Abstract
Purine nucleotides function in a variety of vital cellular and metabolic processes including energy production, cell signaling, synthesis of vitamin-derived cofactors and nucleic acids, and as determinants of cell fate. Unlike their mammalian and insect hosts, Leishmania cannot synthesize the purine ring de novo and are absolutely dependent upon them to meet their purine requirements. The obligatory nature of purine salvage in these parasites, therefore, offers an attractive paradigm for drug targeting, and consequently, the delineation of the pathway has been under scientific investigation for over 30 years. Here we review recent developments that reveal how purines flux in Leishmania and offer a potential ‘Achilles’ heel’ for future validation.
The leishmanial purine salvage pathway revealed
The inability of Leishmania to synthesize the purine ring was established in 1978 by Marr, Berens and Nelson who demonstrated that this genus could not convert [14C]-formate, [14C]-glycine, or [14C]-serine purine ring precursors, into adenylate and guanylate nucleotides [1]. By contrast, the incorporation of [U-14C] glucose into purine nucleotides suggested both a capacity for purine salvage and the synthesis of phosphoribosylpyrophosphate (PRPP), a substrate for several key enzymes of purine salvage [1]. As a consequence of their absolute reliance on an external purine source, Leishmania have developed an extensive purine acquisition pathway that enables them to scavenge purines from their culture or host milieu, and the parasite is capable of incorporating virtually any naturally occurring purine nucleobase or nucleoside into its nucleotide pools [1–5].
The Leishmania donovani purine salvage pathway has been largely delineated using biochemical, molecular, and genetic tools over the past three decades [1–15]. Early metabolic flux experiments with radiolabeled purine precursors helped establish a nearly complete picture of the activities that comprise the purine salvage pathway (Figure 1). The genes for all of these purine salvage pathway components have now been identified using molecular genetics approaches or from the annotated leishmanial genomes [16–19].
Figure 1.
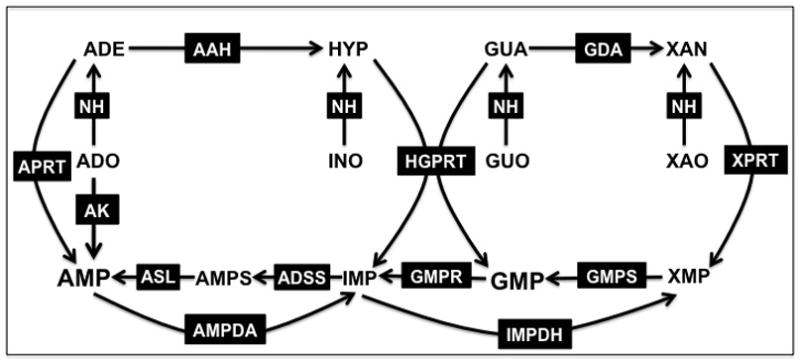
Predicted purine salvage pathway of Leishmania. The purine salvage and interconversion enzymes that have been identified in L. donovani are depicted. Abbreviations: APRT, adenine phosphoribosyltransferase; HGPRT, hypoxanthine-guanine phosphoribosyltransferase; XPRT, xanthine phosphoribosyltransferase; AK, adenosine kinase; AAH, adenine aminohydrolase; GDA, guanine deaminase; ADSS, adenylosuccinate synthetase; ASL, adenylosuccinate lyase; AMPDA, AMP deaminase; IMPDH, inosine monophosphate dehydrogenase; GMPS, GMP synthase; GMPR, GMP reductase; NH, nucleoside hydrolase; ADO, adenosine; ADE, adenine; INO, inosine; HYP, hypoxanthine; GUO, guanosine; GUA, guanine; XAO, xanthosine; XAN, xanthine.
