymal tissue (f, asterisk), but reduced in the fibrous mesenchymal tissue facing the upper joint cavity (f, arrow); IGFBP-5 mRNA was expressed in the outer layer of the perichondrium/fibrous cell zone (h, arrow). i–l, IGFBP-2 mRNA was not significantly expressed (j); IGFBP-4 mRNA was expressed in the perichondrium/fibrous cell zone (k, arrows), and IGFBP-5 mRNA was expressed in the outer layer of the perichondrium/fibrous cell zone (l, arrows). UJC, upper joint cavity; AD, articular disc; LJC, lower joint cavity; MF, mandibular fossa; AC, angular cartilage; MM, masseter muscle; MPM, medial pterygoid muscle. Scale bar: 100 µm.
The mandibular angular cartilage (AC) at E18.5 formed with zones similar to those of the condylar cartilage, and the masseter muscle (MM) and medial pterygoid muscle (MPM) were attached to it (Figure 4i). The joint structure had not formed, but code-like tissue (presumably future stylomandibular ligament) was attached to the posterior end of the angular cartilage (in Figure 4i, asterisk). IGFBP-2 mRNA was not significantly expressed in the angular cartilage (Figure 4j). IGFBP-4 mRNA was expressed in the perichondrium/fibrous cell zone (Figure 4k, arrows) as well as in the code-like tissue (Figure 4k, asterisk). IGFBP-5 mRNA was expressed in the outer layer of the perichondrium/fibrous cell zone (Figure 4l, arrows) and in the code-like tissue (Figure 4l, asterisk). Negative controls using sense probes showed no positive reactions at any stage examined (data not shown). We examined three different samples for each embryonic day and the same results were obtained each time.
Discussion
Expression of IGF-I, IGF-II, and IGF-IR mRNA in developing mandibular condylar cartilage
In the present study, we first demonstrated the expression of IGF-I and IGF-II and IGF-IR mRNA in developing fetal mouse mandibular condylar cartilage. IGF-I and IGF-II mRNA expression was diffusely detected in the mes-enchyme around the mandibular bone at E13.0, but IGF-IR mRNA was not detected within the mandibular bone. The condylar anlage was morphologically identified at E14.0, and formation of the condylar cartilage was first detected within the condylar anlage at E15.0, as previously reported.8,10–12,15 During these periods, IGF-I and IGF-II mRNA expression was restricted to the periosteum and to the outer layer of the condylar anlage/perichondrium/fibrous cell zone, but the IGF-II mRNA-positive areas were broader, indicating that IGF was expressed around the whole condylar process. IGF-IR mRNA was first detected within the condylar anlage at E14.0, suggesting that the presence of IGF-IR under an IGF-rich environment triggers subsequent condylar cartilage formation. Further, IGF-IR was continuously expressed in the upper part of the condylar cartilage, including the embryonic zone at E15.0 and the polymorphic cell zone at E16.0. These IGF-IR mRNA-positive areas have high cell proliferation activity and seem to function as the condylar cartilage growth center.11,38,39 Therefore, IGF-IR expression under an IGF-rich environment is also involved in the subsequent growth of the fetal condylar cartilage.
Previous in vivo studies demonstrated the expression of IGF-I and IGF-II mRNA or their immunostaining in the upper layer of the condylar cartilage in young rat20–22 and in aged mice.23 IGF-IR immunostaining is detected in the fibrous cell zone and upper part of the cartilage.20,23,29 These studies and previous in vitro experiments20,25,26 indicate that the IGF system affects growth, development, and aging, as well as the reaction to the effects of orthopedic appliances on the condylar cartilage21 during the postnatal period. In addition to these functions, the present results indicate that the IGF system is involved in the initial formation of the condylar cartilage. Our model of IGFs and IGF-IR expression during mandibular condylar cartilage formation is summarized in Figure 5a. In murine long bone cartilage, representative primary cartilage, IGF expression in initial chondrogenesis is well investigated by Wang et al.34 They reported IGF-I mRNA expression in surrounding mesenchymal tissue, but not in mesenchymal condensations or newly formed chondrocytes (chondroblasts), whereas strong IGF-II mRNA expression was observed in both mesenchymal condensations and chondroblasts. Additionally, IGF-IR mRNA was expressed in mesenchymal condensations and in chondroblasts. These findings of the initial chondrogenesis of primary cartilage are comparable to the present findings of condylar cartilage, except for the IGF-I mRNA expression in the condylar anlage/perichondrium. Secondary cartilage is defined as cartilage derived from the periosteum, not from primary condensations.5–7 We previously demonstrated that the condylar cartilage is derived from ALP-positive, type I collagen mRNA-expressing periosteum-like cells continuous with the ossifying mandible.10–12,15 Wang et al. also reported that IGF-I and IGF-II mRNA are expressed in osteoblasts in fetal long bone.34 Thus, IGF-I mRNA expression in the present study may reflect the nature of secondary cartilage derived from the periosteum.
