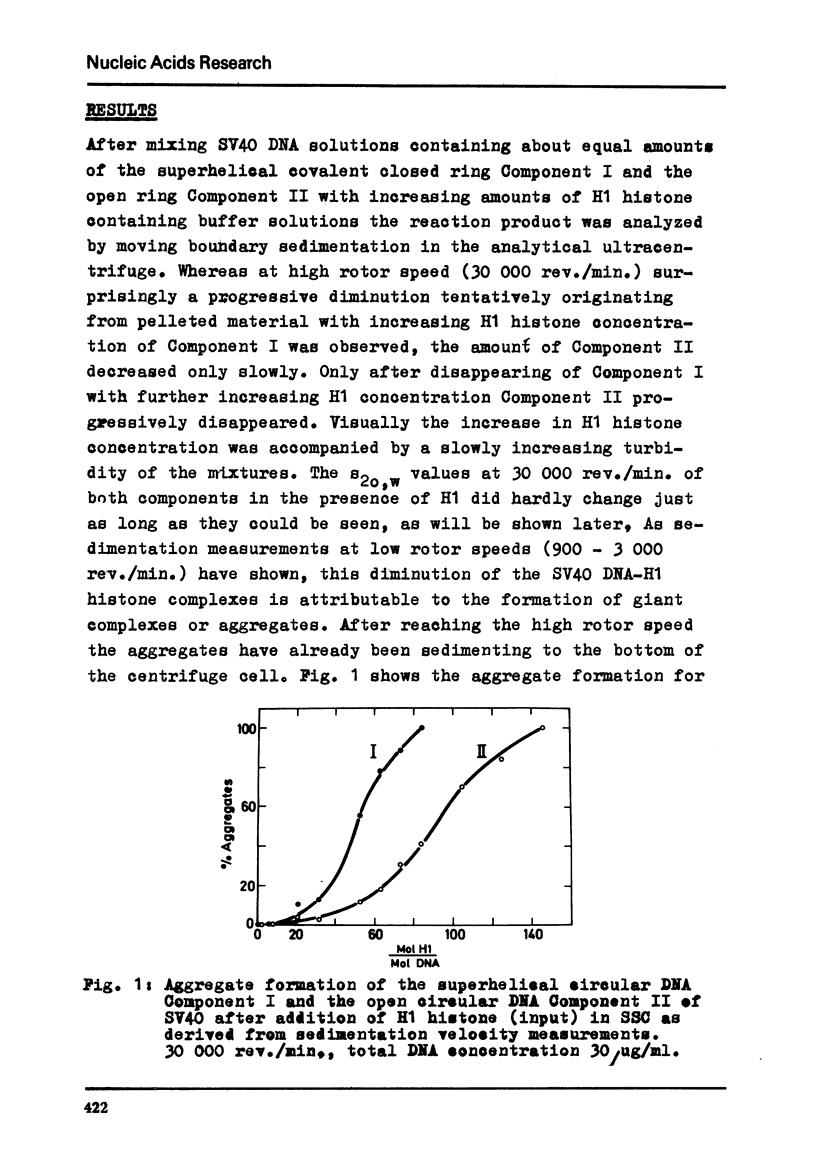
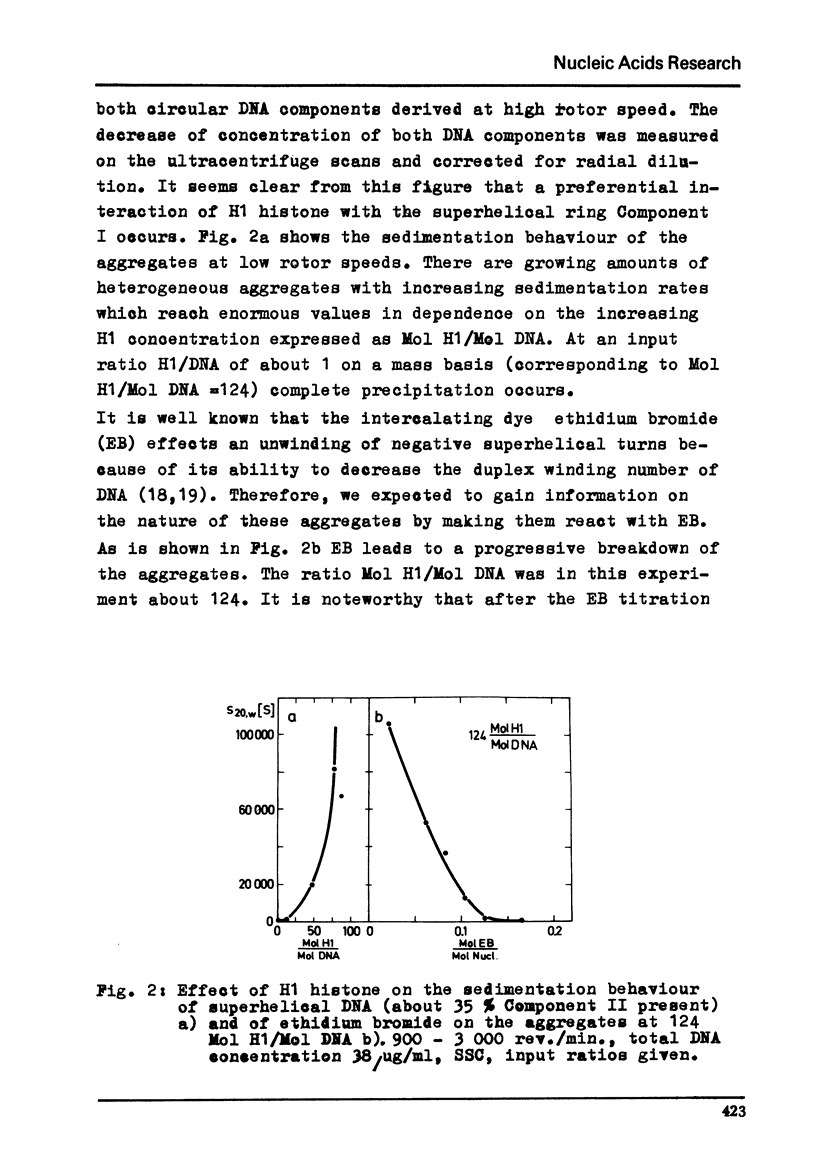
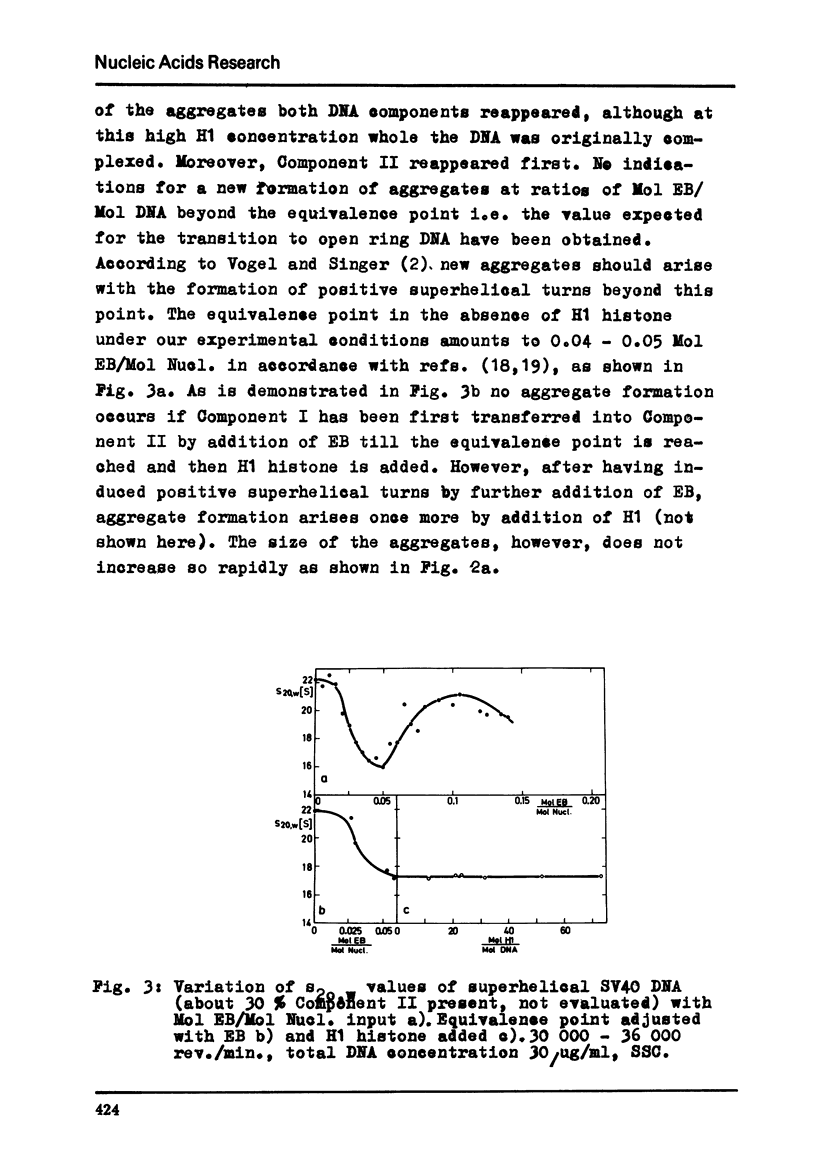
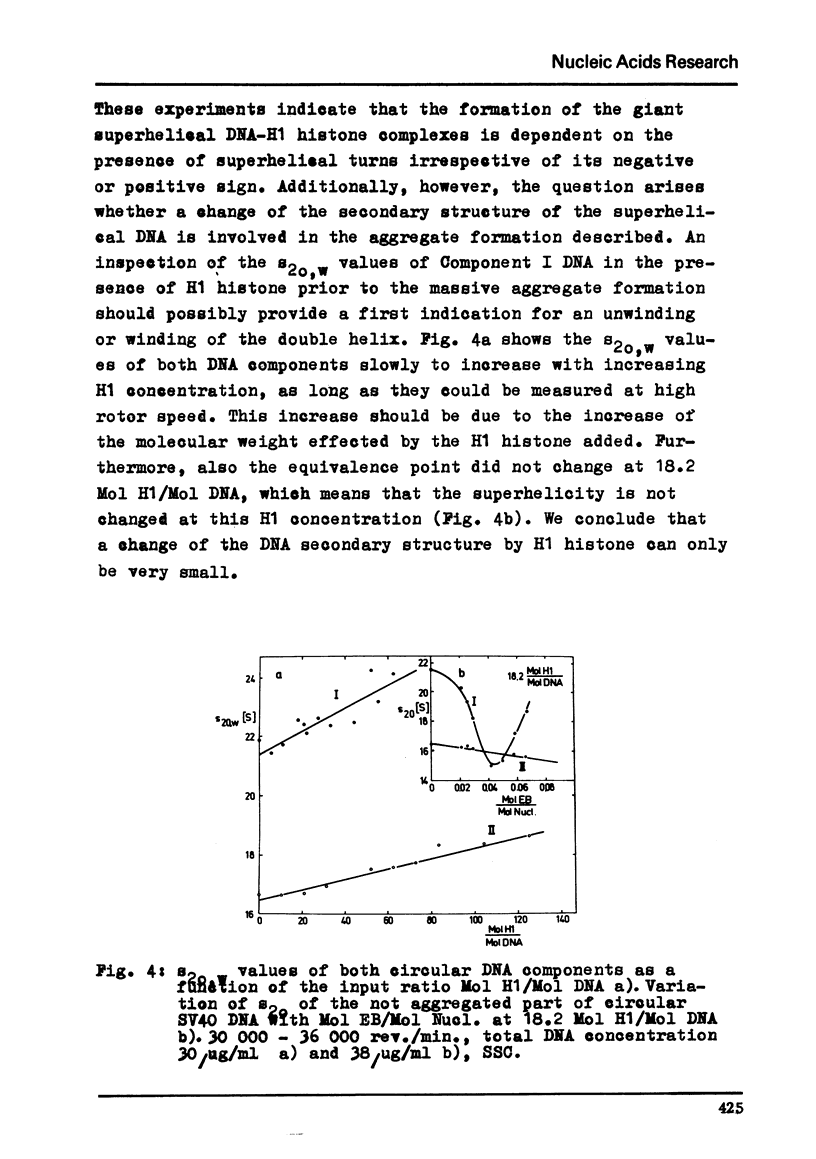
Abstract
By moving boundary sedimentation it is shown that the interaction of H1 histone with superhelical circular SV40 DNA results in the formation of giant heterogeneous aggregates. The size of these aggregates grows with increasing H1 concentration. s20,w values of some 10 000 S were measured. As compared with open relaxed circular DNA a preferential interaction of superhelical DNA with H1 histone is observed, irrespective of the sign of the superhelical turns which was reversed by the addition to DNA of ethidium bromide. The addition to the H1 complexed aggregates of ethidium bromide effects a progressive breakdown of the aggregates. Furthermore, the superhelicity of DNA is not changed by the addition of small amounts of H1 histone.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer W., Vinograd J. The interaction of closed circular DNA with intercalative dyes. I. The superhelix density of SV40 DNA in the presence and absence of dye. J Mol Biol. 1968 Apr 14;33(1):141–171. doi: 10.1016/0022-2836(68)90286-6. [DOI] [PubMed] [Google Scholar]
- Bradbury E. M., Inglis R. J., Matthews H. R. Control of cell division by very lysine rich histone (F1) phosphorylation. Nature. 1974 Feb 1;247(5439):257–261. doi: 10.1038/247257a0. [DOI] [PubMed] [Google Scholar]