Purine salvage and interconversion in L. donovani
The L. donovani purine salvage activities include three phosphoribosyltransferases, hypoxanthine-guanine phosphoribosyltransferase (HGPRT), xanthine phosphoribosyltransferase (XPRT), and adenine phosphoribosyltransferase (APRT), that catalyze the phosphoribosylpyrophosphate (PRPP)-dependent phosphoribosylation of purine bases [1,5], adenosine kinase (AK) that phosphorylates adenosine [20,21], and a multiplicity of purine interconversion enzymes (Glossary) [1–3]. These purine salvage components are summarized in Figure 1. Leishmania express several enzymes that catalyze the breakdown of host nucleosides, nucleotides, and nucleic acids prior to entry into the parasite purine pools. At least four nucleoside hydrolase enzymes have been identified in Leishmania [3,22–25]. IUNH, an inosine-uridine nucleoside hydrolase, also designated as the non-specific nucleoside hydrolase [23,24,26], cleaves inosine, uridine, cytidine, xanthosine, adenosine and guanosine to the corresponding base. L. donovani IUNH has been immunolocalized to specific foci inside the cell membrane, but this location has not been verified by other biochemical methods [23]. Of the other two nucleoside hydrolases annotated in the leishmanial genomes, one is specific for inosine and guanosine [22], and the other recognizes inosine, adenosine, and guanosine [25] (J. M. Boitz, et al., unpublished). A fourth nucleoside hydrolase activity that is specific for 2′-deoxyribonucleosides has also been detected in L. donovani, but its gene and protein have not been identified to date [22]. L. donovani also express two membrane-bound 3′-nucleotidases/nucleases that are located on the external cell surface of the parasite [27–29]. These 3′-nucleotidases/nucleases either generate free nucleosides via the hydrolysis of 3′-nucleotides or hydrolyze nucleic acids to 5′-nucleotides. The 5′-nucleotides are further metabolized by membrane-bound acid phosphatases to their respective nucleosides [30–32], which are subsequently translocated into the parasite by cell surface nucleoside transporters.
Purine transport in L. donovani
The first step in purine acquisition into the parasite involves the transport of purine nucleobases and nucleosides across the parasite plasma membrane. Free nucleosides and nucleobases that are scavenged from the host are translocated into the parasite by a variety of specialized transporters that have distinct specificities for particular ligands [33]. Four discrete transporters have been identified in L. donovani, and these have been designated LdNT1-4 for L. donovani Nucleoside or Nucleobase transporter [33,34]. LdNT1 is specific for adenosine and pyrimidine nucleosides [35], LdNT2 transports the 6-oxopurine nucleosides inosine, guanosine, and xanthosine [36–38], LdNT3 is a purine nucleobase transporter [39,40], and LdNT4, which is homologous to the L. major transporter LmaNT4 that preferentially transports purine nucleobases at acidic pH [39,41], is likely a purine nucleobase transporter. LdNT1-4 share approximately 30% identity and are topologically homologous to members of the mammalian Equilibrative Nucleoside Transporter (ENT) family [33]. LdNT1-4 also display several conserved ENT signature residues that are located within the predicted transmembrane domains of these transporters [33]. However, despite their sequence and topological similarities with their ENT counterparts in higher eukaryotes, the LdNT1-4 are electrogenic symporters, coupling a proton for each molecule of ligand transported and creating a positive inward flux across the parasite plasma membrane [33].
Functional redundancy within the purine salvage pathway
Once transported, exogenous nucleosides and nucleobases are incorporated into the parasite adenylate and guanylate nucleotide pools via a complex and intertwined purine salvage pathway that contains several functional redundancies. For example, adenosine can either be directly phosphorylated by AK to form AMP or catabolized to adenine by at least one nucleoside hydrolase [3,22]. Adenine can be phosphoribosylated by APRT or deaminated to hypoxanthine by adenine aminohydrolase (AAH) and subsequently phosphoribosylated by HGPRT or XPRT [2]. Inosine can be hydrolyzed by at least two nucleoside hydrolases to form hypoxanthine, which in turn is phosphoribosylated to inosine monophosphate (IMP) by HGPRT. Guanosine is cleaved to guanine, which can theoretically be phosphoribosylated by HGPRT or deaminated by guanine deaminase (GDA) to xanthine [2,12], which is converted to xanthine monophosphate (XMP) by XPRT. Xanthosine, which is probably not produced in great quantities by host cells, is catabolized to xanthine and salvaged through XPRT [2]. The intricacy of the purine pathway is further magnified by its compartmentalization between the cytosol and the glycosome, a unique metabolic organelle in these organisms [10,42–49]. The sequestration of cytosolic and glycosomal purine salvage pathway enzymes is discussed in Box 1.
