Abstract
One of the characteristic features of anaplastic lymphoma kinase (ALK)-positive, anaplastic large cell lymphoma (ALK+ALCL) is the constitutive activation of STAT3, a defect believed to be important for the pathogenesis of these tumors. In this report, we describe the existence of an autocrine stimulatory loop involving interleukin-22 (IL-22) that contributes to STAT3 activation and tumorigenicity of ALK+ALCL. The IL-22 receptor, a heterodimer composed of IL-22R1 and IL-10R2, was expressed in all ALK+ALCL cell lines and tumors examined. The expression of IL-22R1 in ALK+ALCL is aberrant, since this protein is absent in benign lymphocytes. While ALK+ALCL cells produce endogenous IL-22, addition of recombinant IL-22 to ALK+ALCL cell lines significantly increased STAT3 activation, cell proliferation and colony formation in soft agar. Opposite biological effects were observed in cells treated with recombinant IL-22BP (a naturally-occurring IL-22 decoy) or IL-22 neutralizing antibody. NPM-ALK, the characteristic fusion gene oncoprotein expressed in ALK+ALCL, directly contributes to the aberrant expression of IL-22R1, since transfection of NPM-ALK in Jurkat cells induced IL-22R1 expression and IL-22-mediated STAT3 activation. To conclude, for the first time, we demonstrate the importance of the IL-22 autocrine pathway in a lymphoid malignancy, and reveal yet another novel function of NPM-ALK.
Keywords: anaplastic large cell lymphoma, IL-22, tumorigenicity, STAT3, NPM-ALK
INTRODUCTION
Anaplastic lymphoma kinase (ALK)-positive anaplastic large-cell lymphoma (ALK+ALCL) is defined as a subtype of T/null-cell non-Hodgkin lymphoma characterized by the consistent expression of CD30. The aberrant expression of ALK in approximately 80% of ALK+ALCL tumors is a result of the reciprocal chromosomal translocation that leads to the fusion of the nucleophosmin (NPM) gene at 5q35 with the ALK gene at 2p23. 1–3 NPM-ALK has been shown to be oncogenic, and it is believed that it contributes to tumorigenesis by exerting its constitutively active tyrosine kinase embedded in the ALK portion of the fusion gene protein. 4 Previous studies have demonstrated that NPM-ALK induces dysregulation of multiple signaling pathways, 4 including that of the signal transducers and activators of transcription-3 (STAT3). 5,6 STAT3 is oncogenic, and constitutive activation of STAT3 has been demonstrated in a variety of human cancers. 7–10 In ALK+ALCL, evidence suggest that the tumorigenic effect of NPM-ALK requires STAT3 activation. 11 While there is little doubt that NPM-ALK directly contributes to STAT3 activation in ALK+ALCL, other mechanisms appear to be involved 12–14.
Interleukin-22 (IL-22) was recently discovered as an IL-9-inducible, T-cell derived cytokine that belongs to the IL-10 gene family. 15 It is known to exert its function by binding to its heterodimeric receptor complex composed of IL-22R1 and IL-10R2. IL-10R2 is ubiquitously expressed, while IL-22R1 expression is restricted and not detectable in normal immune cells including B-cells and T-cells. 16–18 IL-22 stimulation is known to be negatively regulated by the IL-22 binding protein (IL-22BP), a naturally occurring decoy that can antagonize IL-22 binding to its receptor. 19–21 Once bound to its receptor, IL-22 is known to activate a number of signaling pathways including that of STAT3 and mitogen-activated protein kinase (MAPK). 22 Based on the current understanding of the biology of IL-22, it is believed that IL22 produced by T-cells homing to the mucosal surfaces plays an important role in enhancing innate immunity. 23
In our initial study analyzing the pattern of cytokine expression in ALK+ALCL cell lines using oligonucleotide microarrays, we identified the expression of IL-22R1 as well as IL-22. A recent published study using large-scale microarray gene expression profiling also described the expression of IL-22 among ALK− and ALK+ ALCL tumors and cell lines. 24 While the expression of IL-22 is not unexpected for ALK+ALCL, a tumor derived from mature T-cells, IL-22R1 expression in ALK+ALCL cells represents an aberrant event, since this receptor is not detectable in benign lymphoid cells. In light of these findings, we hypothesized that the aberrant expression of IL-22R1 creates an abnormal IL-22 autocrine stimulatory pathway that contributes to STAT3 activation and the tumorigenicity in ALK+ALCL. In this study, we first established that the expression pattern of IL-22, IL-22R1, IL-10R2 and IL-22BP in ALK+ALCL, and examined the biological significance of IL-22 stimulation in ALK+ALCL cell lines. Furthermore, we examined the possibility that NPM-ALK is directly linked to the aberrant expression of IL-22R1.
MATERIALS AND METHODS
1. Cells and IL-22 signaling
Three ALK+ALCL cell lines, SU-DHL-1, Karpas 299, and SUP-M2, were included in this study. HepG2 (a hepatocellular carcinoma cell line) and Jurkat (a T-cell lymphoblastic lymphoma cell line), both of which were obtained from the American Type Culture Collection (Manassas, VA, USA), were used for controls. ALK+ALCL and Jurkat cells were maintained in RPMI-1640 (Sigma-Aldrich, St. Louis, MO, USA). HepG2 was maintained in Dulbecco’s Modified Eagle’s medium (Sigma-Aldrich). Both types of culture media were enriched with 10% fetal bovine serum (FBS)(Gibco, Grand Island, NY, USA) and supplemented with antibiotics (10,000 units/mL penicillin G, 10,000 μg/mL streptomycin, Gibco). All cells were grown at 37°C in 5% CO2. To isolate peripheral blood T- and B-lymphocytes, mononuclear cells harvested from the peripheral blood of healthy donors were isolated using Ficoll-Paque Plus according to the manufacturer’s protocol (GE Healthcare Biosciences, Uppsala, Sweden). CD3-positive T-cells and CD19-positive B-cells were then isolated using the Epics Altra Hypersort system (Beckman Coulter, Mississauga, Ontario, Canada). To assess IL-22 signaling, cells (1 × 106 cells/mL) were initially serum-starved for 16 hours. Subsequently, cells were treated with 5 μg/mL of IL-22BP recombinant protein (R&D Systems, Minneapolis, MN, USA), 10 μg/mL of IL-22 neutralizing antibody, or 10 ng/mL of IL-22 recombinant protein for 0 and 30 minutes, and harvested for Western blot analysis.
2. Western blot analysis and antibodies
Western blot analysis was performed using standard techniques described in details previously. 25 Anti-STAT3 and pSTAT3 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA), Anti-IL-22R1 and anti-β-actin was purchased from Sigma-Aldrich. Anti-pERK1/2, anti-ERK1/2, anti-pJNK/SAPK, anti-JNK/SAPK, anti-phospho-p38 and anti-p38 were all purchased from Cell Signaling Technology (Danvers, MA, USA).
