Abstract
Objective
The purpose of this study was to examine working memory for sequences of auditory and visual stimuli in prelingually deafened pediatric cochlear implant users with at least 4 yr of device experience.
Design
Two groups of 8- and 9-yr-old children, 45 normal-hearing and 45 hearing-impaired users of cochlear implants, completed a novel working memory task requiring memory for sequences of either visual-spatial cues or visual-spatial cues paired with auditory signals. In each sequence, colored response buttons were illuminated either with or without simultaneous auditory presentation of verbal labels (color-names or digit-names). The child was required to reproduce each sequence by pressing the appropriate buttons on the response box. Sequence length was varied and a measure of memory span corresponding to the longest list length correctly reproduced under each set of presentation conditions was recorded. Additional children completed a modified task that eliminated the visual-spatial light cues but that still required reproduction of auditory color-name sequences using the same response box. Data from 37 pediatric cochlear implant users were collected using this modified task.
Results
The cochlear implant group obtained shorter span scores on average than the normal-hearing group, regardless of presentation format. The normal-hearing children also demonstrated a larger “redundancy gain” than children in the cochlear implant group—that is, the normal-hearing group displayed better memory for auditory-plus-lights sequences than for the lights-only sequences. Although the children with cochlear implants did not use the auditory signals as effectively as normal-hearing children when visual-spatial cues were also available, their performance on the modified memory task using only auditory cues showed that some of the children were capable of encoding auditory-only sequences at a level comparable with normal-hearing children.
Conclusions
The finding of smaller redundancy gains from the addition of auditory cues to visual-spatial sequences in the cochlear implant group as compared with the normal-hearing group demonstrates differences in encoding or rehearsal strategies between these two groups of children. Differences in memory span between the two groups even on a visual-spatial memory task suggests that atypical working memory development irrespective of input modality may be present in this clinical population.
Working memory is generally characterized as a limited capacity system used for short-term maintenance of information in memory. A long-standing debate surrounds the question of the extent to which input from the different sensory modalities is channeled independently through the mechanisms of working memory (Fastenau, Conant, & Lauer, 1998; Hale, Bronik, & Fry, 1997; Swanson, 1996). Over the years the theoretical construct of working memory has been “fractionated” into modality-specific pathways in the brain's prefrontal cortex sharing a common “executive” control center (Baddeley, 1986; Smith & Jonides, 1999). Much effort has been expended in trying to understand how sensory experience contributes to these apparent divisions (Lewkowicz & Lickliter, 1994; Stein & Meredith, 1993). Although past research has tended to study developmental changes in working memory in general terms of memory capacity, processing speed, and susceptibility to interference, the modality specificity of these influences is now undergoing extensive examination (e.g., Hale et al., 1997; Kail, 1991; Swanson, 1996).
Clear effects of early sensory experience and learning have been demonstrated by showing how memory performance improves with greater familiarity with the to-be-remembered items and the acquisition of strategies for circumventing capacity limitations (Dempster, 1981; Naus & Ornstein, 1983). Several common encoding strategies, by their very nature, seem to favor use of the auditory input modality. The learned strategy of verbal rehearsal for example, although best suited for the task of remembering the phonological characteristics of spoken or read words, is thought to be a mandatory process that is automatically used by normal-hearing adults whenever verbal labels can be applied (Brandimonte, Hitch, & Bishop, 1992; Carmichael, Hogan, & Walter, 1932). This heavy reliance on verbal encoding strategies has naturally led to interest in whether access to a typical oral/aural-based language environment is necessary for the development of aspects of working memory that are assumed to be modality-independent—Baddeley's “central executive” component, for example (Baddeley, 1992).
Literature published between the 1950s and 1970s suggested that hearing-impaired children in general were less adept overall than normal-hearing children in their working memory for some types of visual (but verbally codeable) sequences because these children lacked effective verbal strategies for rehearsal (Conrad, 1972; Furth, 1966; Marschark & Mayer, 1998; Mayberry, 1992). In the 1980s and 1990s, however, the focus of research shifted to showing that manually signed equivalents to verbal strategies could be adopted to analogous benefit, although the comparable efficiency of these manual strategies, and the frequency of their use by signing individuals came under debate (Hanson, 1990; Hanson & Lichtenstein, 1990; Shand, 1982; Tomlinson-Keasey & Smith-Winberry, 1990).
The present study investigated the processing and short-term storage of auditory and visual-spatial sequences in prelingually deafened pediatric cochlear implant users—profoundly deaf children who were without auditory input during their early development, but who currently use a prosthetic device that presents an electrically coded instantiation of sound directly to their auditory nervous system. Through an intervention program that follows the implant surgery, most cochlear implant users eventually acquire at least an awareness of sound through their implant and many (the majority) go on to develop open-set speech perception skills and are able to produce intelligible speech (Geers et al., 2000; Svirsky, Robbins, Kirk, Pisoni, & Miyamoto, 2000; Tobey et al., 2000). The skills attained through use of a cochlear implant vary quite widely among children, and the variables and processes that contribute to this outcome are not well understood (Pisoni, 2000; Pisoni, Svirsky, Kirk, & Miyamoto, 1997).
Large individual differences in spoken word recognition skills and language development continue to be observed in pediatric users of cochlear implants (Miyamoto et al., 1994; Tyler, Fryauf-Bertschy, Kelsay, Gantz, Woodworth, & Parkinson, 1997). A sizable proportion of this variability can be accounted for in terms of physiological and hardware-related factors. Miyamoto et al. (1994) suggest that about 40% of the variance in open-set speech perception measures can be accounted for in terms of processor type, duration of deafness, communication mode, age of onset of deafness, duration of cochlear implant use, and age at implantation. Other studies, using similar sets of variables, have obtained R-squared values ranging between 37% and 64% (Dowell, Blamey, & Clark, 1995; Snik, Vermeulen, Geelen, Brokx, & van den Broek, 1997). In young children, the age at which the hearing impairment occurred, the length of auditory deprivation, and amount of experience using the implant have all been shown to play important roles in predicting outcome (Fryauf-Bertschy, Tyler, Kelsay, Gantz, & Woodworth, 1997; Miyamoto et al., 1994; Nikolopoulos, O'Donoghue, & Archbold, 1999). From recent reexamination of audiological candidacy requirements, it has also been determined that the presence of even minimal amounts of residual hearing under aided conditions before implantation can also contribute positively to success with an implant (Seghal, Kirk, Pisoni, & Miyamoto, Reference Note 2; Zwolan, Zimmerman-Phillips, Ashbaugh, Hieber, Kileny, & Telian, 1997). However, there is still a large proportion of unexplained variance in this multivariate prediction of speech perception performance (Pisoni et al., 1997).
Our current research program on individual differences in cochlear implant users is motivated by the hypothesis that some significant part of this current error variance can be accounted for by cognitive factors related to the efficiency with which representations of spoken words are stored and retrieved from memory, after identification (Pisoni, 2000). In particular, given the pediatric cochlear implant user's newly acquired access to auditory information, one of our goals has been to tease apart possible contributions from modality-specific versus modality-independent aspects of working memory.
Although it is common practice to administer tests of “general intelligence” before implantation (Miyamoto, Robbins, & Osberger, 1993; Tiber, 1985), the early finding that overall IQ was a poor predictor of speech perception performance seems to have discouraged more detailed investigation of whether certain subscales within the IQ batteries might show more predictive power than others (for exceptions, see Quittner & Steck, 1991; Tiber, 1985; and for general discussion of intelligence measures obtained from pediatric cochlear implant users, see Knutson, Boyd, Goldman, & Sullivan, 1997). New research, however, has begun to explore the cognitive development of children with cochlear implants in greater detail and to investigate the specific information processing skills used in spoken language processing (Pisoni, 2000).
The focus on working memory in the present study was largely motivated by recent findings reported in Pisoni and Geers (2000) on auditory digit span in hearing-impaired children with cochlear implants. Auditory digit span is a simple and widely used task that requires that the subject listen to a list of digits and then repeat back the list items in the correct order. Typically, the length of the list is increased over the course of several trials until the subject can no longer do the task correctly.
Pisoni and Geers found that in a large group of pediatric cochlear implant users (N = 43), simple forward digit span measures (administered live voice with lipreading permitted, and requiring a spoken response during immediate recall) were significantly correlated with open-set spoken word recognition scores even when the most obvious confounding variables were statistically controlled for (simple bivariate r = +0.64; with variance from a test of speech feature discrimination removed r = 0.37). Pisoni and Geers interpreted this finding as evidence for the influence of “processing variables”—that is, skills and abilities “that are used in the encoding, rehearsal, storage, retrieval, and manipulation of the phonological representations of spoken words” (Pisoni & Geers, 2000, p. 93). Their findings on auditory digit spans were replicated more recently in a new sample of cochlear implant users (one of the two groups reported on in the current paper). Reanalysis of the pooled dataset (N = 88) showed that statistically significant correlations of at least r = +0.30 still remained, even with statistical “partialling-out” of variability linked to slight age differences, communication mode, duration of deafness, duration of device use, age of onset of deafness, number of active electrodes, as well as a measure of auditory speech feature discrimination (Pisoni, Cleary, Geers, & Tobey, 2000). Examination of the same dataset using the closely related procedure of factor analysis has led to a similar result (Geers, Reference Note 1).