Figure 5.

a, Schematic representations of expression patterns of IGF-I, -II, and -IR mRNA from E 13.0 to E16.0; appearance of IGF-IR under IGF-rich environment seemed to work as a trigger for subsequent condylar cartilage formation. B, Schematic representation of expression pattern of IGFBP-2, -4, and -5 mRNA in the TMJ at E18.5; IGFBP-5 mRNA is restrictedly expressed in the outer layer of perichondrium/fibrous cell zone; IGFBP-4 mRNA is expressed in the perichondrium, anlagen of articular disc and lower joint cavity and continuous mesenchymal tissue; IGFBP-2 mRNA is expressed in the anlagen of articular disc and lower joint cavity and in the fibrous mesenchymal tissue facing to the upper joint cavity, but not in the perichondrium; IGFBP-2 seems to be involved in the formation of joint cavity.
Expression of IGFBP mRNA in developing condylar cartilage, articular disc, and joint cavities
In the present study, IGFBP-5 mRNA was continuously detected in the outer layer of the periosteum/perichondrium/fibrous cell layer in the mandibular condyle, and IGFBP-4 mRNA tended to be expressed mesenchymal tissues around the condylar cartilage (including the outer layer of the periosteum/perichondrium). This distribution pattern is similar to that of IGF-I and IGF-II mRNA, suggesting that IGFBP-4 and IGFBP-5 interact with these molecules. IGFBP-4 and IGFBP-5 immunostaining is detected in the upper layer of the condylar cartilage (chondroblasts, chondrocytes), especially in the anterior and posterior regions in growing rats35 and aged mice,23 indicating that the functions of these molecules are different from those in fetal mice observed in the present study. IGFBP-5 is complicated in terms of its function; it is reported to both inhibit and enhance IGF actions in bone, and IGF-independent mechanisms are also indicated.40 IGFBP-4 is considered to be a negative regulator of IGF action in a variety of cell types, but IGFBP-4 gene knockout mice have a significantly reduced body size, and therefore the inhibitory action of IGFBP-4 must be evaluated in bone tissue in vivo. A possible explanation for this phenomenon is that IGFBP-4 binds IGFs, serving as a pericellular reservoir, and losses of the reservoir function in the knockout mice results in diminished local IGF-stimulated growth.40 Organ culture systems of developing condylar cartilage accompanied by antisense oligonucleotide or small interference RNA have been established19,39 and may be useful for elucidating the complicated functions of these IGFBPs.
The present findings that IGFBP mRNA was expressed during formation of the articular disc and joint cavities are interesting. IGFBP-4 mRNA was expressed in the anlage of the articular disc showing no sign of inferior joint cavity formation at E15.0, then continuously expressed in until identification of the anlage of the lower joint cavity at E16.0. When the upper joint cavity was formed at E18.5, IGFBP-4 mRNA expression was still detected in the anlagen of the articular disc and lower joint cavity, but reduced in the fibrous mesenchymal tissue facing the upper joint cavity. Meanwhile, the enhanced expression of IGFBP-2 mRNA was first recognized in the anlagen of the articular disc and lower joint cavity at E16.0 and continuously expressed in the anlagen of articular disc/lower joint cavity as well as in the fibrous mesenchymal tissue facing the upper joint cavity.
These expression patterns suggest the involvement of IGFBP-2 and IGFBP-4 in the formation of the TMJ, including the articular disc and joint cavities. In particular, IGFBP-2 seems to be related to joint cavity formation, because no specific expression of IGFBP-2 mRNA was detected in the angular cartilage at E18.5, which does not form joint structures. These findings are the first to suggest that the IGF system is involved in the formation of the TMJ and a schematic model of IGFBP expression at E18.5 is summarized in Figure 5b.
