Abstract
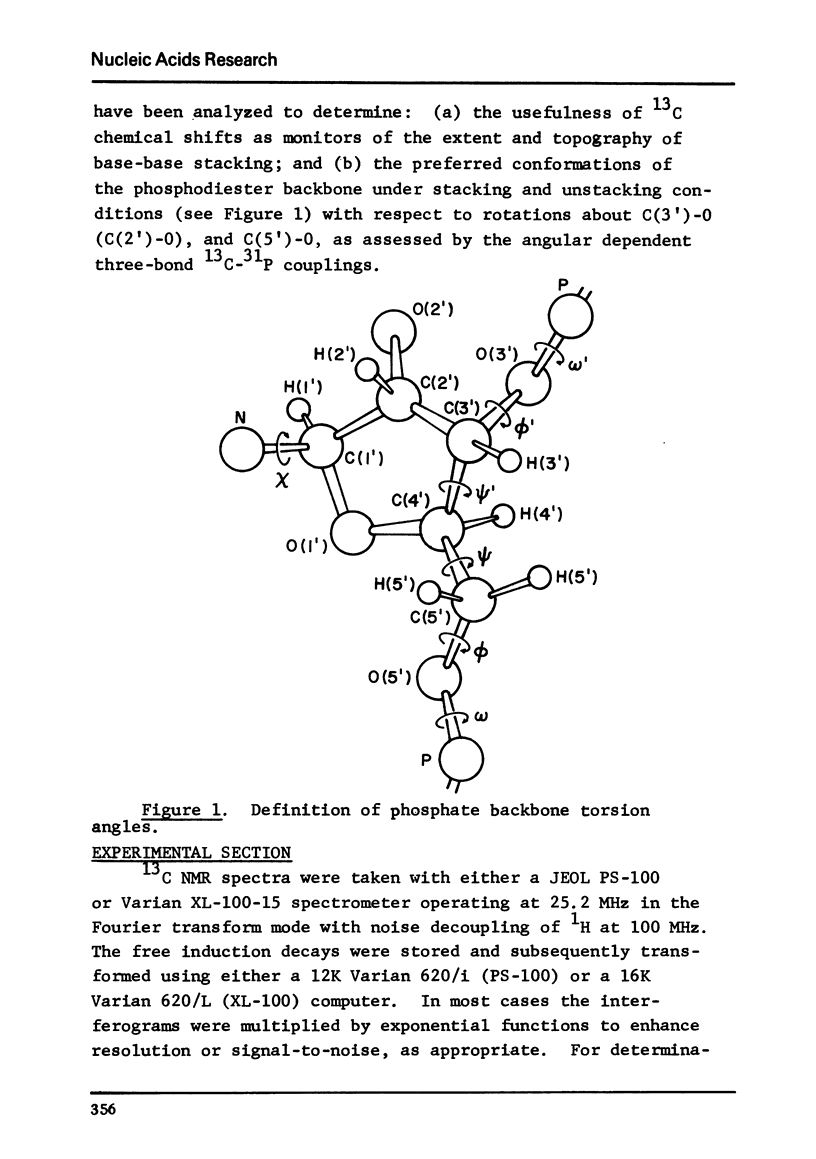
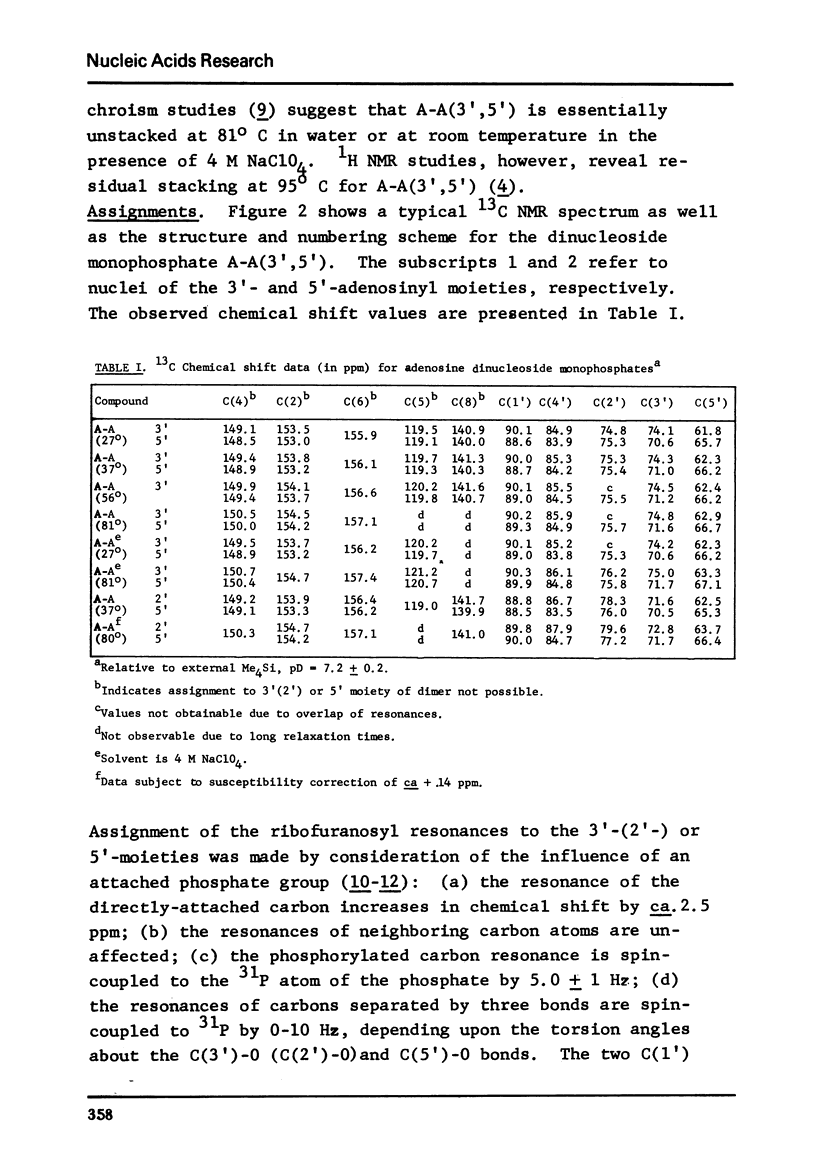
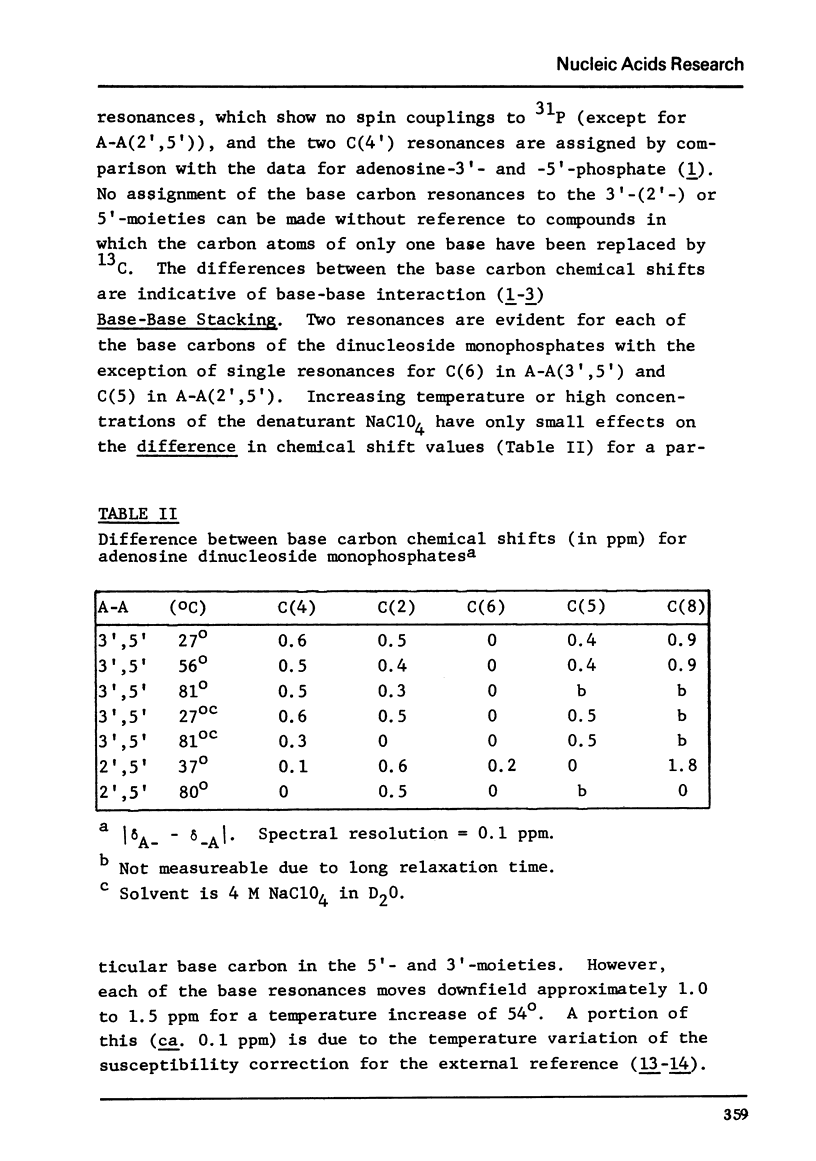
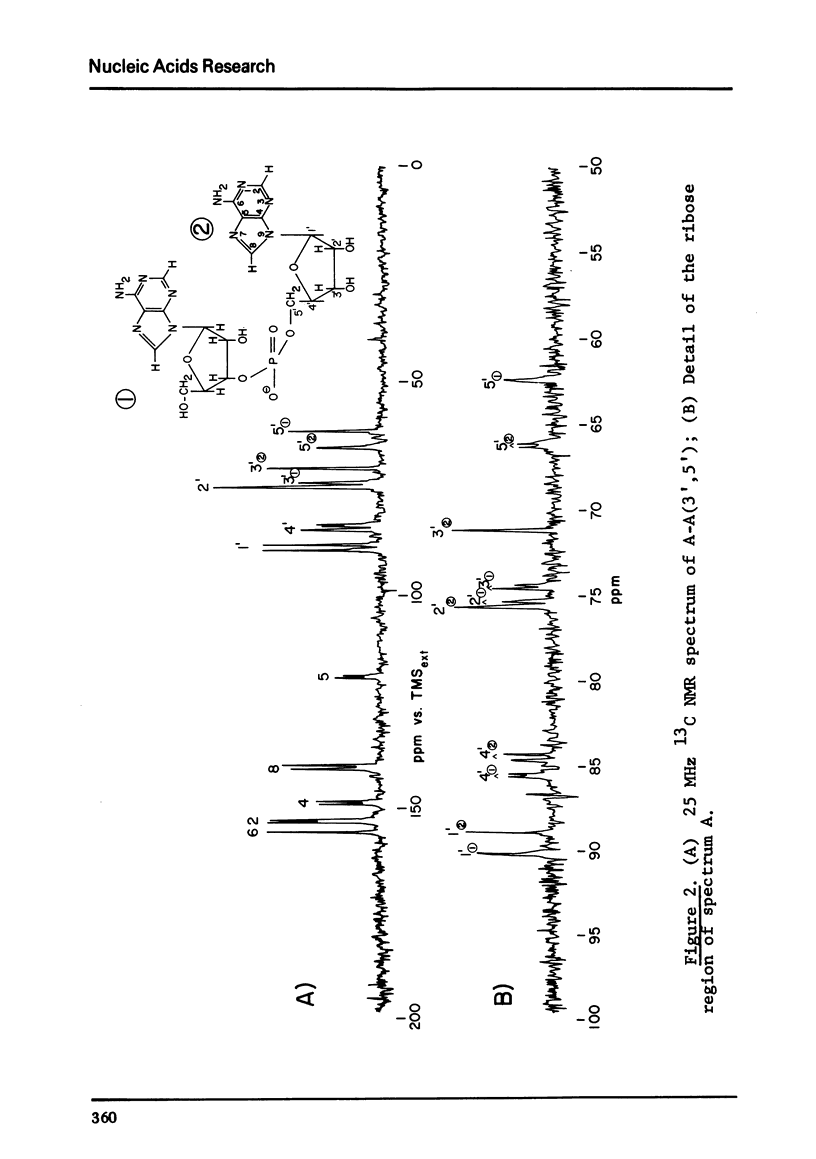
The solution conformation of adenylyl-(3',5')-adenosine and adenylyl-(2',5')-adenosine in both the stacked and unstacked states was studied by carbon-13 magnetic resonance spectroscopy. Large chemical shift differences between the base carbons in the dimers and those in the corresponding monomers are attributed in part to the influence of base-base interaction. Carbon-phosphorus couplings across three bonds revealed the preferred populations for certain backbone rotamers, demonstrating that significant changes in conformation about the "c(3')-O and C(5')-O bonds do not occur in the temperature or salt-induced unstacking of adenylyl-(3',5')-adenosine. However, rotations about the C(2')-O and C(5')-O bonds occur in the temperature-mediated unstacking of adenylyl-(2',5')-adenosine.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bush C. A., Tinoco I., Jr Calculation of the optical rotatory dispersion of dinucleoside phosphates. J Mol Biol. 1967 Feb 14;23(3):601–604. doi: 10.1016/s0022-2836(67)80128-1. [DOI] [PubMed] [Google Scholar]
- Chan S. I., Nelson J. H. Proton magnetic resonance studies of ribose dinucleoside monophoshates in aqueous solution. I. The nature of the base-stacking interaction in adenylyl 3'--5')adenosine. J Am Chem Soc. 1969 Jan 1;91(1):168–183. doi: 10.1021/ja01029a033. [DOI] [PubMed] [Google Scholar]