These recent findings on auditory digit span suggest that approximately 10% of the variance in open set speech perception measures may be accounted for by individual differences in the cognitive skills tapped by the forward digit span memory task, or perhaps some other co-varying but as yet unidentified variable. In making the case for the relevance of memory span measures, Pisoni et al. (2000) have therefore proposed that an important cognitive processing variable related to how young children encode and manipulate the phonological representations of spoken words is contributing to the more general development of oral/aural language skills in pediatric cochlear implant users.
Surprisingly, relatively little other research has focused directly on auditory working memory in users of cochlear implants. Lyxell, Andersson, Arlinger, Bredberg, Harder, and Ronnberg (1996) have, however, examined in adult cochlear implant users whether it might be “possible to predict the level of speech understanding (postimplant) by means of a preoperative cognitive assessment.” Because Lyxell had previously obtained results suggesting that individual differences in processing speed and working memory capacity could account for a portion of the variability found in the speechreading/lipreading scores of normal-hearing adults, he hypothesized that this same relationship might also be observed in users of cochlear implants. Although Lyxell et al. reported negative results for the above study—that is, no evidence of working memory capacity being predictive of speechreading ability—they have, as yet, only examined the case of postlingually deafened adult cochlear implant users, and not prelingually deafened pediatric users of cochlear implants.
In another, more clinically oriented study of postlingually deafened cochlear implant users, Gantz, Woodworth, Abbas, Knutson, and Tyler (1993) identified six preoperative measures that accounted for approximately two-thirds of the between-subject variability in sound-only open-set word recognition scores 9 mo postimplant. The Wechsler Memory Test, a battery of learning, memory, and working memory measures, though initially included in the battery of predictor variables, failed to exhibit any sizable correlation with the word recognition scores. Gantz et al. did, however, report that “the ability to extract information from sequentially arrayed signals and rapidly process that information as measured by the signal detection score from the Visual Monitoring Task, appears to be relevant to word understanding with an implant” (Gantz et al., 1993, p. 915). The visual detection task used by Gantz et al. required participants to monitor a series of visually presented digits shown one at a time, for a specified subsequence—e.g., an odd-even-odd sequence of digits—thus requiring short-term storage of at least two items in recent memory during presentation (Knutson, Hinrichs, Tyler, Gantz, Shartz, & Woodworth, 1991). In another article by this research group on the skills of postlingually deafened adults, the scores on this same visual monitoring task were again found to correlate between +0.30 and +0.40 with auditory-only measures of phoneme discrimination (Knutson et al., 1991).
At first glance it may appear somewhat surprising that a “visual” skill that seems minimally dependent on the verbal ability associated with spoken language would show any relation to subsequent gains made in speech understanding by hearing-impaired listeners. One point that bears discussion, however, is whether hearing subjects typically approach the above visual monitoring task using non-verbal strategies (i.e., do they tend to rehearse or keep a verbal tally using the spoken names of the items immediately prior in the monitored sequence, or can the visual patterns on the screen avoid “verbal mediation” en route to being remembered?) Here it is relevant to consider recent evidence showing that the skills tapped in such “visual monitoring” tasks develop somewhat atypically in at least some pediatric cochlear implant users as compared with normal-hearing children. Quittner, Smith, Osberger, Mitchell, and Katz (1994) used a task similar to Gantz et al.'s (1993) with normal-hearing and hearing-impaired children (cochlear implant and hearing aid users) ages 6 to 13 yr. The participants were required to monitor a series of single-digit numerals on a screen and respond only when a certain specified sequence of two successive numbers was seen. No auditory stimuli were presented in this task. Both groups of hearing-impaired children did significantly worse than the hearing controls in the 6 to 8 yr age range, and although this difference was not statistically significant in the older age range of 9 to 13 yr (probably due to high within-group variability), average performance was still worse in the hearing-impaired groups. Although no word recognition or language development outcome measures were reported for these samples, the results suggest that some pediatric cochlear implant users (even those with several years experience with an implant) may encounter more difficulty than is typical for their age-matched hearing peers even when they are presented with a memory/attention task that could conceivably be performed on the basis of vision alone.
Experiment 1
The task employed in this investigation is neither the traditional verbal auditory digit span measure used in Pisoni and Geers (2000) (although the digit span measure was also gathered for comparison purposes), nor the visual monitoring and attention task used by Quittner et al. (1994) (though it shares some characteristics with this task). Instead, the “memory game” procedure used in this study involves the presentation of a sequence of sounds in conjunction with a sequence of colored lights located behind a series of four large translucent buttons mounted on a response box modeled after the popular Milton Bradley electronic game Simon™ (Fig. 1). The task requires the child to immediately reproduce the target sequence by pressing on the appropriate buttons in the proper order thereby causing the synchronized sounds to be heard again as the buttons are pressed. The difficulty of the task is adjusted by increasing the list length of the sequence to be reproduced when the child is doing well, and shortening it after an error is made. A computer program monitors the child's performance using an adaptive testing algorithm and records the longest list length a child is able to reproduce under a given set of conditions as a measure of “memory span.”
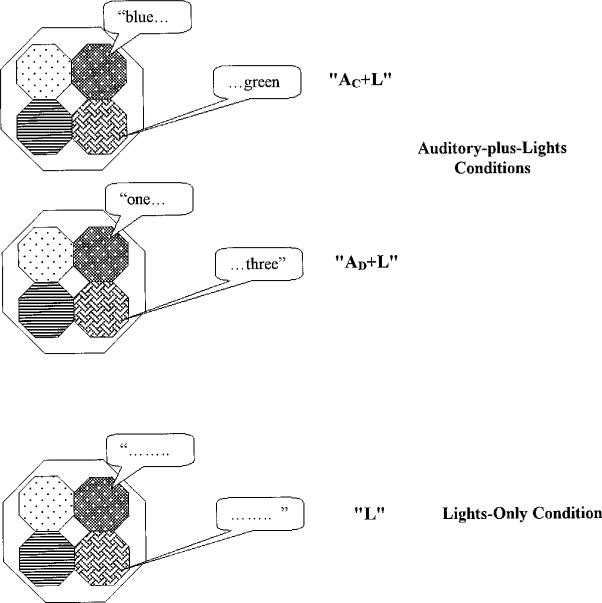
Figure 1.
Diagram of memory game apparatus and experimental conditions given a list length of two items. Each shaded hexagon represents a large colored button back-lit by a light. All auditory stimuli were presented via a loudspeaker located just behind the memory game response box. The verbal labels simply illustrate the consistent mapping between a particular auditory stimulus and a given button location. “AC+L” = auditory color-names-plus-lights; “AD+L” = auditory digit-names-plus-lights; “L” = lights-only.
The motivation for using this particular methodology was to obtain a measure of memory span using a nonlinguistic manual response, rather than a spoken or signed verbal response as is used in traditional digit span tasks. A nonlinguistic manual response was adopted to avoid possible confounds involving individual differences in rate and fluency of articulation and/or manual signing (i.e., productive language skills). The choice of target stimuli was determined by our interest in assessing differences in memory span as a function of whether or not redundant auditory cues in addition to visual-spatial cues were presented as part of the target sequence. That is, we examined the effect of presenting auditory stimuli during a task that could also be performed on the basis of vision alone. We also manipulated whether or not the redundant sound stimuli were semantically congruent with the light sequence. In one condition, whenever a sound was presented, the auditory stimulus was the color-name of the currently illuminated button. In a second multi-modal condition, a spoken digit-name was arbitrarily assigned to each of the four response buttons and consistently presented whenever the matched button was illuminated. Finally, a uni-modal control condition also was included that presented sequences only of lights without any auditory stimuli (Fig. 1).
For the normal-hearing children, we expected that the presence of an informationally redundant auditory stimulus would lead to higher memory span scores compared with the uni-modal stimulus-presentation condition. In the cochlear implant group, our primary focus was on whether those cochlear implant users who could correctly identify the auditory stimuli when presented in isolation would show the same benefit from the addition of the redundant auditory signals as was expected for the normal-hearing group. Because all of the pediatric cochlear implant users in the current study had used their cochlear implants for at least 4 yr, we judged it more likely that the mechanisms of phonological working memory might have developed in some members of this sample, than if relatively inexperienced users had been selected. Although the previous research showing differences in visual monitoring performance in pediatric cochlear implant users was suggestive, the cochlear implant group was not necessarily expected to perform differently from the normal-hearing children on the visual-spatial-only version of the memory game task.
An examination of the relationship between traditional verbal digit span and each of the three different presentation conditions of the memory game task was also planned for both groups of children. Because the digit span task makes no demands on visual-spatial aspects of working memory, smaller correlations between verbal digit spans and auditory-plus-visual-spatial memory game scores were expected for those children who could be shown to be doing the memory game task by vision alone, and larger correlations were anticipated for those children who demonstrated use of the redundant auditory stimuli in this task.