Box 1. Compartmentalization of the purine salvage pathway.
Leishmania, like all members of the kinetoplastid family, harbor fuel-metabolizing organelles called glycosomes [43,46,64]. Although glycosomes contain several archetypal peroxisomal functions, such as fatty acid β-oxidation and ether-lipid biosynthesis, they lack the hallmark H2O2 producing catalases that are a defining feature of peroxisomes [46]. In addition to housing the first seven steps of glycolysis, glycosomes also compartmentalize specific enzymes involved in gluconeogenesis, the pentose phosphate pathway, squalene and sterol synthesis, pyrimidine and polyamine biosynthesis, and purine salvage, although it should be noted that some glycosomal enzymes can also be found in the cytosol [46]. Most glycosomal matrix proteins contain a peroxisomal targeting signal (PTS) that directs their import into the glycosome. This may be either a C-terminal tripeptide PTS1 or a more divergent N-terminal nonapeptide PTS2. For those glycosomal matrix proteins that lack a PTS, a ‘piggybacking’ mechanism through interaction with other glycosome-destined proteins has been proposed [45]. Through bioinformatic searches for potential PTS motifs, as well as from direct experimental validation, it appears that HGPRT, XPRT, IMPDH, [47–49] GMPR (A. Jardim, et al. unpublished) and possibly GMPS are localized within the glycosome (Figure I). This glycosomal targeting is not essential for the function of certain enzymes, as deleting the PTS1 motifs from HGPRT, XPRT, IMPDH or GMPR, does not affect the ability of the truncated products to complement Δhgprt, Δxprt, Δ impdh, [47,48,58], and Δgmpr cells, respectively.
Although the reasons for this dual compartmentalization are unclear, it has been postulated that the ‘tight quarters’ of the glycosome may serve to increase metabolic flux, but this theory, at least for glucose metabolism, has not been supported by the mathematical models produced by Barbara Bakker and colleagues [46]. The bulk of purine nucleotide synthesis is likely to occur within the glycosomal matrix since both HGPRT and XPRT, which catalyze the vast majority of flux through the salvage pathway, reside there. Another intriguing, but as of yet unproven, thesis that has recently been put forth for compartmentalization within the glycosome is that this organelle provides these parasites with more metabolic flexibility and an ability to adapt quickly and efficiently to changes in their host environments [46].
The regulation of purine metabolism in the glycosome may perhaps be achieved through inherent nucleotide sensing mechanisms that are harbored within IMPDH and GMPR [17]. Multiple sequence alignments show that both of these enzymes accommodate a tandemly repeated 60 amino acid cystathionine β-synthase (CBS) regulatory domain, an element that binds ATP, AMP, and S-adenosylmethionine [17] and has been postulated to bind GMP and GTP [49]. Enzyme kinetic studies have revealed that IMPDH activity is not affected by ATP but is dramatically reduced by elevated levels of GMP and GTP [49]. By contrast, in vitro experiments showed that the GMPR activity is accelerated by increased levels of GTP, which would promote the shunting of accumulating levels of GMP into the adenylate pool. Interestingly, the GMPR CBS domain is unique in that it binds both ATP and GTP, directly governing the catalytic response to the adenylate and guanylate levels in the cell (A. Jardim, et al., unpublished). Given that IMPDH and GMPR are glycosomal enzymes, it is tempting to speculate that compartmentalization of these purine interconversion pathways may be a vital mechanism for controlling purine levels in Leishmania.
Genetic analyses reveal fluxes through the purine salvage pathway
The mere existence of a gene or its product cannot point to its functional relevance or its involvement within the purine salvage pathway. However, the evolution and advancement of genetic tools in Leishmania, such as targeted gene replacement [50,51], has enabled a systematic, and nearly complete, genetic dissection of this pathway in L. donovani. The generation of Leishmania mutants that are null for an assortment of enzymes in the purine salvage pathway has facilitated the delineation between functional and functionally redundant routes of purine acquisition and has pinpointed enzymes that might be essential for L. donovani survival. A selection of the L. donovani purine salvage mutants that are either genetically null or enzymatically dysfunctional for purine salvage or interconversion is listed in Table 2.