3. Immunofluorescence staining and confocal microscopy
Cells were grown on the coverslips in 6-well plates. After being fixed with 4% paraformaldehyde in PBS, cells were washed three times with PBS and incubated with 30 μL of anti-IL-22R1 (1:50) overnight. After washings with PBS, cells were incubated with Alexa 488 goat anti-rabbit secondary antibody (1:250) for 1 hour. After washings with PBS, mounting media (Sigma-aldrich) was added to the slides, and cells were visualized with a Zeiss LSM 510 confocal microscope (Oberkochen, Germany). For the double staining experiments, frozen sections from 5 cases of ALK+ALCL were fixed in acetone and washed with PBS. Slides were incubated simultaneously with anti-IL-22R1 (Capralogic, 1:200, anti-goat) and anti-IL-22 (Capralogic, 1:200, anti-rabbit) for 1 hour. After washings with PBS, slides were incubated simultaneously with Alexa 488 goat anti-rabbit and Alexa 555 donkey anti-goat secondary antibodies (1:300) for 30 minutes at room temperature.
4. Reverse transcriptase-polymerase chain reaction (RT-PCR)
Using TRIzol extraction method (Gibco), we extracted total cellular RNA from SU-DHL-1, Karpas 299, SUP-M2, Jurkat, HepG2, as well as a randomly chosen, frozen primary breast tumor. Reverse transcription was performed using 500 ng of total RNA and superscript reverse transcriptase (Invitrogen Life Technologies, Carlsbad, CA, USA). The PCR was performed for 35 cycles, with each consisting of denaturation (94°C for 1 minute), primer annealing (60°C for 1 minute) and DNA extension (72°C for 1 minute for 35 cycles). Amplified products were electrophoresed in 1% agarose and visualized using Alpha Imager 3400 (Alpha Innotech, San Leandro, CA, USA). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and β-globin were used as internal controls. The primer sequences are: IL-22 (expected size, 169 bp) forward: 5′-TTCTCTTGGCCCTCTTGGTA-3′, reverse: 5′-TTCTCCC CAATGAG ACGAAC-3′. IL-22R1 (expected size, 181 bp) forward: 5′-TGCTGACCATCTTGACTGTG-3′, reverse: 5′-TCCCTCTCTCCGTACGTCTTAT-3′. IL-22BP (expected size, 178 bp) forward: 5′-GGAACTCAGTCAACGCATGA-3, reverse: 5′-TTGGCTTCTG GTGAGAGCTT-3′. IL-10R2 (expected size, 219 bp) forward: 5′-TACCACCTCCCGAAAATGTC-3′, reverse: 5′-CAAATTCAGCCCTGACTCTCA-3′. NPM-ALK (expected size, 240 bp) forward: 5′-ATGGAAGATTCGATGGACAG-3′, reverse: 5′-TCAG GGCCCAGGCTGGTT-3′. GAPDH (expected size, 316 bp) forward: 5′-AAGGTCATCCCTGAGCTGAA-3′, reverse: 5′-CCCTGT TGCTGTAGCCAAAT-3′. β-globin (expected size, 343 bp) forward: 5′-CAACTTCATCCACGTTCACC-3′, reverse: 5′-GAAGAGCCAAGGACAGGTAC-3′.
5. IL-22R1 siRNA
SU-DHL-1 cells (1 × 106 cells in 1 mL of culture medium) were transfected with 100 pmol of IL-22R1 or scrambled siRNA (Qiagen) using Lipofectamine 2000 (Invitrogen). The IL-22R1 target sequence is: 5′-CAGGAGCTCCACAGCGGCATA-3′. Cells were harvested 24 hours after the transfection and subjected to Western blot analysis.
6. Gene Transfection of NPM-ALK
The original NPM-ALK plasmid was kindly provided by Dr. S. Morris (St. Jude’ Children Research hospital, Memphis, TN, USA) and the NPM-ALK fusion gene was inserted in a pCDNA vector. 26 Transfection was carried out using the Nucleofector Kit (Amaxa, Cologne, Germany) according to the manufacturer’s protocol for Jurkat cells.
7. ELISA Assay for IL-22
Aliquots of culture medium from 3 ALK+ALCL cell lines were spun at 15,000g, and the supernatant was subjected to ELISA, as per manufacturer’s protocol (R&D systems). Based on the manufacturer’s manual, the specificity of kit has been extensively tested. Results are obtained using a colorimetric plate reader (Biorad Life Science Research Group, Hercules, CA, USA) at an absorbance of 450/570 nm.
8. Cell growth assays
To determine changes in cell growth induced by IL-22, IL-22BP, and IL-22 neutralizing antibody, 25,000 cells/mL in culture medium were treated with one of these three reagents added daily to the cell culture. The number of viable cells was assessed daily with trypan blue exclusion test (Gibco). In addition, at the end of the third day of the experiments, we also performed MTS assay (Promega, Madison, WI, USA) to assess the number of viable cells using a microplate reader (Biorad) at 450 nm.
9. Colony formation in soft agar
The soft agar consisted of two layers, both of which were prepared from a stock 1.2% Bacto agar (Difco, Detroit, MI, USA) that was dissolved in distilled water and autoclaved. For the bottom layer, RPMI 1640 supplemented with 10% FBS was added to the stock agar to achieve a 0.6% agar concentration. For the top layer, cell suspension (20,000 cells in 2 mL of RPMI 1640 supplemented with 10% FBS) was mixed with the stock agar to achieve a final agar concentration of 0.3%. Cells in the agar were fed with 500 μL of RPMI 1640/10% FBS every 4 days. Colonies were stained and visualized with 0.05% crystal violet after 2 weeks of culture.
10. ALK+ALCL tumors and immunohistochemistry
Paraffin-embedded ALK+ALCL tumors were retrieved from the Department of Laboratory Medicine and Pathology, Cross Cancer Institute, and the use of these tissues have been approved by the institutional ethics committee. The diagnosis of ALK+ALCL was based on the criteria established by the World Health Organization Classification scheme, and all cases were ALK-positive by immunohistochemistry. Immunohistochemical staining was performed using standard techniques previously described. 25 Anti-IL-22R1 and anti-IL-22 were purchased from Capralogics (Hardwick, MA, USA). Competition experiment was performed to further assess the specificity of anti-IL-22 used for immunohistochemistry. Briefly, we pre-incubated the antibody with different concentrations of recombinant IL-22, and we found that this treatment abrogated the IL-22 immunostaining. The specificity of the anti-IL-22R1 used in this study was confirmed by Western blots and has been previously tested by the manufacturer (www.capralogic.com).