Method
Participants
Forty-five pediatric cochlear implant users were recruited as part of a large ongoing study by the Central Institute for the Deaf (CID). Participants were preselected by CID to demonstrate relatively low variability in chronological age (between 8 and 9 yr of age), age at onset of deafness (before age 3), and device experience (at least 4 yr of use) (Geers et al., 1999). Participant characteristics for the cochlear implant group, excluding one child whose data were incomplete, are shown more fully in Table 1. Nineteen children were female and 25 were male. Each child's degree of exposure to Oral- only communication methods was quantified by determining the type of communication environment experienced by the child in the year just before implantation, each year over the first 3 yr of cochlear implant use, and then in the yr just before the current testing. A score was assigned to each yr, with a “1” corresponding to the use of “total communication” with a sign emphasis (that is, extensive use of manual signs in addition to spoken language), and a “6” indicating an auditory-verbal environment with a strong emphasis on auditory communication without the aid of lipreading (Geers et al., 1999). Communication methods intermediate between these two extremes were assigned intermediate scores ranging from 2 to 5. These scores were then averaged over the five points in time. The mean communication mode score for the group over the five intervals was approximately 3.8 on this 6-point scale. A wide range of communication mode backgrounds was, however, present within the sample (range of average communication mode scores = 1.6 to 6.0). All children in this group used a Nucleus 22 cochlear implant containing between 6 and 20 active electrodes, with the group mean being 17+ active electrodes.
TABLE 1.
Participant characteristics for Experiment 1, cochlear implant group (N = 44).
| Range | Mean | |
|---|---|---|
| Chronological age at testing | 8;1–9;10 | 8;10 |
| Age at onset of deafness | 0;0–3;0 | 0;4 |
| Duration of deafness | 0;3–5;0 | 3;0 |
| Length of cochlear implant use | 4;3–6;9 | 5;5 |
Forty-five normal-hearing children also were recruited for this study as a comparison group. The normal-hearing children were matched for gender and chronological age with the children in the CID group. The mean age for both groups was 106 mo (8 yr, 10 mo). A hearing screening was conducted for each normal-hearing child at 250, 500, 1000, 2000, and 4000 Hz at a level of 20 dB HL using a Maico Hearing Instruments pure-tone audiometer (MA27) and TDH-39P headphones (a response at 25 dB HL was accepted for 250 Hz due to ambient room noise). Left and right ears were screened separately. Of 53 normal-hearing participants initially recruited, data from eight children were not included in the analysis due to one of the following reasons: failure to pass the hearing screening, equipment malfunction, experimenter error, or lack of a suitably matched child in the cochlear implant group.
Materials and Procedure
The auditory stimuli were created by recording utterances from a male speaker of American English in a sound-attenuated, single-walled anechoic recording chamber (Industrial Acoustics Company Audiometric Testing Room, Model 402) using a head-mounted close-talking microphone (Shure, Model SM98). The talker was recorded saying the words “red,” “blue,” “green,” “yellow,” “one,” “three,” “five,” and “eight” in a clear voice at a moderate rate of speech. Each word was spoken in isolation. The durations of the stimuli were not artificially equated, but were retained in their original form. The color-names ranged between 360 msec and 400 msec in length, whereas the digit-names ranged more widely between 290 msec and 490 msec in length. The recordings were digitally sampled online at 22.05 kHz with 16-bit amplitude quantization using a Tucker-Davis Technologies System II with an A-to-D converter (DD1), and a low-pass filter of 10.4 kHz (anti-aliasing filter, FT5), controlled by an updated version of Dedina's 1987 “Speech Acquisition Program” (Dedina, 1987; Hernandez, 1995). The amplitudes of the individually edited speech files were then adjusted using a digital leveling procedure such that the average RMS amplitudes for each file were approximately equated. All auditory signals were presented via a single loudspeaker (Advent AV280, 10 Watts amplifier output power, THD < 1%, frequency response 70 Hz to 20 kHz) at approximately 70 dB SPL as determined via a hand-held sound level meter (Triplett Model 370) held at approximately the level of the child's head.
Presentation of the stimuli was controlled by a computer program specially created for this purpose, running on a PC computer. The response box used to collect the child's button presses consisted of a large round disk-like plastic case approximately 10 inches in diameter housing four wide plastic buttons on its surface. The four buttons were each approximately one quarter of the surface area of the response box in size and could be easily depressed by a child. Each button was made of a different color plastic and could be illuminated by a light located beneath its surface. The colors of the buttons matched the color-names that were recorded as stimuli. The button response box was interfaced to the PC computer so that the control program could illuminate the lights when the sound stimuli were played, and turn off the lights once the stimuli ceased outputting. In the case of the lights-only presentation condition, the control program illuminated each light for the same stimulus duration as used in the color-name presentation condition. The computer recorded all button presses and automatically tracked the subject's performance using an adaptive testing procedure further described below.
The cochlear implant users were tested by professional clinicians and researchers experienced in working with hearing-impaired children as part of a larger study being conducted by the CID (see Geers, Reference Note 1). The normal-hearing children were tested at the Speech Research Laboratory at Indiana University by graduate and undergraduate research assistants. All children were tested individually in a quiet room. Each presentation condition of the memory game task took approximately 4 minutes to complete.
Identification Testing
Before the memory game was administered, each child was asked to identify the recorded tokens of the digits and color-names by pointing to one of four numerals printed in large lettering on a piece of paper or one of four large colored squares. These stimulus tokens were presented one at a time through the same loudspeaker as was used for the memory game. The digit-names and color-names were presented separately in two sets. If a child correctly identified all four items in a set on the first attempt, no further identification testing was administered. If one or more errors were made, the identification task was repeated up to three times, or until zero mistakes were made on a given set of stimuli, whichever occurred first. Twenty-one cochlear implant children identified all four digit-names correctly on their first try. Twenty-two cochlear implant children did the same for the four color-names. Identification of the stimulus “eight” seemed to pose a problem for many of the pediatric cochlear implant users. Eighteen of the 44 cochlear implant children misidentified this item one or more times during this pretest. Errors on the set of color-names were randomly distributed across the set of four stimuli. None of the normal-hearing children misidentified any of the test stimuli from either set during the same identification task. Regardless of identification errors made, the memory game task was administered next.
Memory Game Task
Participants were shown how the buttons on the response box could be pressed and were told that they would be hearing sounds through the loudspeaker and seeing the buttons light up. They were then instructed to “pay attention and copy exactly what the computer does by pressing on the buttons.” A hypothetical example was given to ensure that the children understood what they were supposed to do: “So for example, if you hear ‘blue’ and then ‘green’ (pointing consecutively to two buttons), I want you press the blue button and then the green button. Do you understand?”
Sequences used for the memory game task were generated pseudo-randomly by a computer program, with the stipulation that no single item would be repeated consecutively in a given list. A very brief inter-stimulus interval of 100 msec was used between sequence items. However, because the individual stimuli had been recorded at a moderate speech rate, the rate of presentation was actually about 2 items per second. Each child started with a list length of one item. If two lists in a row at a given length were correctly reproduced, the next list presented was increased by one item in length. If on any trial the list was incorrectly reproduced by the child, the next trial used a list one item shorter in length. This “adaptive tracking procedure” is similar to methods typically used in psychophysical testing (Levitt, 1970). If the child made no response within 4 sec after the last target item was presented (for example if the child turned away temporarily from the task), the sequence was presented again up to two additional times. The child was not permitted to wait for re-presentation of the sequence (e.g., a second, or third time) if he or she had attended to the first presentation. The computer recorded when and if a child was permitted an extra repetition of the list. Very few trials involved such repetitions. Twenty unique trials were presented total, for a maximum possible list length of 10 items. Although the experimenter provided no explicit feedback regarding the accuracy of the child's responses, whenever the child pressed a button during the response period the button was illuminated and the appropriately mapped sound was played out. Verbal encouragement was given only to keep the child on task during the procedure.
The independent variable of presentation condition (color-names-plus-lights versus digit-names-plus-lights versus lights-only) was administered within subject. All children were presented with 20 lists to reproduce under each of these three conditions. The lights-only condition was always completed last in the series of three conditions, whereas the two auditory-plus-visual-spatial conditions (color-names and digit-names) were counterbalanced across subjects. This procedure was followed based on practice effects evident in pilot testing, and a desire to not encourage purely visual-spatial encoding in a third of the children by introducing them first to the task in which no auditory stimuli were presented. Because the lights-only condition was assumed to be the most difficult of the three conditions, placing it last in the series, when coupled with practice effects raising scores later in the testing session, can be reasoned to conservatively work against finding significant differences among the three conditions.