Regarding TMJ formation and growth factors, Shibukawa et al. demonstrated failed formation of the articular disc and joint cavities fails in Indian hedgehog (IHH)-deficient mice, and suggested the involvement of IHH signaling in TMJ formation.14 Ikeda et al. investigated the development of synovial membrane cells on joint cavity surfaces.41 Furthermore, Matsuda et al. investigated cavity formation in terms of apoptosis, and concluded that apoptosis was not directly related.42 These phenomena are closely related to the regions expressing IGFBPs in the present study, suggesting that they may be regulated by the IGF system. Thus, analysis of the IGF system may be useful for further studies of TMJ formation.
In conclusion, the findings of the present study demonstrated mRNA expression for IGF molecules in the initial formation of the condylar cartilage, articular disc, and joint cavities.
Acknowledgements:
this work was supported by the following grants: 1) High-Tech Research Center Project for Private Universities: matching fund subsidy from MEXT, 2007–2011; 2) Grant for Supporting Project for Strategic Research of Nihon University School of Dentistry at Matsudo by the Ministry of Education, Culture, Sports, Science and Technology, 2008–2012; 3) Grant-in-Aid for Scientific Research (No.19791342 and 22592044) from Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1.Meloto CB, Serrano PO, Ribeiro-DaSilva MC, Rizzatti-Barbosa CM. Genomics and the new perspectives for temporomandibular disorders. Arch Oral Biol. 2011;56:1181–91. doi: 10.1016/j.archoralbio.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Kiga N, Tojyo I, Matsumoto T, Hiraishi Y, Shinohara Y, Makino S, Fujita S. Expression of lumican and fibromodulin following interleukin-1 beta stimulation of disc cells of the human temporomandibular joint. Eur J Histochem. 2011;55:e11–e11. doi: 10.4081/ejh.2011.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Moraes LO, Lodi FR, Gomes TS, Marques SR, Oshima CT, Lancellotti CL, et al. Immunohistochemical expression of types I and III collagen antibodies in the temporomandibular disc of human foetuses. Eur J Histochem. 2011;55:e24–e24. doi: 10.4081/ejh.2011.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Proffit WR. Contemporary Orthodontics. 4th ed. St. Louis, MO, USA: Mosby; 2007. The development of orthodontic problems; pp. 27–71. [Google Scholar]
- 5.Beresford WA. Baltimore, MD, USA: Urban & Schwarzenberg; 1981. Chondroid bone, secondary cartilage and metaplasia; pp. 23–47. [Google Scholar]
- 6.Vinkka H. Secondary cartilages in the facial skeleton of the rat. Proc Finn Dent Soc. 1982;78(Suppl.7):1–137. [PubMed] [Google Scholar]
- 7.Hall BK. San Diego, CA, USA: Elsevier Academic Press; 2005. Bones and cartilage: developmental and evolutionary skeletal biology; pp. 149–65. [Google Scholar]
- 8.Shibata S, Suzuki S, Tengan T, Ishii M, Kuroda T. A histological study of the developing condylar cartilage of the fetal mouse mandible using coronal sections. Arch Oral Biol. 1996;41:47–54. doi: 10.1016/0003-9969(95)00105-0. [DOI] [PubMed] [Google Scholar]
- 9.Miyake T, Cameron AM, Hall BK. Stage-specific expression patterns of alkaline phosphatase during development of the first arch skeleton in inbred C57BL/6 mouse embryos. J Anat. 1997;190:239–60. doi: 10.1046/j.1469-7580.1997.19020239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shibata S, Fukada K, Suzuki S, Yamashita Y. Immunohistochemistry of collagen types II and X, and enzyme-histochemistry of alkaline phosphatase in the developing condylar cartilage of the fetal mouse mandible. J Anat. 1997;191:561–70. doi: 10.1046/j.1469-7580.1997.19140561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukada K, Shibata S, Suzuki S, Ohya K, Kuroda T. In situ hybridisation study of type I, II, X collagens and aggrecan mRNAs in the developing condylar cartilage