11. Statistical Analysis
The association between IL-22 and cell growth was evaluated using Student’s t-test. A p-value of <0.05 is considered to be statistically significant.
RESULTS
1. Expression of IL-22R1, IL10R2, and IL-22 in ALK+ALCL cell lines and tumors
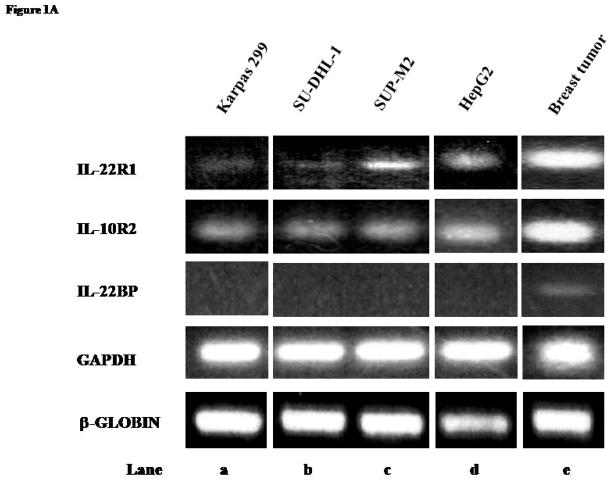
The expression of IL-22R1 and IL-10R2 in 3 ALK+ALCL cell lines was assessed. As shown in Figure 1A, RT-PCR was performed and amplifiable products for IL-22R1 and IL-10R2 were detectable in all 3 ALK+ALCL cell lines (lane a–c). While the IL-10R2 bands were of relatively even intensity among these 3 cell lines, SUP-M2 showed the strongest intensity for IL-22R1. HepG2 and a case of breast cancer served as the positive controls for IL-22R1 (lane d and e). IL-22BP was not detected in any of the ALK+ALCL cell lines, but expressed in a case of breast cancer (lane e).
Figure 1. Expression of IL-22 and IL-22 receptors in ALK+ ALCL cell lines and tumors.
A) RT-PCR studies demonstrated the presence of IL-22R1 and IL10R2 mRNA and the absence of IL-22BP mRNA in 3 ALK+ALCL cell lines. HepG2 was included as a positive control for IL-22R1 and IL-10R2 and a breast cancer tumor was included as a positive control for IL-22BP. GAPDH and β-globin were included as internal controls.
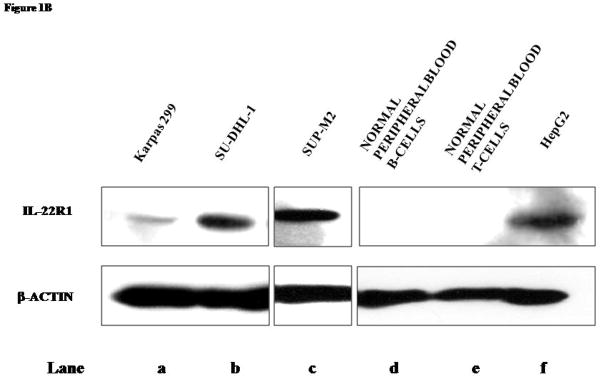
B) Western blot analysis confirmed the expression of IL-22R1 in all 3 ALK+ALCL cell lines examined. T- and B-cells harvested from the peripheral blood healthy individuals did not express IL-22R1. HepG2 was included as a positive control.
C) Immunofluorescence staining using antibodies directed against IL-22R1 (left panel) or control IgG (right panel) demonstrated the expression of IL-22R1 in ALK+ cell lines, SU-DHL-1, Karpas 299, and SUP-M2 (confocal microscopy).
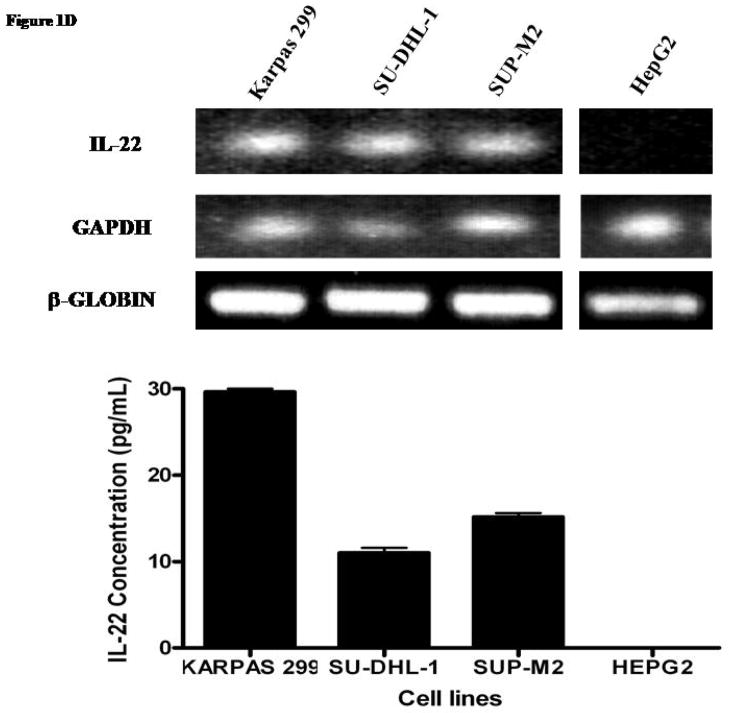
D) RT-PCR studies demonstrated the presence of IL-22 mRNA in ALK+ALCL cell lines. HepG2 was included as a negative control. GAPDH and β-globin were included as internal controls. ELISA performed revealed autocrine expression of IL-22 in ALK+ALCL cells. HepG2 was included as a negative control.
E) Immunohistochemical staining using a case of ALK+ALCL tumor confirmed the expression of IL-22 and IL-22R1 in ALK+ALCL cells. In picture I, the white arrow highlights the sinusoid infiltrated by IL-22-expressing ALK+ALCL lymphoma cells, whereas the black dotted arrow highlights a residual B-cell follicle, which was IL-22 negative. On high magnification (II), IL-22 staining was cytoplasmic. In picture III, the infiltrating lymphoma cells were positive for IL-22R1 whereas a residual B-cell follicle (dotted black arrow) was negative. Picture IV shows a high magnification view of the lymphoma cells expressing IL-22R1.
F) Double immunofluorescence staining was performed using frozen sections of an ALK+ALCL tumor. Co-expression of IL-22R1 (red signals) and IL-22 (green signals) were found in the same cell population located in the same area of the tissue section. Staining with omission of the primary antibody served as the negative control.