Table 2.
The capacity of L. donovani purine salvage mutants to grow in a range of purine sources
| Cell Line | Purine Source
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ADE | ADE + dCF | ADO | ADO + dCF | GUA | GUO | HYP | INO | XAN | HYP + XAN | |
| Wild Type | + | + | + | + | + | + | + | + | + | + |
| Δaah | + | + | + | + | + | + | + | + | + | ND |
| ak− | + | ND | + | ND | + | + | + | + | + | ND |
| aprt− | + | ND | + | ND | + | + | + | + | + | ND |
| Δaprt | + | − | + | + | + | + | + | + | + | ND |
| Δadss | − | + | − | + | − | − | − | − | − | ND |
| Δasl | − | + | − | + | − | − | − | − | − | ND |
| Δgda | + | + | + | + | + | + | + | + | + | ND |
| Δgmpr | + | + | + | + | − | − | + | + | − | + |
| Δhgprt | + | + | + | + | + | + | + | + | + | ND |
| Δimpdh | − | ND | − | ND | + | + | − | − | + | + |
| Δxprt | + | + | + | + | − | + | + | + | − | ND |
| aprt−/Δxprt | + | ND | + | ND | − | + | + | + | − | ND |
| ak−/Δaprt | + | ND | + | ND | + | + | + | + | + | ND |
| ak−/Δhgprt | + | ND | + | ND | + | + | + | + | + | ND |
| ak−/Δxprt | + | ND | + | ND | − | + | + | + | − | ND |
| Δhgprt/Δaprt | + | ND | + | ND | + | + | + | + | + | ND |
| Δhgprt/Δxprt | − | + | − | + | − | − | − | − | − | ND |
| Δaah/Δadss | + | + | − | + | − | − | − | − | − | ND |
| Δgmpr/Δimpdh | − | − | − | − | − | − | − | − | − | + |
| Δhgprt/Δaprt/ak− | + | ND | + | ND | + | + | + | + | + | ND |
| Δxprt/Δaprt/ak− | + | ND | + | ND | − | + | + | + | − | ND |
| Δaah/Δhgprt/Δxprt | + | + | + | + | − | − | − | − | − | ND |
Abbreviations: (+), continuous growth could be maintained; (−), growth could not be sustained; (ND), growth not determined; ADE, adenine; dCF, 2′ deoxycoformycin; ADO, adenosine; GUA, guanine; GUO, guanosine; HYP, hypoxanthine; INO, inosine; XAN, xanthosine
Mutational and gene targeting schemes in L. donovani have clearly established that none of the four known enzymes capable of converting host purine nucleobases or nucleosides to the nucleotide level, HGPRT, APRT, XPRT, or AK, is by itself essential and that parasites with a deficiency in any one of these enzymes can still effectively salvage most, if not all, purines, although the routes of purine metabolism are affected by these lesions [12,20]. The phenotypic characterization of the Δhgprt, Δaprt, and Δxprt null mutants, in particular, has revealed some unusual and novel features about purine salvage in Leishmania, and most importantly, provided clear evidence for how purines are funneled through the pathway [12]. For example, although cell lysates prepared from Δhgprt parasites cannot incorporate [14C]hypoxanthine into purine nucleotides over a short time course, intact Δhgprt cells are still capable of incorporating [14C]guanine at rates similar to wild type levels and can proliferate indefinitely in guanine as a sole purine source [12]. This ability to salvage and grow in guanine can be explained by the presence of XPRT, which, in fact, plays a key but indirect role in guanine salvage by L. donovani. The significance of XPRT in guanine salvage is supported by the fact that Δxprt L. donovani are unable to grow in guanine, as well as xanthine and xanthosine, providing genetic proof that XPRT is perhaps the only physiologically important route by which these three purine nutrients are incorporated into the parasite nucleotide pools [12]. Even though kinetic studies have revealed that guanine is a high affinity substrate for L. donovani HGPRT [52], the fact that Δxprt parasites fail to grow in guanine indicates that guanine is deaminated to xanthine, a dead end substrate in the Δxprt background [12]. A robust guanine deaminating activity has been described [1,2], and a gene encoding a presumptive GDA has been annotated in the genomes of all Leishmania species [16,17,19]. Thus, it appears that the majority of intracellular guanine is preferentially transformed to xanthine by GDA and elevated to the nucleotide level by XPRT.