of fetal mouse mandible. J Anat. 1999;195:321–9. doi: 10.1046/j.1469-7580.1999.19530321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukuoka H, Shibata S, Suda N, Yamashita Y, Komori T. Bone morphogenetic protein rescues lack of secondary cartilage in Runx2-deficient mice. J Anat. 2007;211:8–15. doi: 10.1111/j.1469-7580.2007.00739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silbermann M, Reddi AH, Hand AR, Leapman RD, von der Mark K, Franzen A. Further characterization of the extracellular matrix in the mandibular condyle in neonatal mice. J Anat. 1987;151:169–88. [PMC free article] [PubMed] [Google Scholar]
- 14.Shibukawa Y, Young B, Wu C, Yamada S, Long F, Pacifici M, et al. Temporo - mandibular joint formation and condylar growth require Indian hedgehog signaling. Dev Dyn. 2007;236:426–34. doi: 10.1002/dvdy.21036. [DOI] [PubMed] [Google Scholar]
- 15.Shibata S, Suda N, Suzuki S, Fukuoka H, Yamashita Y. An in situ hybridization study of Runx2, Osterix, and Sox9 at the onset of condylar cartilage formation in fetal mouse mandible. J Anat. 2006;208:169–77. doi: 10.1111/j.1469-7580.2006.00525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shibata S, Yokohama-Tamaki T. An in situ hybridization study of Runx2, Osterix, and Sox9 in the anlagen of mouse mandibular condylar cartilage in the early stages of embryogenesis. J Anat. 2008;213:274–83. doi: 10.1111/j.1469-7580.2008.00934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oka K, Oka S, Sasaki T, Ito Y, Bringas P, Jr, Nonaka K, et al. The role of TGF-beta signaling in regulating chondrogenesis and osteogenesis during mandibular development. Dev Biol. 2007;303:391–404. doi: 10.1016/j.ydbio.2006.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oka K, Oka S, Hosokawa R, Bringas P, Jr, Brockhoff HC, 2nd, Nonaka K, et al. TGF-beta mediated Dlx5 signaling plays a crucial role in osteo-chondroprogenitor cell lineage determination during mandible development. Dev Biol. 2008;321:303–9. doi: 10.1016/j.ydbio.2008.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogawa T, Shimokawa H, Fukada K, Suzuki S, Shibata S, Ohya K, et al. Localization and inhibitory effect of basic fibroblast growth factor on chondrogenesis in cultured mouse mandibular condyle. J Bone Miner Metab. 2003;21:145–53. doi: 10.1007/s007740300023. [DOI] [PubMed] [Google Scholar]
- 20.Fuentes MA, Opperman LA, Bellinger LL, Carlson DS, Hinton RJ. Regulation of cell proliferation in rat mandibular condylar cartilage in explant culture by insulin-like growth factor-1 and fibroblast growth factor-2. Arch Oral Biol. 2002;47:643–54. doi: 10.1016/s0003-9969(02)00052-3. [DOI] [PubMed] [Google Scholar]
- 21.Hajjar D, Santos MF, Kimura ET. Propulsive appliance stimulaties the syntheses of insulin-like growth factors I and II in the mandibular condylar cartilage of young rats. Arch Oral Biol. 2003;48:635–42. doi: 10.1016/s0003-9969(03)00128-6. [DOI] [PubMed] [Google Scholar]
- 22.Visnapuu V, Peltomäki T, Rönning O, Syrjänen Distribution of insulin-like growth factor-1 mRNA in the mandibular condyle and rib cartilage of the rat during growth. Arch Oral Biol. 2002;47:791–8. doi: 10.1016/s0003-9969(02)00115-2. [DOI] [PubMed] [Google Scholar]
- 23.Götz W, Dühr S, Jäger A. Distribution of component of the insulin-like growth factor system in the temporomandibular joint of the aging mouse. Growth Dev Aging. 2005;69:67–79. [PubMed] [Google Scholar]