We then assessed the protein expression of IL-22R1 in ALK+ALCL cell lines using Western blots. As shown in Figure 1B, IL-22R1 protein was detectable in all ALK+ALCL cell lines (lane a–c) but not in normal peripheral blood T- and B-cells (lane d–e). HepG2 showed strong expression of IL-22R1 (lane f). To determine the sub-cellular localization of IL-22R1, we performed confocal microscopy. As shown in Figure 1C, IL-22R1 had a cytoplasmic and membranous pattern in all 3 ALK+ALCL cell lines.
We next sought to determine whether ALK+ALCL cells secrete IL-22. As shown in Figure 1D, RT-PCR revealed that IL-22 mRNA was expressed in all three ALK+ALCL cell lines examined. The amplifiable PCR products were sequenced which were confirmed to be IL-22. In addition, ELISA assay to quantify secreted IL-22 in the supernatant was performed using these 3 cell lines; soluble IL-22 was detectable in all three ALK+ALCL cell lines with the highest level found in Karpas 299. HepG2 was included as a negative control.
To evaluate the expression of IL-22R1 and IL-22 in ALK+ALCL tumors, we employed 10 paraffin-embedded tumors and immunohistochemistry. IL-22 was expressed in all tumors examined, as illustrated in Figure 1E (upper panel). Of note, a residual benign B-cell follicle included was not reactive with the anti-IL-22 (I), in keeping with the concept that IL-22 is a T-cell derived cytokine. As illustrated in III and IV, scattered ALK+ALCL cells were reactive with anti-IL-22R1. IL-22R1 had a membranous and cytoplasmic staining pattern. To further support that IL-22R1 and IL-22 were expressed in the same cell population, we employed double immunofluorescence staining and confocal microscopy using five cases of ALK+ALCL; results are illustrated in Figure 1F.
2. IL-22 modulated cell growth in ALK+ALCL
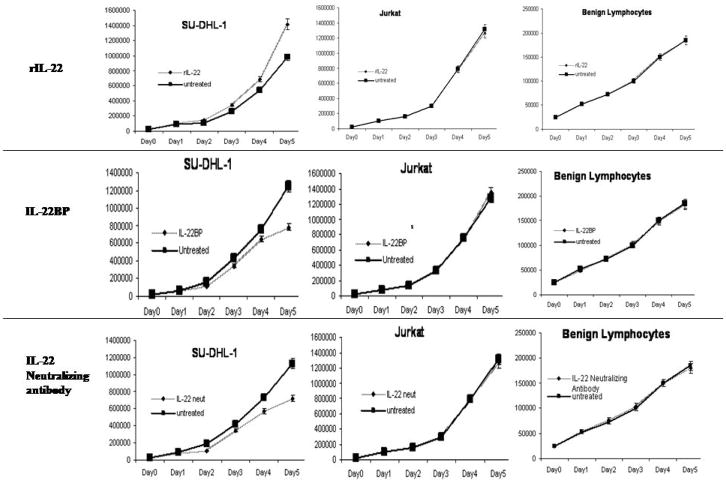
To assess the biological effects of IL-22 stimulation, all three ALK+ALCL cell lines were subjected to daily treatment with 10 ng/mL of recombinant IL-22 protein. Daily manual counts of viable cells were performed using trypan blue exclusion. Jurkat cells, a T-lymphoblast cell line that lacks IL-22R1 expression (data not shown), and normal peripheral blood lymphocytes, were included as negative controls. A significant increase in the viable cell numbers was observed in all 3 cell lines on day 3, and the results from SU-DHL-1 is illustrated in Figure 2. In contrast, no significant change was observed in Jurkat cells and benign lymphocytes. To support our manual cell count data, we performed MTS assay 3 days after adding recombinant IL-22 to the cell culture. Similar results were obtained: the cell count was significantly higher with IL-22 treatment in both SU-DHL-1 and Karpas 299 (p values, 0.0001, 0.028, respectively, Student’s t-test); no significant change was seen for Jurkat cells and benign lymphocytes.
Figure 2. IL-22 modulated cell proliferation in ALK+ALCL cell lines.
Treatment of SU-DHL-1 with recombinant IL-22 induced a significant increase in the number if viable cells, whereas treatment with IL-22BP or IL-22 neutralizing antibody resulted in a significant decrease in viable cells (trypan blue exclusion assay). No significant change was seen in Jurkat cells and benign peripheral blood lymphocytes.
To explore the effects on cell growth after IL-22 blockade, the same cell lines were subjected to daily treatment with 5 μg/mL of IL-22BP protein or 10 μg/mL of IL-22 neutralizing antibody. As shown in Figure 2 (middle and lower panels), all ALK+ALCL cell lines showed a significantly lower viable cell number on day 4, whereas no significant differences were detected between the treated and untreated cells in Jurkat and benign lymphocytes. MTS assay performed on day 3 again showed consistent results with the use of IL-22BP (p values, 0.02 for both SU-DHL-1 and Karpas 299) or IL-22 neutralizing antibody (p values, 0.03 and 0.007 for SU-DHL-1 and Karpas 299, respectively); no significant differences were detected for Jurkat cells and benign lymphocytes.
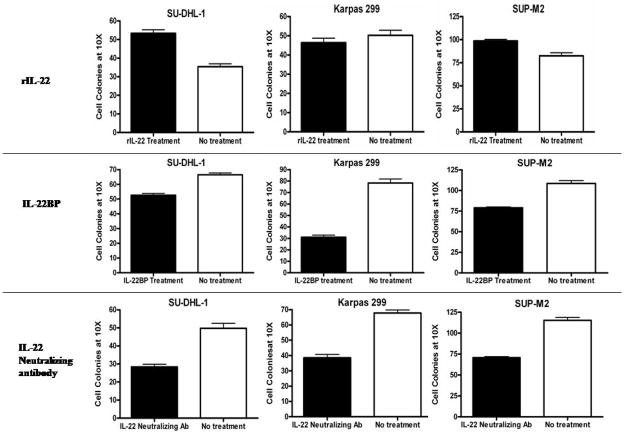
3. IL-22 modulated tumorigenicity assessed by soft agar colony formation
To determine the biological effects and significance of IL-22 autocrine stimulation, we employed soft agar colony formation assay. The results are summarized in Figure 3. When IL-22 was blocked using 5 μg/mL of IL-22BP recombinant protein or 10 μg/mL of IL-22 neutralizing antibody, there was a significant decrease in the number of colonies in all three ALK+ALCL cell lines on Day 14 (p values = 0.0001 for all 3 cell lines) (Figure 3), with the greatest difference seen in Karpas 299. In contrast, treatment of these cells with recombinant IL-22 (10 ng/mL) led to a significant increase in the number of colonies in SUP-M2 (p value = 0.0001), SU-DHL-1 (p value = 0.0001), but not Karpas 299 (p value = 0.339). The lack of significant effect in Karpas 299 is likely related to the fact that these cells produce the highest level of endogenous IL-22 among the 3 ALK+ALCL cell lines, as described above.