WISC Digit Span
Children in both the cochlear implant and normal-hearing groups also completed the WISC Digit Span Supplementary Verbal Sub-test of the Wechsler Intelligence Scale for Children, Third Edition (WISC-III) (Wechsler, 1991). This memory span task requires the child to repeat back a list of digits as spoken live-voice by an experimenter at a rate of approximately one digit per second (WISC-III Manual, Wechsler, 1991). In the “digits-forward” section of the task, the child is required to simply repeat back the list as heard. In the “digits-backward” section of the task, the child is told to “say the list backward.” In both parts of the WISC task, the lists begin with two items, and are increased in length on successful repetition until a child gets two lists incorrect at a given length, at which point testing stops. Points are awarded for each list correctly repeated with no partial credit.
Although the CID research group gathered a large number of other speech and language-related measures from the cochlear implant children as part of their larger study (Geers et al., 1999), only the working memory tasks (the memory game and WISC digit span) are reported here. Other analyses will appear in future reports.
Vocabulary Screening Measure
Finally, the Peabody Picture Vocabulary Test, a measure of receptive vocabulary (PPVT-III Form A, Dunn & Dunn, 1997) was administered to all normal-hearing children to obtain a general measure of verbal language development. Every normal-hearing child tested obtained a standardized score no lower than one standard deviation below the published norm mean for his or her age.
Results and Discussion
One cochlear implant participant failed to complete the memory game task, reducing the number of participants in that group to 44. The matched normal-hearing child was also dropped from the final analysis. We had planned that our primary dependent measure of performance on the memory game task in each condition would be the longest list length that the child was able to correctly reproduce at least once during that condition. After our initial analysis, however, we became concerned that the “at least once” scoring method was overly “coarse” and could be collapsing over meaningful differences in performance between individual children. Therefore, we also computed a “weighted” span score for each child. This weighted score was calculated by finding the proportion of lists correct at each list length (e.g., the child correctly reproduced four of six lists presented at a list length of four items = 0.667), and this proportion was summed across all list lengths. (The use of an adaptive testing algorithm entailed that different subjects experienced a variable number of lists of any one given length, but all subjects received 20 lists per condition.) Across all 88 children, this weighted scoring method correlated r = +0.95 with the “at least once” measure and is much more continuously and normally distributed. We will not consider the “at least once” scoring method any further in this report, but qualitatively equivalent results are obtained using the original scoring procedure.
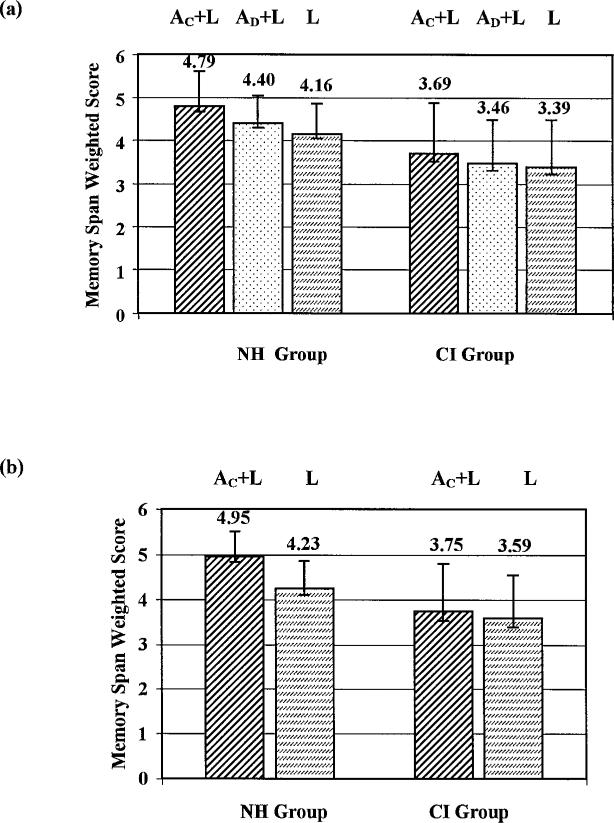
Figure 2A displays mean scores for the normal-hearing and cochlear implant groups as a function of presentation condition. An alpha level of 0.05 was adopted for all statistical tests reported below. In our first set of analyses, we included the full set of 44 cochlear implant users, regardless of their performance on the identification task. A 2 × 3 mixed factorial repeated measures analysis of variance for hearing status (normal-hearing versus cochlear implant) and presentation condition (color-names, digit-names, lights-only) demonstrated a within-subjects main effect of presentation condition, F(2,172) = 11.77, p < 0.001, and a between-subjects main effect of hearing status, F(1,86) = 32.9, p < 0.001. Normal-hearing children obtained significantly higher scores than the pediatric cochlear implant users in every condition of the memory game task, including the lights-only condition (t(86) = 3.937, p < 0.001). No significant interaction was found between presentation condition and hearing status (F(2,172) = 1.43, p = 0.24), indicating that the advantage of the normal-hearing children compared with the cochlear implant group was not significantly greater in the auditory-plus-lights conditions than in the lights-only condition. Post hoc Bonferroni pair-wise comparisons between the three presentation conditions over both hearing status groups indicated a significant difference between the means for the color-name and lights-only conditions (p = 0.001), and a significant difference between the color-name and digit-name conditions (p = 0.008). No statistically significant difference was obtained between the digit-name and lights-only conditions (p = 0.33), indicating that the addition of the auditory digit-names had no significant effect on performance over lights-alone.
Figure 2.
Comparison of performance on the memory game task across different presentation conditions in the normal-hearing (NH) and pediatric cochlear implant (CI) groups. Mean span scores from the NH group are shown on the left, mean span scores from the CI group on the right. “AC+L” = auditory color-names-plus-lights; “AD+L” = auditory digit-names-plus-lights; “L” = lights-only. Mean scores from all participants in each group are shown in the top panel (a). Mean scores from the subset of 22 CI participants who made no errors on the color-name identification pretest and the 22 normal-hearing children with whom they were matched are shown in (b). Positive error bars indicate 1 SD from the mean. Error bars in the negative direction indicate 1 standard error.
Although no significant interaction was obtained in the overall analysis when all three presentation conditions were considered, the overall statistical picture was complicated by the inclusion of the digit-name condition, and also by the inclusion of children who had made errors on the stimulus identification pretest. We therefore also conducted a 2 × 2 mixed factorial repeated measures analysis of variance for hearing status versus presentation format excluding the digit-name condition, for only the 22 cochlear implant children who correctly identified all color-name stimuli with no errors and the 22 normal-hearing children with whom they were matched. This analysis demonstrated a within-subjects main effect of presentation condition, F(1,42) = 13.5, p = 0.001, a between-subjects main effect of hearing status, F(1,42) = 18.15, p < 0.001, and, in this case, a significant interaction between presentation condition and hearing status (F(1,42) = 5.39, p = 0.025. The source of this interaction was the lack of significant gain from the addition of the semantically redundant auditory color-names in the cochlear implant group (paired-samples t-test, t(21) < 1, p = 0.40), along with a reliable “redundancy gain” demonstrated by the normal-hearing group (t(21) = 4.91, p < 0.001). The size of this redundancy gain was +0.72 “memory span units” for the normal-hearing group, but only +0.16 units for the cochlear implant group (Fig. 2B).
These results show that, as expected, the normal-hearing children did obtain longer “spans” on average, when auditory stimuli accompanied the visual-spatial light sequence. However, this advantage, or “redundancy gain,” was only statistically reliable when the sounds were “semantically redundant” with salient characteristics of the spatial locations to be remembered. That is to say, merely providing a consistently mapped auditory stimulus (i.e., the spoken digit-names) did not provide reliable benefit for either group of children. Examination of Figure 2 suggests that although the differences in performance among the presentation conditions followed the same overall pattern in the cochlear implant group, the within-group variability among the pediatric cochlear implant users was too large for the presentation format manipulation to yield reliable differences. Children in the cochlear implant group did not seem to use the informationally redundant auditory cues as effectively as the normal-hearing children even if they could correctly identify all of the auditory signals when presented in isolation.
Table 2 lists the intercorrelations for the children's scores in color-names-plus-lights and lights-only conditions of the memory game task as a function of hearing status. The correlations are shown both for all children tested, as well as for, in parentheses, the subset of 22 cochlear implant children who correctly identified the color-name stimuli in isolation and their matched normal-hearing counterparts. The large positive correlation observed between the color-names-plus-lights and lights-only conditions of the memory game would be expected in the cochlear implant group, if it were the case that these children were approaching both conditions in the same manner using a visual-spatial encoding strategy regardless of whether the additional auditory component were present. On the other hand, the normal-hearing children demonstrate a low correlation between these two memory game conditions, suggesting the use of different encoding or rehearsal strategies in each condition.
TABLE 2.