- Davis R. C., Tinoco I., Jr Temperature-dependent properties of dinucleoside phosphates. Biopolymers. 1968;6(2):223–242. doi: 10.1002/bip.1968.360060206. [DOI] [PubMed] [Google Scholar]
- Dorman D. E., Roberts J. D. Nuclear magnetic resonance spectroscopy: 13C spectra of some common nucleotides. Proc Natl Acad Sci U S A. 1970 Jan;65(1):19–26. doi: 10.1073/pnas.65.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans F. E., Lee C. H., Sarma R. H. 300 MHz NMR study on the effect of base stacking on backbone conformational flexibility in oxy- and deoxy- adenyl dinucleosides. Biochem Biophys Res Commun. 1975 Mar 3;63(1):106–114. doi: 10.1016/s0006-291x(75)80017-9. [DOI] [PubMed] [Google Scholar]
- Giessner-Prettre C., Pullman B. Intermolecular nuclear shielding values for protons of purines and flavins. J Theor Biol. 1970 Apr;27(1):87–95. doi: 10.1016/0022-5193(70)90130-x. [DOI] [PubMed] [Google Scholar]
- Giessner-Prettre C., Pullman B. Regions of negative values of intermolecular nuclear shielding for protons of purines. J Theor Biol. 1970 May;27(2):341–342. doi: 10.1016/0022-5193(70)90145-1. [DOI] [PubMed] [Google Scholar]
- Johnson N. P., Schleich T. Circular dichroism studies of the conformational stability of dinucleoside phosphates and related compounds in aqueous neutral salt solutions. Biochemistry. 1974 Feb 26;13(5):981–987. doi: 10.1021/bi00702a023. [DOI] [PubMed] [Google Scholar]