Figure 3. IL-22 signaling modulated the ability of colony formation by ALK+ALCL cells.
Treatment of the two ALK+ALCL cell lines, SU-DHL-1 and SUP-M2, with IL-22 recombinant protein induced a significant increase in colony formation. There was no significant change in the colony number in Karpas 299 cells after two weeks. Treatment of ALK+ALCL cell lines with IL-22BP or IL-22 neutralizing antibody induced a significant decrease in colony formation.
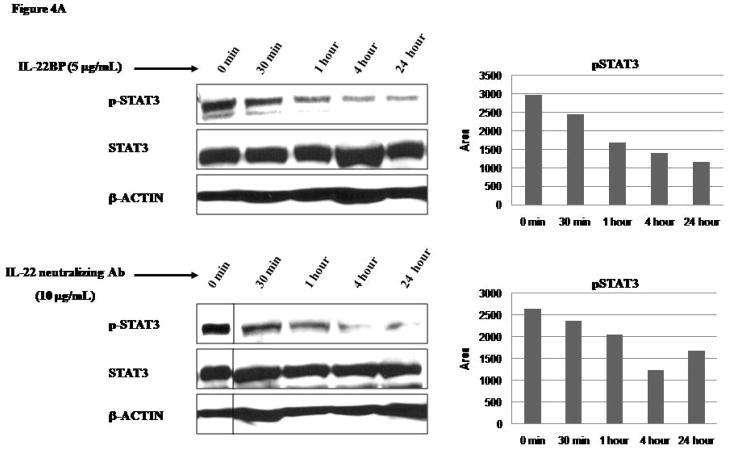
4. IL-22 modulated STAT3 and MAPK signaling
Because IL-22 have been reported to activate STAT3 in a number of non-hematologic cell lines, 15,21,22,27 we sought to determine if IL-22 also contribute to STAT3 activation, a mechanism know to contribute to tumorigenesis in ALK+ALCL. As illustrated in Figure 4A, IL-22 blockade using IL-22BP or an IL-22 neutralizing antibody led to a time-dependent decrease in pSTAT3 in all ALK+ALCL cell lines. In contrast, using Western blots, we detected a substantial increase in the pSTAT3 levels with the addition of IL-22 for 30 minutes to the cell culture (Figure 4B). Jurkat cells and benign lymphocytes were included as negative controls; no pSTAT3 was detectable in these cell types with or without IL-22 (data not shown). In the same experiment, we also demonstrated an increase in the phosphorylated forms of ERK1/2, JNK/SAPK, and p38 kinase after 30 minute exposure to recombinant IL-22 protein (illustrated in Figure 4B).
Figure 4. Effects of IL-22 on STAT3 and MAPK pathways.
A) Western blots revealed that SU-DHL-1 cells treated with IL-22BP (5 μg/mL) or IL-22 neutralizing antibody (10 μg/mL) resulted in a gradual and time-dependent decrease in pSTAT3. On the right panel, densitometry results for pSTAT3 were included.
B) Western blot revealed that SU-DHL-1 cells treated with recombinant IL-22 (10 ng/mL) for 30 minutes increased the level of pSTAT3. Three other signaling proteins in the MAPK kinase pathway (ERK1/2, JNK/SAPK, p38) were also activated. On the right panel, densitometry results for pSTAT3, pJNK/SAPK, pERK1/2, and phoshpho-p38 were included.
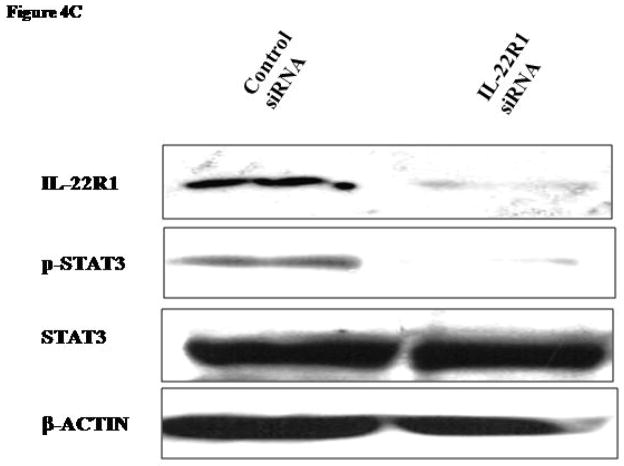
C) Compared to the scrambled siRNA, IL-22R1 siRNA downregulated both IL-22R1 and pSTAT3 (cell lysates prepared 24 hours after transfection). The STAT3 and β-actin expression levels were equal in both lanes.
To further evaluate the role of IL-22R1 in ALK+ALCL, we downregulated IL-22R1 expression using siRNA. 24 hours after siRNA transfection, there was a substantial decrease in IL-22R1 cells transfected with IL-22R1 siRNA but not scrambled siRNA (Figure 4C). Correlating with this change, pSTAT3 was decreased in cells treated with IL-22R1 siRNA.
5. NPM-ALK induced IL-22R1 expression
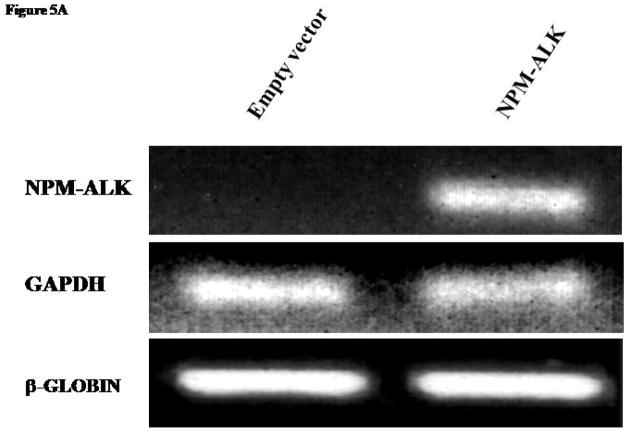
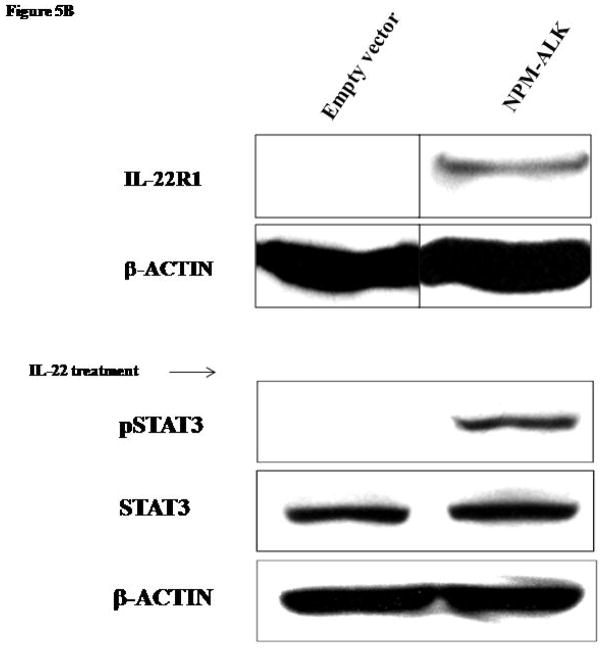
Because NPM-ALK is the characteristic oncoprotein in this tumor type, we asked if NPM-ALK is responsible for the aberrant expression of IL-22R1 in ALK+ALCL. We subjected Jurkat cells to transfection with either NPM-ALK vector or an empty vector. The expression of the NPM-ALK transgene was confirmed by RT-PCR (Figure 5A) in cells transfected with NPM-ALK but not with the empty vector. In cells transfected with NPM-ALK, but not those transfected with the empty vector, IL-22R1 protein expression was detectable by Western blots (Figure 5B).