Intercorrelations obtained in Experiment 1 between WISC forward digit span, memory game conditions, and WISC backward digit span.
| Cochlear Implant Group N = 43 (Subgroup Values in Parentheses, N = 22) | |||
|---|---|---|---|
| Memory Game |
|||
| WISC Forward Digit Span | Color-Names-Plus-Lights | Lights-Only | |
| Memory game | |||
| Color-names-plus-lights | 0.09 (0.14) | — | |
| Lights-only | –0.02 (–0.07) | 0.71** (0.60**) | — |
| WISC backward digit span | 0.52** (0.47*) | 0.46** (0.50*) | 0.48** (0.57**) |
| Normal-Hearing Group N = 44 (Subgroup Values in Parentheses, N = 22) | |||
|---|---|---|---|
| Memory Game |
|||
| WISC Forward Digit Span | Color-Names-Plus-Lights | Lights-Only | |
| Memory game | |||
| Color-names-plus-lights | 0.58** (0.54*) | — | |
| Lights-only | 0.01 (0.03) | 0.20 (0.30) | — |
| WISC backward digit span | 0.32* (0.08) | 0.36* (0.52*) | 0.19 (0.22) |
p < 0.05
p < 0.01.
Although age in months has been partialled out of the calculations, age showed only a small positive correlation in the normal-hearing group and a near-zero correlation in the cochlear implant group, making the simple correlations almost identical to those shown above.
Table 2 also shows that forward digit span scores were only significantly positively correlated with memory game scores in the case of the normal-hearing group's performance in the color-names-plus-lights condition. In this condition, but not in the lights-only condition, the normal-hearing children seem to have used strategies or processes also used in the traditional verbal digit span task. The failure to find this same pattern of correlations among the cochlear implant participants, taken together with the absence of a significant redundancy gain in the color-name condition, suggests that the pediatric cochlear implant users processed the sequences differently, using primarily visual-spatial cues to perform the memory game task even when auditory cues were available to them.
Table 2 also includes a row showing the correlations between each of the memory tasks just discussed and the backward digit span scores. The common theoretical interpretation of the backward span task is that because it requires an effortful “strategic” manipulation of the presented items, it is a better measure of “working memory” than the forward digit span task (Lezak, 1995). Table 2 shows that there are consistent small to moderate correlations between the memory game task and backward digit span. This relationship is present in the cochlear implant group for both memory game presentation conditions. In the normal-hearing group, only the color-names-plus-lights condition of the memory game is significantly correlated with backward digit span. Although we suspect that the overall pattern of correlations is due to a component of backward digit span that requires “strategic spatial manipulation” of the items to effect the reversed list, the lack of a significant correlation between the scores of the normal-hearing group in the spatially-based lights-only condition of the memory game and backward digit span is not entirely consistent with this explanation.
Calculation of simple correlations also indicated that the pediatric cochlear implant users’ forward digit span scores, which are primarily auditory and require verbal coding, were positively correlated with their auditory-only open-set word recognition scores as measured via the LNT (Kirk, Pisoni, & Osberger, 1995) in both the full sample of children (r = +0.53, p < 0.001) as well as in the smaller subset of 22 cochlear implant children (r = +0.48, p = 0.023). However, none of the memory game conditions, each of which incorporated a visual-spatial component, were significantly correlated with the word recognition scores. This result is consistent with the finding that although significant positive correlations were obtained in this cochlear implant group between degree of exposure to an oral-only communication environment and WISC verbal digit spans (e.g., r = +0.45, p = 0.037, for N = 22) (see also, Pisoni & Geers, 2000), these correlations were not observed between the memory game measures of span and communication mode. Thus, amount of exposure to an oral-only language environment was not found to be associated with higher memory span scores when the memory game task included visual-spatial signals.
Conclusions
We found in Experiment 1 that normal-hearing school-age children were sensitive to the informational redundancy present in the color-names-plus-lights condition of the memory game task. The hearing-impaired pediatric cochlear implant users that participated in our study did not reliably demonstrate the same sensitivity to the relationship between the auditory and visual stimuli. The data also indicated that the cochlear implant group did less well than the normal-hearing group even on a condition of the memory game task that provided only visual-spatial target sequences to be remembered. This was an interesting and somewhat unexpected finding.
After looking at the results obtained from the cochlear implant group, we realized that the visual-spatial component of the memory game task needed to be reduced to obtain a measure of more purely auditory working memory from children who use cochlear implants. To begin to address this issue, a subset of our normal-hearing subjects were asked to complete an additional condition of the memory game task, as described below in Experiment 2.
Experiment 2
To try to discourage purely visual-spatial processing of the target sequences, a subset of the normal-hearing participants tested in Experiment 1 was asked to play an additional “round” of the memory game in which sequences of color-names were played through the loudspeaker. This time, however, the auditory stimuli were presented without the lights flashing synchronously on the memory game response box. Thus, on a given trial, a child might hear the list “red, green, blue, red,” output via the loudspeaker, but no lights would be presented on the response box as the target sequence was played out. The child would then have to manually reproduce the sequence of color-names by pressing the appropriately colored buttons on the response box.
We planned to compare memory spans obtained in this presentation condition with spans obtained when lights were presented at the same time as the color-names. Lower spans were expected for the “auditory-only” condition. This finding, if obtained, would provide additional demonstration of the informational redundancy present in the original color-name condition used in Experiment 1.
Method
Participants
Twenty-seven of the original 45 normal-hearing children participated in this condition. This task was added after the data from the cochlear implant users in Experiment 1 had been collected, and the collection of data from the matched group of normal-hearing children had already begun.
Materials and Procedure
On each trial, the target sequence consisted of an auditory list of color-names presented without illumination of the colored lights. The mapping between auditory stimulus and spatial button location was thus made less visually salient during the target list presentation. The child's task was still to reproduce the sequence by pressing the color-matched buttons in the proper order. Both sound and light were initiated by the subject's button press responses to convey that each press had registered and to provide the subject with the same minimal amount of feedback as had been present in the original task.
The administration of this task was not counter-balanced across subjects, but was always run after the three memory game presentation conditions described earlier had been completed, after a short break. All hardware and stimulus materials were otherwise identical to those used with the normal-hearing children in Experiment 1. The child was told before starting that “you will not see the lights light up this time, so listen carefully, and copy what you hear by pressing on the buttons just like before.”
Results and Discussion
Figure 3 (left panel) shows the mean span for the new “auditory-only” version of the task (“AC”) plotted alongside the mean spans obtained by this group of children in Experiment 1 for the color-names-plus-lights condition (“AC+L”) and the lights-only condition (“L”). Although the auditory-only scores were reduced relative to the multi-modal condition, the normal-hearing children were clearly able to complete the task without the visual stimuli present in the target sequence. A t-test for paired-samples comparing the weighted scores in the “AC” versus “AC+L” conditions approached significance (t(26) = 1.82, p = 0.08). Figure 3 also shows that the normal-hearing children's mean performance for the “auditory-only” condition was actually quite similar to their mean span in the lights-only condition. Because the conditions were not counterbalanced in administration, these results must be interpreted cautiously. We suggest, however, that these findings provide further evidence that the difference in performance shown by the normal-hearing children in the single-modality versus multi-modal presentation conditions used in Experiment 1 was, in fact, due to the presence of redundant information in the latter condition. Use of only a single modality of presentation appears to reduce the normal-hearing children's memory span performance on this task by about the same amount, regardless of which modality is omitted. Scores on the “auditory-only” presentation condition of the memory game were, moreover, significantly correlated with the children's WISC forward digit spans (r = +0.41, p = 0.036), despite the differences in response format between the two memory tasks.
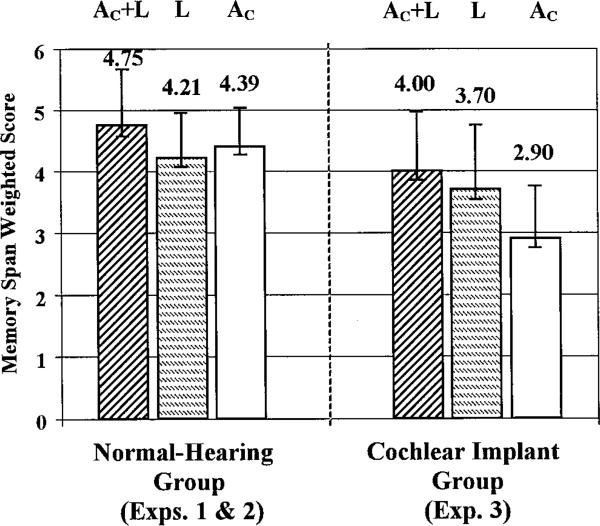
Figure 3.
Memory game task group means for the color-names-plus-lights presentation condition (“AC+L”), the lights-only presentation condition (“L”), and the auditory-only presentation condition using color-name stimuli (“AC”). Results from the 27 normal-hearing children in Experiment 2 are shown in the left-hand panel. Results from the cochlear implant group in Experiment 3 are shown in the right-hand panel (N = 37). Positive error bars indicate 1 SD from the mean. Error bars in the negative direction indicate 1 standard error. (The two leftmost bars representing the “AC+L” and “L” conditions are a subset of the data reported in Experiment 1 for the normal-hearing children.)
From this last set of results, we were eager to try the “auditory-only” version of the memory game task with a new sample of pediatric cochlear implant users. To reproduce the sequence of color-names, the cochlear implant users would be forced to verbally encode the auditory stimuli because no visual-spatial information would be available at the time of initial encoding.