Similarly, analysis of purine metabolism by Δaprt L. donovani demonstrates that this null mutant could efficiently incorporate [14C]adenine into purine nucleotides, supporting the existence of at least one additional route for adenine metabolism in the parasite [12]. The deamination of adenine to hypoxanthine via AAH has been documented [2,15,53], and this route of adenine flux in the Δaprt parasites was confirmed by the addition of 2′-deoxycoformycin (dCF), a known inhibitor of AAH, to the culture medium [12]. The addition of dCF to Δaprt parasites obliterated [14C]adenine incorporation into nucleotides by preventing adenine hydrolysis to hypoxanthine and subsequent phosphoribosylation of the 6-oxypurine by HGPRT. The ability of adenine to serve as the purine nutrient for Δaprt parasites indicates that the conversion of adenine to hypoxanthine by AAH is sufficiently robust for maintenance of purine nucleotide pools and to sustain the continuous growth of the null parasites [12]. Recent experiments have demonstrated that purified, recombinant AAH, as well as live parasites, catalyzes the robust conversion of adenine to hypoxanthine [10] and its pivotal role in funneling salvaged purines through hypoxanthine to IMP. Although AAH is homologous to the mammalian adenosine deaminase enzyme, the L. donovani AAH does not recognize adenosine as a substrate. Despite its central role in adenine metabolism, AAH is not essential for parasite viability, since Δaah parasites are capable of utilizing the full spectrum of salvageable purine nucleobases and nucleosides, implying L. donovani can divert adenine and adenosine metabolism through APRT and possibly AK in the absence of AAH [10].
These genetic studies in promastigotes underscore the significance of both GDA and AAH in purine interconversion and salvage by L. donovani and indicate that the majority of intracellular guanine and adenine is metabolized to xanthine and hypoxanthine, respectively, and then converted to the nucleotide level by XPRT and/or HGPRT. These mutational analyses also imply that the formation of nucleotides from xanthine and hypoxanthine are the primary salvage routes utilized by L. donovani. A depiction of the purine salvage pathway showing the relative flux and presumptive fates of metabolites is shown in Figure 2.
Figure 2.
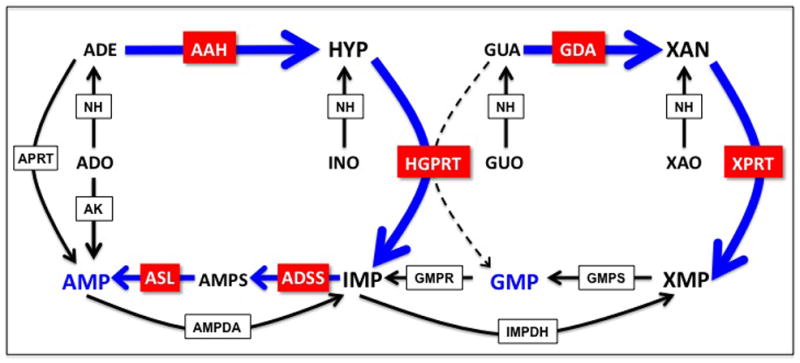
Metabolic flux through the purine salvage pathway in L. donovani. Heavy blue arrows highlight the major flux of substrates through the purine salvage pathway as deduced from genetic and biochemical experiments. The minor activities are represented by black arrows, and the dashed line indicates that the phosphoribosylation of guanine most likely does not occur under physiological conditions in Leishmania. Abbreviations: APRT, adenine phosphoribosyltransferase; HGPRT, hypoxanthine-guanine phosphoribosyltransferase; XPRT, xanthine phosphoribosyltransferase; AK, adenosine kinase; AAH, adenine aminohydrolase; GDA, guanine deaminase; ADSS, adenylosuccinate synthetase; ASL, adenylosuccinate lyase; AMPDA, AMP deaminase; IMPDH, inosine monophosphate dehydrogenase; GMPS, GMP synthase; GMPR, GMP reductase; NH, nucleoside hydrolase; ADO, adenosine; ADE, adenine; INO, inosine; HYP, hypoxanthine; GUO, guanosine; GUA, guanine; XAO, xanthosine; XAN, xanthine.