Figure 5. Induction of IL-22R1 expression in NPM-ALK activated cell line.
A) RT-PCR studies confirmed the expression of NPM-ALK in Jurkat cells after transfection. Jurkat cells transfected with an empty vector was included as a negative control.
B) Transfection of Jurkat cells with NPM-ALK induced expression of IL-22R1 detectable by Western blots. After stimulation with IL-22 recombinant protein, pSTAT3 levels were detected in Jurkat cells transfected with NPM-ALK, but not in cells transfected with an empty vector.
To assess the ability of IL-22R1 to stimulate STAT3 activation by NPM-ALK, Jurkat cells transfected with either NPM-ALK or an empty vector were treated with IL-22 for 30 minutes and subjected to Western blot. Only cells transfected with NPM-ALK but not with the empty vector showed detectable pSTAT3 after treatment with IL-22 (Figure 5B).
DISCUSSION
IL-22, a recently discovered cytokine, 15 is a member of the IL-10 family proteins largely expressed by activated T- and NK-cells. 28,29 IL-22R1 is characteristically absent in benign immune cells including lymphocytes. 16–18 in various epithelial cell lines, IL-22 has been shown to promote cell proliferation and activate a number of signaling pathways including those of STAT3, MAPK and AKT. 22,30 It is currently believed that the physiological function of IL-22 is related to the maintenance of innate immunity. 23
One of the significant findings of this study is that of aberrant expression of IL-22R1 in ALK+ALCL, a type of mature T-cell neoplasm. The expression of IL-22R1 in ALK+ALCL, initially identified in our oligonucleotide array studies using ALK+ALCL cell lines, was confirmed by several different methods such as RT-PCR, Western blots, confocal microscopy and immunohistochemistry. We also identified consistent expression of IL-22 by these tumors, a finding that is not too surprising considering that ALK+ALCL is a T-cell neoplasm. A recently published study also reported the presence of IL-22 in ALK+ALCL. 24 Lastly, our results indicate that IL-22BP, the IL-22 decoy receptor, is absent in all 3 ALK+ALCL cell line examined. Taken together, our findings provide evidence to support the existence of the IL-22 autocrine stimulatory pathway that is functional and ‘un-checked’ in ALK+ALCL. Our functional assays including the soft agar colony formation assay support the concept that this pathway indeed contributes to its tumorigenicity in ALK+ALCL.
In keeping with the previous findings of IL-22 mediated activation of STAT3 in a number of epithelial cell types, 22,30,31 we found that STAT3 activation can be enhanced by IL-22 in ALK+ALCL cells. The importance of IL-22 in contributing to STAT3 activation in these cells is further supported by our findings that blockade of IL-22 signaling using siRNA, IL-22BP or an IL-22 neutralizing antibody substantially decreased STAT3 activation. STAT3 activation in ALK+ALCL is multi-factorial; in addition to NPM-ALK, JAK3 and loss of SHP1 contributes to deregulate this signaling pathway, 32–35 it is not too surprising that the addition or blockade of IL-22 can only partially change the pSTAT3 levels in these cells. Since constitutive activation of STAT3 is crucial to the pathogenesis of ALK+ALCL and NPM-ALK mediated lymphomagenesis, 5,11 enhancement of STAT3 activation in these cells correlates well with the observed increased tumorigenicity induced by IL-22. Of note, other than STAT3, IL-22 is also known to activate other signaling pathways such as the three major MAPK pathways, ERK1/2, JNK, and p38 kinase pathways. 22,36,37 These pathways, which have been implicated in transformation and tumor progression in many human cancers 38–40, were confirmed to be activated by IL-22 in ALK+ALCL cell lines. NPM-ALK is also a known activator of the ERK1/2 signaling pathway and has been shown to enhance cell proliferation and survival. 41 Stimulation of these pathways likely further promotes the tumorigenic effects of IL-22.
Since NPM-ALK plays a key role in the pathogenesis of ALK+ALCL, we investigated if NPM-ALK is directly responsible for the aberrant expression of IL-22R1 expression. Our transfection experiments using Jurkat cells strongly support that NPM-ALK expression can induce IL-22R1 expression, which in turn can activate STAT3 (Figure 5B). Thus, our results have clearly shown that NPM-ALK can convert an ‘IL-22 unresponsive phenotype’ into an ‘IL-22 responsive phenotype’. The mechanism by which NPM-ALK mediates this effect is unclear, but it is likely that specific signaling pathways may be implicated. This area is currently under investigation in our laboratory. Nevertheless, our results illustrate a novel pathway by which NPM-ALK promotes tumorigenesis.
Lastly, more recent studies in our laboratory have shown that the aberrant expression of IL-22R1 is not restricted to ALK+ALCL. In fact, one of the ALK-ALCL cell lines, MAC-2A, showed IL-22R1 expression (data not shown). Thus, mechanisms other than NPM-ALK likely induce aberrant IL-22R1 expression in lymphoma cells. Of note, a recent paper using cDNA microarray has demonstrated that ALK−ALCL produces IL-22. 24 It is highly likely that the IL-22 autocrine stimulatory pathway also carries important biological significance in the lymphomagenesis of ALK−ALCL, and this area warrants further studies.
Acknowledgments
This study is supported by operating research grants from the Alberta Cancer Foundation, Canadian Cancer Society/National Cancer Institute of Canada, and the Canadian Institute for Health Research awarded to RL. PG is a research fellow supported by the Lymphoma Foundation of Canada. MA is a clinical research fellowship supported by the Canadian Cancer Society and the National Cancer Institute of Canada. HMA is a recipient of the University of Texas M.D. Anderson Physician Scientist Program Award as well as a K08 award from the National Cancer Institute (CA114395).