- Jones A. J., Winkley M. W., Grant D. M., Robins R. K. Carbon-13 nuclear magnetic resonance: naturally occurring nucleosides. Proc Natl Acad Sci U S A. 1970 Jan;65(1):27–30. doi: 10.1073/pnas.65.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo N. S., Holmes H. M., Stempel L. M., Ts'o O. P. Influence of the phosphodiester linkage (3'-5', 2'-5', and 5'-5') on the conformation of dinucleoside monophosphate. Biochemistry. 1970 Sep 1;9(18):3479–3498. doi: 10.1021/bi00820a002. [DOI] [PubMed] [Google Scholar]
- Lapper R. D., Smith I. C. A 13 C and 1 H nuclear magnetic resonance study of the conformations of 2',3'-cyclic nucleotides. J Am Chem Soc. 1973 May 2;95(9):2878–2880. doi: 10.1021/ja00790a024. [DOI] [PubMed] [Google Scholar]
- Lee C. H., Evans F. E., Sarma R. H. The gain in conformational purity and loss in flexibility as a result of 3',5' polymerization between the component mononucleotides - a 300 MHz 1H and 40.5 MHz 31 P NMR comparative study of the dynamic solution conformation of dinucleoside monophosphates and their component monomers. FEBS Lett. 1975 Mar 1;51(1):73–79. doi: 10.1016/0014-5793(75)80857-x. [DOI] [PubMed] [Google Scholar]
- Mantsch H. H., Smith I. C. Fourier-transformed 13 C NMR spectra of polyuridylic acid, uridine, and related nucleotides--the use of 31 POC 13 C couplings for conformational analysis. Biochem Biophys Res Commun. 1972 Jan 31;46(2):808–815. doi: 10.1016/s0006-291x(72)80213-4. [DOI] [PubMed] [Google Scholar]