- 24.Hinton RJ, Serrano M, So S. Differential gene expression in the perichondrium and cartilage of the neonatal mouse temporomandibular joint. Orthod Craniofac Res. 2009;12:168–77. doi: 10.1111/j.1601-6343.2009.01450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delatte M, Von den Hoff JW, Maltha JC, Kuijpers-Jagtman K. Growth stimulator of mandibular condyles and femoral heads of newbon rats by IGF-1. Arch Oral Biol. 2004;49:165–75. doi: 10.1016/j.archoralbio.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Major G, Hochberg Z, Silbermann M. Insulin-like growth factor-I accelerates proliferation and differentiation of cartilage progenitor cells. J Endocrinol. 1993;137:21–6. doi: 10.1530/acta.0.1280056. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki S, Itoh K, Ohyama K. Local administration of IGF-I stimulates the growth of mandibular condyle in mature rats. J Orthod. 2004;31:138–43. doi: 10.1179/146531204225020436. [DOI] [PubMed] [Google Scholar]
- 28.Yokota T, Shimokawa H, Shibata S, Itoh K, Baba Y, Ohya K, et al. Insulin-like growth factor I regulaties apoptosis in condylar cartilage. J Dent Res. 2008;87:159–63. doi: 10.1177/154405910808700216. [DOI] [PubMed] [Google Scholar]
- 29.Visnapuu V, Peltomäki T, Rönning O, Vahlberg T, Helenius H. Growth hormone and insulin-like growth factor I receptor in the temporomandibular joint of the rat. J Dent Res. 2001;80:1903–7. doi: 10.1177/00220345010800100801. [DOI] [PubMed] [Google Scholar]
- 30.Maki RG. Small is beautiful: insulin-like growth factors and their growth, development and cancer. J Clin Oncol. 2010;28:4985–95. doi: 10.1200/JCO.2009.27.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le Roith D, Bondy C, Yakar S, Liu JL, Butler A. The somatomedin hypothesis: 2001. Endocr Res. 2001;22:53–74. doi: 10.1210/edrv.22.1.0419. [DOI] [PubMed] [Google Scholar]
- 32.Kaplan SA, Cohen P. The somatomedin hypothesis 2007: 50 years later. J Clin Endocrinol Metab. 2007;92:4529–35. doi: 10.1210/jc.2007-0526. [DOI] [PubMed] [Google Scholar]
- 33.Firth SM, Baxter RC. Celular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23:824–54. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- 34.Wang E, Wang J, Chin E, Zhou J, Bondy CA. Cellular patterns of insulin-like growth factor system gene expression in murine chondrogenesis and osteogenesis. Endocrinology. 1995;136:2741–51. doi: 10.1210/endo.136.6.7750499. [DOI] [PubMed] [Google Scholar]
- 35.Hajjar D, Santos MF, Kimura ET. Mandibular repositioning modulates IGFBP-3, -4, -5 and -6 expression in the mandibular condylar cartilage of young rats. Biorheology. 2006;43:311–21. [PubMed] [Google Scholar]
- 36.Suzuki Y, Takeda M, Sakakura Y, Suzuki N. Distinct expression pattern of insulin-like growth factor family in rodent taste buds. J Comp Neurol. 2005;482:74–84. doi: 10.1002/cne.20379. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki Y. Expression of IGFBPs in the developing mouse submandibular and von Ebner's glands. Anat Embryol. 2006;211:189–96. doi: 10.1007/s00429-005-0071-z. [DOI] [PubMed] [Google Scholar]
- 38.Luder HU, Leblond CP, von der Mark K. Cellular stages in cartilage formation as revealed by morphometry, radioautography and type II collagen immunostaining of the mandibular condyle from weanling rats. Am J Anat. 1988;182:197–214. doi: 10.1002/aja.1001820302. [DOI] [PubMed] [Google Scholar]
- 39.Yokohama-Tamaki T, Maeda T, Tanaka TS, Shibata S. Functional analysis of CTRP3/cartducin in Meckel's cartilage and developing condylar cartilage in the fetal mouse mandible. J Anat. 2011;211:517–33. doi: 10.1111/j.1469-7580.2011.01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Conover CA. Insulin-like growth factor-binding proteins and bone metabolism. Am J Endocrinol Metab. 2008;294:E10–E14. doi: 10.1152/ajpendo.00648.2007. [DOI] [PubMed] [Google Scholar]
- 41.Ikeda N, Nozawa-Inoue K, Takagi R, Maeda T. Development of the synovial membrane in the rat tenporomandibular joint as demonstrated by immunocytochemistry for heat shock protein 25. Anat Rec Part A. 2004;279:623–35. doi: 10.1002/ar.a.20043. [DOI] [PubMed] [Google Scholar]
- 42.Matsuda S, Mishima K, Yoshimura Y, Hatta T, Otani H. Apoptosis in the development of the temporomandibular joint. Anat Embryol. 1997;196:383–91. doi: 10.1007/s004290050106. [DOI] [PubMed] [Google Scholar]