References
- 1.Morris SW, Kirstein MN, Valentine MB, Dittmer KG, Shapiro DN, Saltman DL, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science. 1994;263:1281–1284. doi: 10.1126/science.8122112. [DOI] [PubMed] [Google Scholar]
- 2.Moser B, Wolf M, Walz A, Loetscher P. Chemokines: multiple levels of leukocyte migration control. Trends Immunol. 2004;25:75–84. doi: 10.1016/j.it.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Shiota M, Fujimoto J, Semba T, Satoh H, Yamamoto T, Mori S. Hyperphosphorylation of a novel 80 kDa protein-tyrosine kinase similar to Ltk in a human Ki-1 lymphoma cell line, AMS3. Oncogene. 1994;9:1567–1574. [PubMed] [Google Scholar]
- 4.Slupianek A, Nieborowska-Skorska M, Hoser G, Morrione A, Majewski M, Xue L, et al. Role of phosphatidylinositol 3-kinase-Akt pathway in nucleophosmin/anaplastic lymphoma kinase-mediated lymphomagenesis. Cancer Res. 2001;61:2194–2199. [PubMed] [Google Scholar]
- 5.Amin HMMT, Ma Y, Lin Q, Fujio Y, Kunisada K, Leventaki V, Das P, Rassidakis GZ, Cutler C, Medeiros LJ, Lai R. Selective inhibition of STAT3 induces apoptosis and G(1) cell cycle arrest in ALK-positive anaplastic large cell lymphoma. Oncogene. 2004;23:5426–5434. doi: 10.1038/sj.onc.1207703. [DOI] [PubMed] [Google Scholar]
- 6.Zamo A, Chiarle R, Piva R, Howes J, Fan Y, Chilosi M, et al. Anaplastic lymphoma kinase (ALK) activates Stat3 and protects hematopoietic cells from cell death. Oncogene. 2002;21:1038–1047. doi: 10.1038/sj.onc.1205152. [DOI] [PubMed] [Google Scholar]
- 7.Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, et al. Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 8.Burke WM, Jin X, Lin HJ, Huang M, Liu R, Reynolds RK, et al. Inhibition of constitutively active Stat3 suppresses growth of human ovarian and breast cancer cells. Oncogene. 2001;20:7925–7934. doi: 10.1038/sj.onc.1204990. [DOI] [PubMed] [Google Scholar]
- 9.Catlett-Falcone RLT, Oshiro MM, Turkson J, Levitzki A, Savino R, Ciliberto G, Moscinski L, Fernandez-Luna JL, Nunez G, Dalton WS, Jove R. Constitutive Activation of Stat3 Signaling Confers Resistance to Apoptosis in Human U266 Myeloma Cells. Immunity. 1999;10:105–115. doi: 10.1016/s1074-7613(00)80011-4. [DOI] [PubMed] [Google Scholar]
- 10.Levy DE, Lee CK. What does Stat3 do? J Clin Invest. 2002;109:1143–1148. doi: 10.1172/JCI15650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiarle R, Simmons WJ, Cai H, Dhall G, Zamo A, Raz R, et al. Stat3 is required for ALK-mediated lymphomagenesis and provides a possible therapeutic target. Nat Med. 2005;11:623–629. doi: 10.1038/nm1249. [DOI] [PubMed] [Google Scholar]
- 12.Amin HMML, Ma Y Feretzaki M, Das P, Leventaki V, Rassidakis GZ, O’Connor SL, McDonnell TJ, Lai R. Inhibition of JAK3 induces apoptosis and decreases anaplastic lymphoma kinase activity in anaplastic large cell lymphoma. Oncogene. 2003;22:5399–5407. doi: 10.1038/sj.onc.1206849. [DOI] [PubMed] [Google Scholar]
- 13.Cussac D, Greenland C, Roche S, Bai RY, Duyster J, Morris SW, et al. Nucleophosmin-anaplastic lymphoma kinase of anaplastic large-cell lymphoma recruits, activates, and uses pp60c-src to mediate its mitogenicity. Blood. 2004;103:1464–1471. doi: 10.1182/blood-2003-04-1038. [DOI] [PubMed] [Google Scholar]
- 14.Bonvini P, Gastaldi T, Falini B, Rosolen A. Nucleophosmin-anaplastic lymphoma kinase (NPM-ALK), a novel Hsp90-client tyrosine kinase: down-regulation of NPM-ALK expression and tyrosine phosphorylation in ALK(+) CD30(+) lymphoma cells by the Hsp90 antagonist 17-allylamino,17-demethoxygeldanamycin. Cancer Res. 2002;62:1559–1566. [PubMed] [Google Scholar]
- 15.Dumoutier L, Louahed J, Renauld JC. Cloning and characterization of IL-10-related T cell-derived inducible factor (IL-TIF), a novel cytokine structurally related to IL-10 and inducible by IL-9. J Immunol. 2000;164:1814–1819. doi: 10.4049/jimmunol.164.4.1814. [DOI] [PubMed] [Google Scholar]
- 16.Wolk K, Kunz S, Asadullah K, Sabat R. Cutting edge: immune cells as sources and targets of the IL-10 family members? J Immunol. 2002;168:5397–5402. doi: 10.4049/jimmunol.168.11.5397. [DOI] [PubMed] [Google Scholar]
- 17.Wolk K, Witte E, Reineke U, Witte K, Friedrich M, Sterry W, et al. Is there an interaction between interleukin-10 and interleukin-22? Genes Immun. 2005;6:8–18. doi: 10.1038/sj.gene.6364144. [DOI] [PubMed] [Google Scholar]
- 18.Wolk K, Witte E, Wallace E, Docke WD, Kunz S, Asadullah K, et al. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol. 2006;36:1309–1323. doi: 10.1002/eji.200535503. [DOI] [PubMed] [Google Scholar]
- 19.Dumoutier L, Lejeune D, Colau D, Renauld JC. Cloning and characterization of IL-22 binding protein, a natural antagonist of IL-10-related T cell-derived inducible factor/IL-22. J Immunol. 2001;166:7090–7095. doi: 10.4049/jimmunol.166.12.7090. [DOI] [PubMed] [Google Scholar]
- 20.Gruenberg BH, Schoenemeyer A, Weiss B, Toschi L, Kunz S, Wolk K, et al. A novel, soluble homologue of the human IL-10 receptor with preferential expression in placenta. Genes Immun. 2001;2:329–334. doi: 10.1038/sj.gene.6363786. [DOI] [PubMed] [Google Scholar]
- 21.Kotenko SV, Izotova LS, Mirochnitchenko OV, Esterova E, Dickensheets H, Donnelly RP, et al. Identification of the functional interleukin-22 (IL-22) receptor complex: the IL-10R2 chain (IL-10Rbeta) is a common chain of both the IL-10 and IL-22 (IL-10-related T cell-derived inducible factor, IL-TIF) receptor complexes. J Biol Chem. 2001;276:2725–2732. doi: 10.1074/jbc.M007837200. [DOI] [PubMed] [Google Scholar]