- Böttger M., Bierwolf D., Wunderlich V., Graffi A. New calibration correlations for molecular weights of circular DNA: the molecular weight of the DNA of an oncogenic papova virus of the Syrian hamster. Biochim Biophys Acta. 1971 Feb 25;232(1):21–31. doi: 10.1016/0005-2787(71)90487-4. [DOI] [PubMed] [Google Scholar]
- Crawford L. V., Waring M. J. Supercoiling of polyoma virus DNA measured by its interaction with ethidium bromide. J Mol Biol. 1967 Apr 14;25(1):23–30. doi: 10.1016/0022-2836(67)90276-8. [DOI] [PubMed] [Google Scholar]
- Fenske H., Eichhorn I., Böttger M., Lindigkeit R. Evidence of altered histone interactions, as investigated by removal of histones, in chromatin isolated from rat liver nuclei by a conventional method. Nucleic Acids Res. 1975 Oct;2(10):1975–1985. doi: 10.1093/nar/2.10.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germond J. E., Hirt B., Oudet P., Gross-Bellark M., Chambon P. Folding of the DNA double helix in chromatin-like structures from simian virus 40. Proc Natl Acad Sci U S A. 1975 May;72(5):1843–1847. doi: 10.1073/pnas.72.5.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith J. D. Chromatin structure: deduced from a minichromosome. Science. 1975 Mar 28;187(4182):1202–1203. doi: 10.1126/science.187.4182.1202. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- JENSEN F. C., GIRARDI A. J., GILDEN R. V., KOPROWSKI H. INFECTION OF HUMAN AND SIMIAN TISSUE CULTURES WITH ROUS SARCOMA VIRUS. Proc Natl Acad Sci U S A. 1964 Jul;52:53–59. doi: 10.1073/pnas.52.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns E. W. A method for the selective extraction of histone fractions f2(a)1 and f2(a)2 from calf thymus deoxyribonucleoprotein at pH7. Biochem J. 1967 Nov;105(2):611–614. doi: 10.1042/bj1050611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns E. W. Studies on histones. 7. Preparative methods for histone fractions from calf thymus. Biochem J. 1964 Jul;92(1):55–59. doi: 10.1042/bj0920055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan C. F., Lerman L. S., Venable J. H. Structure and circular dichroism of DNA in concentrated polymer solutions. Nat New Biol. 1972 Mar 22;236(64):67–70. doi: 10.1038/newbio236067a0. [DOI] [PubMed] [Google Scholar]
- Lake R. S., Barban S., Salzman N. P. Resolutions and identification of the core deoxynucleoproteins of the simian virus 40. Biochem Biophys Res Commun. 1973 Sep 18;54(2):640–647. doi: 10.1016/0006-291x(73)91471-x. [DOI] [PubMed] [Google Scholar]
- Littau V. C., Burdick C. J., Allfrey V. G., Mirsky S. A. The role of histones in the maintenance of chromatin structure. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1204–1212. doi: 10.1073/pnas.54.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Venable J. H., Jr, Lerman L. S. The structure of psi DNA. J Mol Biol. 1974 Mar 25;84(1):37–64. doi: 10.1016/0022-2836(74)90211-3. [DOI] [PubMed] [Google Scholar]