Experiment 3
Results from Experiment 1 suggested that the memory game task in its original form (that is, with the light stimuli always included in the target sequence) provided measures of primarily visual-spatial memory span from the pediatric cochlear implant users rather than tapping mechanisms for auditory verbal working memory. Experiment 3 was carried out to confirm this result with a new sample of 8- and 9-yr-old users of cochlear implants, but also, more interestingly, to test their performance using the auditory-only version of the memory game task as introduced in Experiment 2. Because the redundancy gain obtained from the auditory digit-names was minimal even for the normal-hearing children studied in Experiment 1, we eliminated the digit-name condition in this subsequent study.
Method
Participants
Forty-five hearing-impaired pediatric users of cochlear implants participated in this study. None of the children in the current experiment took part in Experiment 1. All children were participants in a larger study currently being conducted at CID. Participant characteristics are shown in Table 3 for the 37 children who completed the stimulus identification pretest without errors and whose data were subsequently retained in our final analysis. Eighteen female and 19 male children completed the task. The mean communication mode score for the 37 participants was approximately 4.2 on the scale described in Experiment 1, indicating somewhat more exposure to oral-only communication methods in this sample than in the previous cochlear implant group. All children used a Nucleus 22 or Nucleus 24 device.
TABLE 3.
Participant characteristics for Experiment 3 (N = 37).
| Range | Mean | |
|---|---|---|
| Chronological age at testing | 7;11 –9;11 | 8;9 |
| Age at onset of deafness | 0;0 –3;0 | 0;2 |
| Duration of deafness | 0;7 –5;2 | 3;0 |
| Length of cochlear implant use | 4;1–6;10 | 5;7 |
Materials and Procedure
Identification Testing
The stimulus identification pretest for color-names was run as described in Experiment 1 except that each child was only asked to identify a given stimulus once, this time, by pointing to one of the four colored buttons on the response box. Eight of the 45 children tested made at least one error on the color-name identification task. The remaining 37 children correctly identified all four stimuli after only one presentation.
Memory Game Task
The stimulus materials and hardware used for this study were the same as those used in Experiments 1 and 2. Unlike Experiments 1 and 2, however, in Experiment 3 all three presentation conditions were counterbalanced in administration.
Results and Discussion
The memory data from the eight children who made errors on the stimulus identification pretest were not included in the statistical analysis. The mean scores obtained by the remaining 37 pediatric cochlear implant users in each of three presentation conditions (auditory-plus-lights “AC+L,” lights-only “L,” and auditory-only “AC”) are shown in the right-hand panel of Figure 3. A 1-way repeated measures analysis of variance with weighted memory game scores as the dependent measure showed a significant effect of presentation condition, F(2,72) = 29.25, p < 0.001. A paired-samples t-test between the lights-only versus AC+L conditions indicated that this sample of children did show evidence of a “semantic redundancy gain” from the addition of the auditory signal to the visual sequence, t(36) = 2.223, p = 0.033. This mean difference was only +0.30 units, however. For comparison purposes, the average gain shown by 37 normal-hearing children sub-selected from our earlier experiment to closely match the age and genders of the children in the current study, was +0.69 units, more than double that of the current sample of cochlear implant users.
Although many of the cochlear implant users were able to do quite well on the auditory-only color-name condition, paired-samples t-tests indicated that the group's performance in the auditory-only condition was significantly lower on average than performance in either the lights-only or the AC+L conditions (t(36) = 4.65, p < 0.001 and, t(36) = 7.98, p < 0.001, respectively). As shown in the right-hand panel of Figure 3, the hearing-impaired children's memory spans in the auditory-only condition of the memory game were quite reduced relative to when they were provided cues from both modalities.
Several additional analyses were conducted to determine whether the revised task utilized the desired modality. The performance of the pediatric cochlear implant users on the auditory-only condition of the memory game correlated r = +0.42, p = 0.01, with their performance on the traditional WISC digit-span task, confirming that the two tasks make use of similar processes. As already suggested, the memory game task may be preferable to traditional digit span measures because of its use of automated presentation of recorded stimuli and avoidance of the articulatory component inherent in the WISC digit span response format. These results provide evidence that it is feasible to use this manual response task with a cochlear implant population with the assurance that it will measure phonological working memory span using available auditory input rather than purely visual-spatial memory span. Although it is true that the auditory-only condition as described here does not necessarily preclude the child from looking at the appropriate buttons on the response box as the auditory stimuli are played in sequence on each trial, the elimination of the illuminated lights clearly made the task more difficult than when information from both modalities or only the visual modality was provided during target list presentation.
Because Pisoni and Geers (2000) had reported significant correlations between WISC forward digit span and communication mode, we also computed simple correlations between the communication mode score described in the Participant section, and scores in each of the memory game presentation conditions. Degree of exposure to oral-only communication methods correlated r = +0.20 (ns) with performance in the lights-only condition, r = +0.37 (p < 0.05) with performance in the AC+L condition, and r = +0.40 (p < 0.05) with performance in the auditory-only condition, indicating that the strength of the relationship increased with the degree to which the memory span task required auditory processing. Open-set word recognition performance on the LNT-Easy word lists was also significantly correlated with auditory-only memory game scores (r = +0.39, p = 0.016).
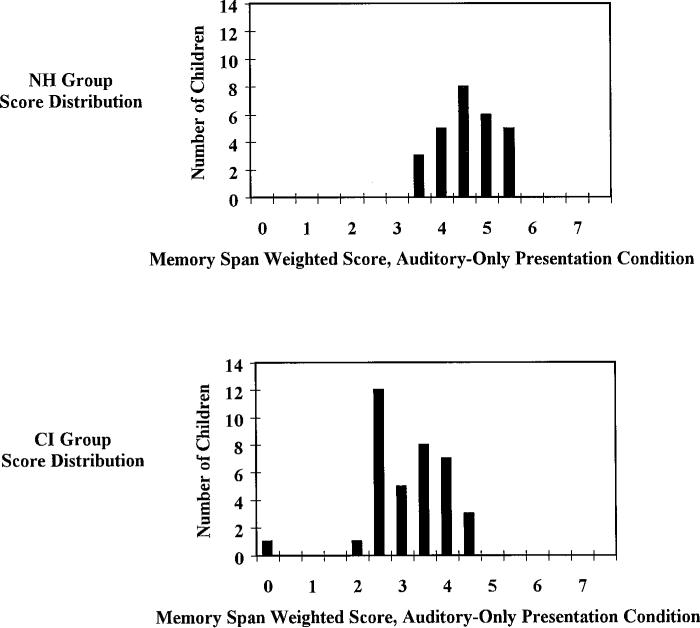
Finally, as a point of interest, we present in Figure 4, the distribution of individual subject scores for the auditory-only condition of the memory game task for both the normal-hearing subjects in Experiment 2 (N = 27) (top panel) as well as the cochlear implant users in Experiment 3 (N = 37) (bottom panel). These groups of children are only approximately matched for age and gender, but there is an interesting conclusion that we feel can legitimately be drawn from this figure. Note that despite their profound deafness as infants and subsequent use of a cochlear implant, almost half of the children in the cochlear implant group are demonstrating auditory memory spans better than or equivalent to the bottom 50% of the children in the normal-hearing distribution. Although not shown here, plotting the WISC forward digit spans of the two groups of children tested in Experiment 1 yields a very similar graph with about the same amount of overlap in scores between the cochlear implant and normal-hearing groups. Although statistical tests show that the means of these distributions differ significantly between the cochlear implant and normal-hearing groups (with or without inclusion of the one cochlear implant group outlier score), the overlap of these distributions is greater than might perhaps be inferred solely from the comparison of distribution means.
Figure 4.
Histograms showing the distribution of scores obtained in Experiments 2 and 3 using the auditory-only presentation condition of the memory game task. Normal-hearing (NH) children are shown in the top panel, pediatric cochlear implant (CI) users are shown in the bottom panel.
General Discussion
One of the long-term goals of our current research program is to develop a practical methodology for assessing and tracking the development of verbal working memory in pediatric cochlear implant users over time. Our interest in this problem partially stems from recent debate in the area of cognitive development having to do with the role of working memory in language development and lexical acquisition (Baddeley, Gathercole, & Papagno, 1998; Gupta & Dell, 1999). The results presented in this article address some methodological and theoretical issues regarding the assessment of working memory in a special population of children for whom the auditory modality has been partially compromised since birth. We found that experienced school-age users of cochlear implants did not integrate a semantic redundancy present in a memory span task across the auditory and visual sensory modalities as effectively as age-matched normal-hearing children. This finding suggests that fundamentally different sensory encoding and/or rehearsal processes may be operating in these two populations. Although the cochlear implant is now providing the cochlear implant children with access to sound and spoken language, their atypical early sensory and perceptual experiences are still evident in how they perceive and encode sensory information even after more than 4 yr of experience with a cochlear implant.