- 22.Lejeune D, Dumoutier L, Constantinescu S, Kruijer W, Schuringa JJ, Renauld JC. Interleukin-22 (IL-22) activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a rat hepatoma cell line. Pathways that are shared with and distinct from IL-10. J Biol Chem. 2002;277:33676–33682. doi: 10.1074/jbc.M204204200. [DOI] [PubMed] [Google Scholar]
- 23.Gurney AL. IL-22, a Th1 cytokine that targets the pancreas and select other peripheral tissues. Int Immunopharmacol. 2004;4:669–677. doi: 10.1016/j.intimp.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 24.Lamant L, De Reynies A, Duplantier MM, Rickman DS, Sabourdy F, Giuriato S, et al. Gene expression profiling of systemic anaplastic large cell lymphoma reveals differences depending on ALK status and two distinct morphological ALK+ subtypes. Blood. 2007;109:2156–2164. doi: 10.1182/blood-2006-06-028969. [DOI] [PubMed] [Google Scholar]
- 25.Dien J, Amin HM, Chiu N, Wong W, Frantz C, Chiu B, et al. Signal transducers and activators of transcription-3 up-regulates tissue inhibitor of metalloproteinase-1 expression and decreases invasiveness of breast cancer. Am J Pathol. 2006;169:633–642. doi: 10.2353/ajpath.2006.051109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han Y, Amin HM, Frantz C, Franko B, Lee J, Lin Q, et al. Restoration of shp1 expression by 5-AZA-2′-deoxycytidine is associated with downregulation of JAK3/STAT3 signaling in ALK-positive anaplastic large cell lymphoma. Leukemia. 2006;20:1602–1609. doi: 10.1038/sj.leu.2404323. [DOI] [PubMed] [Google Scholar]
- 27.Xie MH, Aggarwal S, Ho WH, Foster J, Zhang Z, Stinson J, et al. Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptor-related proteins CRF2-4 and IL-22R. J Biol Chem. 2000;275:31335–31339. doi: 10.1074/jbc.M005304200. [DOI] [PubMed] [Google Scholar]
- 28.Kotenko SV. The family of IL-10-related cytokines and their receptors: related, but to what extent? Cytokine Growth Factor Rev. 2002;13:223–240. doi: 10.1016/s1359-6101(02)00012-6. [DOI] [PubMed] [Google Scholar]
- 29.Brand S, Dambacher J, Beigel F, Zitzmann K, Heeg MH, Weiss TS, et al. IL-22 mediated liver cell regeneration is abrogated by SOCS-1/3 overexpression in vitro. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1019–G1028. doi: 10.1152/ajpgi.00239.2006. [DOI] [PubMed] [Google Scholar]
- 30.Weber GF, Gaertner FC, Erl W, Janssen KP, Blechert B, Holzmann B, et al. IL-22-mediated tumor growth reduction correlates with inhibition of ERK1/2 and AKT phosphorylation and induction of cell cycle arrest in the G2-M phase. J Immunol. 2006;177:8266–8272. doi: 10.4049/jimmunol.177.11.8266. [DOI] [PubMed] [Google Scholar]
- 31.Nagalakshmi ML, Rascle A, Zurawski S, Menon S, de Waal Malefyt R. Interleukin-22 activates STAT3 and induces IL-10 by colon epithelial cells. Int Immunopharmacol. 2004;4:679–691. doi: 10.1016/j.intimp.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 32.Amin HM, Medeiros LJ, Ma Y, Feretzaki M, Das P, Leventaki V, et al. Inhibition of JAK3 induces apoptosis and decreases anaplastic lymphoma kinase activity in anaplastic large cell lymphoma. Oncogene. 2003;22:5399–5407. doi: 10.1038/sj.onc.1206849. [DOI] [PubMed] [Google Scholar]
- 33.Han Y, Amin HM, Franko B, Frantz C, Shi X, Lai R. Loss of SHP1 enhances JAK3/STAT3 signaling and decreases proteosome degradation of JAK3 and NPM-ALK in ALK+ anaplastic large-cell lymphoma. Blood. 2006;108:2796–2803. doi: 10.1182/blood-2006-04-017434. [DOI] [PubMed] [Google Scholar]
- 34.Amin HM, Lai R. Pathobiology of ALK+ anaplastic large-cell lymphoma. Blood. 2007;110:2259–2267. doi: 10.1182/blood-2007-04-060715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Q, Raghunath PN, Xue L, Majewski M, Carpentieri DF, Odum N, et al. Multilevel dysregulation of STAT3 activation in anaplastic lymphoma kinase-positive T/null-cell lymphoma. J Immunol. 2002;168:466–474. doi: 10.4049/jimmunol.168.1.466. [DOI] [PubMed] [Google Scholar]
- 36.Andoh A, Zhang Z, Inatomi O, Fujino S, Deguchi Y, Araki Y, et al. Interleukin-22, a member of the IL-10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology. 2005;129:969–984. doi: 10.1053/j.gastro.2005.06.071. [DOI] [PubMed] [Google Scholar]
- 37.Ikeuchi H, Kuroiwa T, Hiramatsu N, Kaneko Y, Hiromura K, Ueki K, et al. Expression of interleukin-22 in rheumatoid arthritis: potential role as a proinflammatory cytokine. Arthritis Rheum. 2005;52:1037–1046. doi: 10.1002/art.20965. [DOI] [PubMed] [Google Scholar]
- 38.Du L, Lyle CS, Obey TB, Gaarde WA, Muir JA, Bennett BL, et al. Inhibition of cell proliferation and cell cycle progression by specific inhibition of basal JNK activity: evidence that mitotic Bcl-2 phosphorylation is JNK-independent. J Biol Chem. 2004;279:11957–11966. doi: 10.1074/jbc.M304935200. [DOI] [PubMed] [Google Scholar]
- 39.Nagata Y, Todokoro K. Requirement of activation of JNK and p38 for environmental stress-induced erythroid differentiation and apoptosis and of inhibition of ERK for apoptosis. Blood. 1999;94:853–863. [PubMed] [Google Scholar]
- 40.Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signal. 2000;12:1–13. doi: 10.1016/s0898-6568(99)00071-6. [DOI] [PubMed] [Google Scholar]
- 41.Marzec M, Kasprzycka M, Liu X, Raghunath PN, Wlodarski P, Wasik MA. Oncogenic tyrosine kinase NPM/ALK induces activation of the MEK/ERK signaling pathway independently of c-Raf. Oncogene. 2007;26:813–821. doi: 10.1038/sj.onc.1209843. [DOI] [PubMed] [Google Scholar]