Given the plethora of dominant selectable markers for Leishmania gene targeting [13,51,54–56], as well as the capacity to manipulate the purine sources in the cell culture medium, it has been possible to introduce multiple mutations into the purine salvage pathway in many different combinations (Table 2). The ability to generate viable Δhgprt/Δaprt/ak− [9] and Δxprt/Δaprt/ak− [11] L. donovani, each with mutations in three of the four salvage enzymes, revealed that the parasite can rely solely on XPRT or HGPRT, respectively, to meet all of its nutritional requirements and genetically validated that exogenous purines are funneled to hypoxanthine and/or xanthine by L. donovani prior to incorporation into the parasite nucleotide pools. Strikingly, however, our initial selection strategies did not enable selection of a combined Δhgprt/Δxprt genotype [8,9,11]. The Δhgprt/Δxprt mutant was, however, eventually isolated in the presence of adenine and dCF, which enabled mutant parasite survival and growth by inhibiting adenine deamination and thereby obliging adenine salvage through APRT. Growth of the Δhgprt/Δxprt mutant was conditional upon the presence of dCF and either adenine or adenosine as the purine source, since no other purine supported the survival or growth of the double knockout either in the absence or presence of dCF [11]. Despite the conditional growth phenotype, sub-populations of very slow growing Δhgprt/Δxprt cells were selected on plates containing inosine and hypoxanthine [57]. These subpopulations accommodated extrachromosomal amplifications of APRT and were, surprisingly, capable of converting hypoxanthine to IMP through APRT, although the Km of APRT for hypoxanthine was three orders of magnitude greater than that for adenine [52,57]. The Δaah cell line was also used as a background strain to generate a Δaah/Δhgprt/Δxprt triple knockout, which unlike the Δhgprt/Δxprt mutant, is not dependent on dCF for growth, providing unequivocal evidence that AAH is the cellular target for dCF [10].
An ‘Achilles’ Heel’ revealed?
The conditionally lethal growth phenotype of the Δhgprt/Δxprt double knockout established that salvageable purines are funneled to substrates of the two missing enzymes. Thus, the majority of purine nucleobases and nucleosides in wild type L. donovani are first fluxed to IMP and XMP and then converted to AMP and GMP, respectively (Figures 1 and 2), insinuating that the nucleotide interconversion enzymes, inosine monophosphate dehydrogenase (IMPDH), adenylosuccinate synthetase (ADSS), adenylosuccinate lyase (ASL), GMP reductase (GMPR) and GMP synthase (GMPS) might be essential to the parasite. Using rational selective strategies, Δimpdh [58], Δadss, Δasl, and Δgmpr, null mutants were easily generated under appropriate permissive growth conditions. Permissive and restrictive growth conditions for the Δadss, and Δasl promastigotes were, as anticipated, the same as those for the Δhgprt/Δxprt parasites (J.M. Boitz, et al., unpublished), while xanthine and guanine were permissive and adenine and hypoxanthine restrictive for growth of the Δimpdh promastigotes [58], Table 2. Unexpectedly, however, we were unable to create a Δgmps knockout even with the addition of excess guanine, which we conjectured would circumvent the genetic lesion, in the culture medium. Therefore, we concluded, based on previous genetic and metabolic flux studies [2,10–12], that a homozygous Δgmps mutation could be lethal to the parasite because of the presence of GDA. GDA would effectively deaminate guanine to xanthine, which in turn would be phosphoribosylated by XPRT to XMP, a dead end substrate for a Δgmps mutant, thereby starving the mutant parasites for guanylate nucleotides regardless of the nature of the purine supplementation of the growth medium (Figure 2). We proposed, therefore, that introduction of a Δgda mutation into the parasite would drive guanine to GMP through HGPRT to GMP, thereby circumventing the potentially lethal Δgmps lesion. This hypothesis is currently being tested. The observation that Leishmania funnel all salvageable guanine to xanthine, even when XMP accumulates as a dead-end metabolite in Δgmps cells, points to a lack of regulational control of GDA and XPRT activity. Given that mammalian cells lack XPRT, it is tempting to postulate that the robust activity of the L. donovani GDA may have evolved for the benefit of the parasite, since conversion of guanine to xanthine in essence acts to trap this metabolite in a form only usable by the parasite.