The cochlear implant children reported on in this study performed more poorly as a group overall than the normal-hearing children even when the memory game task used only visual-spatial stimuli. This was somewhat unexpected as the literature regarding the development of working memory in hearing-impaired populations suggests that the performance of deaf children on memory span tasks lacking a verbal component should not be impaired relative to that of normal-hearing children (Furth, 1966; Mayberry, 1992). When differences are, in fact observed, investigators tend to find that the visual/spatial stimuli used in the particular task lent themselves to linguistic (verbal) labeling. When verbal labeling is possible, hearing-impaired children seem to be at a disadvantage relative to their normal-hearing counterparts (see discussion in Mayberry, 1992). Several studies have also suggested that hearing-impaired children who have grown up around spoken language sometimes attempt rehearsal/encoding strategies used by normal-hearing persons, involving self-generated verbal labels for even visual-spatial stimuli (Mayberry, 1992). Because the verbal skills of hearing-impaired children with oral/aural backgrounds are often not as fully developed as normal-hearing children their age (i.e., with regard to processing speed, efficiency, robustness, etc.), their performance on a memory span task using self-generated verbal labels would be expected to be reduced relative to that of normal-hearing children. It is possible that this occurred in the Quittner et al. (1994) study discussed in our introduction involving visual monitoring of a stream of orthographically presented digits. This situation could also have arisen in the current study—the cochlear implant users may have attempted to encode the “lights-only” sequences using verbal names for the colors being illuminated even though the auditory stimuli were not explicitly presented.
We acknowledge this as a possible explanation for the reduced memory spans of the cochlear implant group in the lights-only condition, but if this relatively sophisticated verbal coding approach were truly being attempted, we would have expected to see a larger effect of informational redundancy in the cochlear implant group. The fact that no significant differences were found as a function of presentation condition for the cochlear implant children in Experiment 1, and that only a small advantage was observed in Experiment 3 for the multi-modal presentation condition over the lights-only presentation condition, suggests to us that these children relied primarily on visual-spatial encoding of the target sequence to perform the task. These results were obtained despite the fact that many of these cochlear implant children did well on the auditory WISC digit span task and on the auditory-only presentation condition of the memory game.
In summary, the present results suggest that even those cochlear implant children who are able to accurately identify speech signals in isolation, may not have phonological working memory mechanisms or processing strategies that are developed to a point equivalent to chronologically age-matched normal-hearing children. This outcome would not exactly be surprising, as many important milestones in the development of speech perception and memory are reached during the first 2 yr of life (Aslin, Jusczyk, & Pisoni, 1998; Jusczyk, 1997). Despite their prelingually deafened status, most of the cochlear implant users reported on in this paper received their implant at a point in time when the FDA did not permit implantation of children under 2 yr of age. Additionally, because the implantation procedure requires that candidates show a demonstrated failure to benefit from conventional hearing aids, we can be fairly certain that most of these 8- and 9-yr-old children had received only minimal auditory input for at least one quarter to one third of their lives. It should not be surprising, then, that the encoding strategies and working memory mechanisms of pediatric cochlear implant users seem to differ measurably from those of normal-hearing children.
Ongoing research in our lab is attempting to describe in more detail how these encoding/rehearsal mechanisms differ, and what kind of developmental changes can be observed or effected in these children. Increasingly, clinicians are beginning to see pediatric cochlear implant users that have reached ceiling levels of performance on the traditional standardized measures of speech perception and spoken word recognition that are typically used with this population—and yet these children are still clearly having problems with reading and other more advanced language skills that are based on listening, phonological encoding, and other metalinguistic abilities. Further investigation of how pediatric cochlear implant users engage in cognitive processing of information originating from this reintroduced sensory input modality may help us develop new assessment and treatment techniques (Pisoni, 2000). Eventually we would like to answer the question of whether individual differences in the function of particular components of working memory within the pediatric cochlear implant population might have a meaningful causal relation to the level of verbal language skill attained by individual children. The present research begins to address this important issue because it provides some of the first behavioral data on working memory in pediatric cochlear implant users involving tasks in which the potential contribution of each available sensory modality was varied.
ACKNOWLEDGMENTS
Work supported by NIDCD Research Grant DC00111, and NIDCD T32 Training Grant DC00012 to Indiana University Bloomington, Bloomington, IN and by NIH Research Grant DC00064 to the Indiana University School of Medicine and NIH Research Grant DC03100 to the Central Institute for the Deaf. We gratefully acknowledge the technical assistance of L. Hernandez, and the help of G. Torretta, B. Staley, and L. B. Jones with data collection.
References
- Aslin RN, Jusczyk PW, Pisoni DB. Speech and auditory processing during infancy: Constraints on and precursors to language. In: Damon W, Kuhn D, Siegler RS, editors. Handbook of Child Psychology, 5th Edition, Volume 2: Cognition, Perception, and Language. John Wiley & Sons; New York: 1998. pp. 147–198. [Google Scholar]
- Baddeley AD. Working Memory. Oxford University Press; London: 1986. [Google Scholar]
- Baddeley AD. Working memory. Science. 1992;255:556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Gathercole S, Papagno C. The phonological loop as a language learning device. Psychological Review. 1998;105:158–173. doi: 10.1037/0033-295x.105.1.158. [DOI] [PubMed] [Google Scholar]
- Brandimonte MA, Hitch GJ, Bishop DV. Verbal recoding of visual stimuli impairs mental image transformations. Memory and Cognition. 1992;20:449–455. doi: 10.3758/bf03210929. [DOI] [PubMed] [Google Scholar]
- Carmichael L, Hogan HP, Walter AA. An experimental study of the effect of language on the reproduction of visually perceived forms. Journal of Experimental Psychology. 1932;15:73–86. [Google Scholar]
- Conrad R. Short-term memory in the deaf: A test for speech coding. British Journal of Psychology. 1972;63:173–180. doi: 10.1111/j.2044-8295.1972.tb02097.x. [DOI] [PubMed] [Google Scholar]
- Dedina MJ. Research on Speech Perception Progress Report No. 13. Speech Research Laboratory, Indiana University; Bloomington, IN: 1987. SAP: A speech acquisition program for the SRL-VAX. pp. 331–337. [Google Scholar]
- Dempster FN. Memory span: Sources of individual and developmental differences. Psychological Bulletin. 1981;89:63–100. [Google Scholar]
- Dowell RC, Blamey P,J, Clark GM. Potential and limitations of cochlear implants in children. Annals of Otology, Rhinology and Laryngology Supplement. 1995;166:324–327. [PubMed] [Google Scholar]
- Dunn LM, Dunn LM. Peabody Picture Vocabulary Test, Third Edition. American Guidance Service; Circle Pines, Minnesota: 1997. [Google Scholar]
- Fastenau PS, Conant LL, Lauer RE. Working memory in young children: Evidence for modality-specificity and implications for cerebral reorganization in early childhood. Neuropsychologia. 1998;36:643–652. doi: 10.1016/s0028-3932(97)00151-6. [DOI] [PubMed] [Google Scholar]
- Fryauf-Bertschy H, Tyler RS, Kelsay DM, Gantz BJ, Woodworth GG. Cochlear implant use by prelingually deafened children: The influences of age at implant and length of device use. Journal of Speech, Language, and Hearing Research. 1997;40:183–199. doi: 10.1044/jslhr.4001.183. [DOI] [PubMed] [Google Scholar]
- Furth HG. Thinking Without Language: Psychological Implications of Deafness. The Free Press; New York: 1966. [Google Scholar]
- Gantz BJ, Woodworth GG, Abbas PJ, Knutson JF, Tyler RS. Multivariate predictors of audiological success with multichannel cochlear implants. Annals of Otology, Rhinology and Laryngology. 1993;102:909–916. doi: 10.1177/000348949310201201. [DOI] [PubMed] [Google Scholar]