- Olins D. E. Interaction of lysine-rich histones and DNA. J Mol Biol. 1969 Aug 14;43(3):439–460. doi: 10.1016/0022-2836(69)90351-9. [DOI] [PubMed] [Google Scholar]
- Oudet P., Gross-Bellard M., Chambon P. Electron microscopic and biochemical evidence that chromatin structure is a repeating unit. Cell. 1975 Apr;4(4):281–300. doi: 10.1016/0092-8674(75)90149-x. [DOI] [PubMed] [Google Scholar]
- Pett D. M., Estes M. K., Pagano J. S. Structural proteins of simian virus 40. I. Histone characteristics of low-molecular-weight polypeptides. J Virol. 1975 Feb;15(2):379–385. doi: 10.1128/jvi.15.2.379-385.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sponar J., Fric I. Complexes of histone F1 with DNA in 0.15M NaCl. Circular dichroism and structure of the complexes. Biopolymers. 1972;11(11):2317–2330. doi: 10.1002/bip.1972.360111110. [DOI] [PubMed] [Google Scholar]
- Vogel T., Singer M. F. Interaction of f1 histone with superhelical DNA. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2597–2600. doi: 10.1073/pnas.72.7.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel T., Singer M. The interaction of histones with simian virus 40 supercoiled circular deoxyribonucleic acid in vitro. J Biol Chem. 1975 Jan 25;250(2):796–798. [PubMed] [Google Scholar]