- Perahia D., Pullman B., Saran A. Molecular orbital calculations on the conformation of nucleic acids and their constituents. IX. The geometry of the phosphate group: key to the conformation of polynucleotides? Biochim Biophys Acta. 1974 Mar 27;340(3):299–313. doi: 10.1016/0005-2787(74)90275-5. [DOI] [PubMed] [Google Scholar]
- Pullman B., Perahia D., Saran A. Molecular orbital calculations on the conformation of nucleic acids and their constituents. 3. Backbone structure of di- and polynucleotides. Biochim Biophys Acta. 1972 Apr 26;269(1):1–14. doi: 10.1016/0005-2787(72)90068-8. [DOI] [PubMed] [Google Scholar]
- Tewari R., Nanda R. K., Govil G. Quantum chemical studies on the conformational structure of nucleic acids. IV. Calculation of backbone structure by CNDO method. J Theor Biol. 1974 Jul;46(1):229–239. doi: 10.1016/0022-5193(74)90149-0. [DOI] [PubMed] [Google Scholar]
- Ts'o P. O., Kondo N. S., Schweizer M. P., Hollis D. P. Studies of the conformation and interaction in dinucleoside mono- and diphosphates by proton magnetic resonance. Biochemistry. 1969 Mar;8(3):997–1029. doi: 10.1021/bi00831a033. [DOI] [PubMed] [Google Scholar]
- Vernet R. D., Boekelheide V. Nuclear magnetic resonance spectroscopy. Ring-current effects on carbon-13 chemical shifts. Proc Natl Acad Sci U S A. 1974 Aug;71(8):2961–2964. doi: 10.1073/pnas.71.8.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]