- Geers AE, Nicholas J, Tye-Murray N, Uchanski R, Brenner C, Crosson J, Davidson LS, Spehar B, Torretta G, Tobey EA, Sedey A, Strube M. Central Institute for the Deaf Research Periodic Progress Report No. 35. Central Institute for the Deaf; St. Louis, MO: 1999. Center for Childhood Deafness and Adult Aural Rehabilitation, Current research projects: Cochlear implants and education of the deaf child, second-year results. pp. 5–20. [Google Scholar]
- Geers AE, Nicholas J, Tye-Murray N, Uchanski R, Brenner C, Davidson LS, Torretta G, Tobey EA. Effects of communication mode on skills of long-term cochlear implant users. Annals of Otology, Rhinology and Laryngology Supplement. 2000;185:89–92. doi: 10.1177/0003489400109s1239. [DOI] [PubMed] [Google Scholar]
- Gupta P, Dell GS. The emergence of language from serial order and procedural memory. In: MacWhinney B, editor. The Emergence of Language. Lawrence Erlbaum; Mahwah, NJ: 1999. pp. 447–481. [Google Scholar]
- Hale S, Bronik MD, Fry AF. Verbal and spatial working memory in school-age children: Developmental differences in susceptibility to interference. Developmental Psychology. 1997;33:364–371. doi: 10.1037//0012-1649.33.2.364. [DOI] [PubMed] [Google Scholar]
- Hanson VL. Recall of order information by deaf signers: Phonetic coding in temporal order recall. Memory and Cognition. 1990;18:604–610. doi: 10.3758/bf03197103. [DOI] [PubMed] [Google Scholar]
- Hanson VL, Lichtenstein EH. Short-term memory coding by deaf signers: The primary language coding hypothesis reconsidered. Cognitive Psychology. 1990;22:211–224. doi: 10.1016/0010-0285(90)90016-w. [DOI] [PubMed] [Google Scholar]
- Hernandez L. Research on Spoken Language Processing Progress Report No. 20. Speech Research Laboratory, Indiana University; Bloomington, IN: 1995. Current computer facilities in the Speech Research Laboratory. pp. 389–393. [Google Scholar]
- Jusczyk P. The Discovery of Spoken Language. MIT Press; Cambridge, MA: 1997. [Google Scholar]
- Kail R. Developmental changes in speed of processing during childhood and adolescence. Psychological Bulletin. 1991;109:490–501. doi: 10.1037/0033-2909.109.3.490. [DOI] [PubMed] [Google Scholar]
- Kirk KI, Pisoni DB, Osberger MJ. Lexical effects on spoken word recognition by pediatric cochlear implant users. Ear & Hearing. 1995;16:470–481. doi: 10.1097/00003446-199510000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson JF, Boyd RC, Goldman M, Sullivan PM. Psychological characteristics of child cochlear implant candidates and children with hearing impairments. Ear & Hearing. 1997;18:355–363. doi: 10.1097/00003446-199710000-00001. [DOI] [PubMed] [Google Scholar]
- Knutson JF, Hinrichs JV, Tyler RS, Gantz BJ, Shartz HA, Woodworth G. Psychological predictors of audiological outcomes of multichannel cochlear implants. Annals of Otology, Rhinology and Laryngology. 1991;100:817–822. doi: 10.1177/000348949110001006. [DOI] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. Journal of the Acoustical Society of America. 1970;49:467–477. [PubMed] [Google Scholar]
- Lewkowicz DJ, Lickliter R. The Development of Intersensory Perception: Comparative Perspectives. Lawrence Erlbaum; Hillsdale, NJ: 1994. [Google Scholar]
- Lezak MD. Neuropsychological Assessment, Third Edition. Oxford University Press; New York: 1995. [Google Scholar]
- Lyxell B, Andersson J, Arlinger S, Bredberg G, Harder H, Ronnberg J. Verbal information-processing capabilities and cochlear implants: Implications for preoperative predictors of speech understanding. Journal of Deaf Studies and Deaf Education. 1996;1:190–203. doi: 10.1093/oxfordjournals.deafed.a014294. [DOI] [PubMed] [Google Scholar]
- Marschark M, Mayer TS. Mental representation and memory in deaf adults and children. In: Marschark J, Clark MD, editors. Psychological Perspectives on Deafness. Vol. 2. Lawrence Erlbaum Associates; Mahwah, NJ: 1998. [Google Scholar]
- Mayberry RI. The cognitive development of deaf children: recent insights. In: Segalowitz SJ, Rapin I, editors. Handbook of Neuropsychology. Vol. 7. Elsevier Science Publishers; New York: 1992. [Google Scholar]
- Miyamoto RT, Osberger JJ, Todd SL, Robbins AM, Stroer BS, Zimmerman-Phillips S, Carney AE. Variables affecting implant performance in children. Laryngoscope. 1994;104:1120–1124. doi: 10.1288/00005537-199409000-00012. [DOI] [PubMed] [Google Scholar]
- Miyamoto R, Robbins AM, Osberger MJ. Cochlear Implants. In: Cummings CW, editor. Otolaryngology, Head and Neck Surgery, 2nd Edition, Vol. 4. Mosby Publishers; St. Louis, MO: 1993. [Google Scholar]
- Naus MJ, Ornstein PA. Development of memory strategies: Analysis, questions, and issues. In: Chi MT, editor. Trends in memory development research. Karger; New York: 1983. pp. 1–30. [Google Scholar]
- Nikolopoulos TP, O'Donoghue GM, Archbold S. Age at implantation: Its importance in pediatric cochlear implantation. Laryngoscope. 1999;109:595–9. doi: 10.1097/00005537-199904000-00014. [DOI] [PubMed] [Google Scholar]
- Pisoni DB. Cognitive factors and cochlear implants: Some thoughts on perception, learning, and memory in speech perception. Ear & Hearing. 2000;21:70–78. doi: 10.1097/00003446-200002000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisoni DB, Cleary M, Geers A, Tobey E. Individual differences in effectiveness of cochlear implants in children who are prelingually deaf: New process measures of performance. Volta Review. 2000;101:111–164. [PMC free article] [PubMed] [Google Scholar]
- Pisoni DB, Geers AE. Working memory in deaf children with cochlear implants: Correlations between digit span and measures of spoken language processing. Annals of Otology, Rhinology and Laryngology Supplement. 2000;185:92–93. doi: 10.1177/0003489400109s1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisoni DB, Svirsky MA, Kirk KI, Miyamoto RT. Research on Spoken Language Processing Progress Report No. 21 (1996–1997) Speech Research Laboratory; Bloomington, IN: 1997. Looking at the “Stars”: A first report on the intercorrelations among measures of speech perception, intelligibility, and language development in pediatric cochlear implant users (pp. 51–91). [Google Scholar]
- Quittner AL, Smith LB, Osberger MJ, Mitchell TV, Katz DN. The impact of audition on the development of visual attention. Psychological Science. 1994;5:347–353. [Google Scholar]
- Quittner AL, Steck JT. Predictors of cochlear implant use in children. The American Journal of Otology. 1991;12(Suppl.):89–94. [PubMed] [Google Scholar]
- Shand MA. Sign-based short-term coding of American Sign Language signs and printed English words by congenitally deaf signers. Cognitive Psychology. 1982;14:1–12. doi: 10.1016/0010-0285(82)90002-0. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Snik AF, Vermeulen AM, Geelen CP, Brokx JP, van den Broek P. Speech perception performance of children with a cochlear implant compared to that of children with conventional hearing aids. II. Results of prelingually deaf children. Acta-Otolaryngol-Stockh. 1997;117:755–759. doi: 10.3109/00016489709113473. [DOI] [PubMed] [Google Scholar]
- Stein BE, Meredith MA. The Merging of the Senses. MIT Press; Cambridge, MA: 1993. [Google Scholar]
- Svirsky MA, Robbins AM, Kirk KI, Pisoni DB, Miyamoto RT. Language development in profoundly deaf children with cochlear implants. Psychological Science. 2000;11:153–158. doi: 10.1111/1467-9280.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson HL. Individual and age-related differences in children's working memory. Memory and Cognition. 1996;24:70–82. doi: 10.3758/bf03197273. [DOI] [PubMed] [Google Scholar]
- Tiber N. A psychological evaluation of cochlear implants in children. Ear & Hearing. 1985;6(Suppl.):48S–51S. doi: 10.1097/00003446-198505001-00009. [DOI] [PubMed] [Google Scholar]
- Tobey EA, Geers AE, Morchower B, Perrin J, Skellett R, Brenner C, Torretta G. Factors associated with speech intelligibility in children with cochlear implants. Annals of Otology, Rhinology and Laryngology Supplement. 2000;185:28–30. doi: 10.1177/0003489400109s1212. [DOI] [PubMed] [Google Scholar]
- Tomlinson-Keasey C, Smith-Winberry C. Cognitive consequences of congenital deafness. Journal of Genetic Psychology. 1990;151:103–115. doi: 10.1080/00221325.1990.9914647. [DOI] [PubMed] [Google Scholar]
- Tyler RS, Fryauf-Bertschy H, Kelsay DMR, Gantz BJ, Woodworth GP, Parkinson A. Speech perception by prelingually deaf children using cochlear implants. Otolaryngology Head and Neck Surgery. 1997;117:180–187. doi: 10.1016/s0194-5998(97)70172-4. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children, Third Edition (WISC-III) The Psychological Corporation; San Antonio, TX: 1991. [DOI] [PubMed] [Google Scholar]
- Zwolan TA, Zimmerman-Phillips S, Ashbaugh CJ, Hieber SJ, Kileny PR, Telian SA. Cochlear implantation of children with minimal open-set speech recognition skills. Ear & Hearing. 1997;18:240–251. doi: 10.1097/00003446-199706000-00007. [DOI] [PubMed] [Google Scholar]
- 1.Geers A. Effects of educational intervention on the benefits of cochlear implants.. Paper presented at the 6th International Cochlear Implant Conference; Miami, FL. February 3–5, 2000.2000. [Google Scholar]
- 2.Sehgal ST, Kirk KI, Pisoni DB, Miyamoto RT. Effect of residual hearing on children's speech perception abilities with a cochlear implant.. Poster presented at the Fifth International Cochlear Implant Conference; New York City, New York. May 1–3, 1997.1997. [Google Scholar]