Purine salvage and the purine nutritional environment within the mammalian host
Leishmania spp. are digenetic parasites, existing as highly motile, flagellated promastigotes that reside freely within the midgut of the sandfly vector and as amotile amastigotes that replicate within the phagolysosome of the mammalian macrophage. Most of the initial investigations on the purine pathway were undertaken with an attenuated L. donovani clone [59] derived from the 1S-2D strain that was originally adapted to axenic culture by Dennis Dwyer [60–62]. Consequently, studies on parasite infectivity and virulence were precluded. Recently, LdBob, a highly virulent, rapidly growing 1S-2D sub-clone that can axenically cycle between promastigotes and amastigotes and that can infect macrophages and mice, has become available [12,63]. LdBob has proven invaluable for directly assessing the nutritional role of purine salvage enzymes in the amastigote and for determining the nature of host purines available for salvage in host cells [61–63].
To determine whether any single purine salvage or interconversion enzyme is essential for purine nutrition and parasite viability in the infectious form of the parasite, the Δhgprt, Δaprt, Δxprt, Δaah, Δimpdh [10,12,58], Δadss, and Δasl (J. M. Boitz et al., unpublished) mutants, were created within the LdBob background. All of these knockouts retained their ability to transform into axenic amastigotes and exhibited purine growth profiles identical to those of their promastigote counterparts. Therefore, the purine salvage pathway does not appear to deviate significantly between the two life cycle stages – at least in vitro. Likewise the Δhgprt/Δxprt mutant constructed in the LdBob background also exhibited an absolute dependence on dCF and exogenous adenine or adenosine in its axenic amastigote form, strongly implying that AAH is active in both life cycle stages and contrasting to earlier reports indicating that AAH was promastigote-specific [3,10,12].
Several of these mutants have also been evaluated, either singly or in combination, for their impact on the ability of the parasite to infect and replicate within primary murine macrophages or establish and maintain infections in mice. Of the mutants tested thus far, including Δaah, Δimpdh [10,58], Δadss, and Δasl (J. M. Boitz et al., unpublished), only the Δasl mutant exhibits a severely reduced parasite burden in both macrophages and mice. This contrasts with the infectivity phenotype of the Δadss knockout, which also accommodates a genetic lesion in IMP to AMP conversion (Figures 1 and 2) but is only marginally impacted in its capacity to infect macrophages and mice. The discrepancy between the infectivity capacities of the two mutants defective in IMP to AMP synthesis can be explained by the accumulation of adenylosuccinate observed in the Δasl mutant in vitro. This implies that the conversion of IMP to adenylosuccinate by ADSS is unregulated and that unchecked adenylosuccinate accumulation is a lethal event. These data also shed light on the host purine environment, signifying that sufficient host-derived adenine or adenosine is available for direct salvage to AMP, which can fulfill the requirement for adenylate nucleotides in the Δadss and Δasl parasites (J. M. Boitz et al., unpublished).
Of the various L. donovani strains harboring multiple mutations, only the Δhgprt/Δxprt and Δaah/Δhgprt/Δxprt lines have been tested in macrophages and mice. The Δhgprt/Δxprt double knockout exhibited an extremely reduced parasite burden in both primary murine macrophages and mice, but parasite numbers were restored to wild type levels by complementation with XPRT expressed in trans from an episomal vector [11]. Since XPRT is able to metabolize both hypoxanthine and xanthine to the nucleotide level [14], it is unclear whether XPRT complementation of the infectivity deficit indicates that the primary purine salvage insufficiency in the Δhgprt/Δxprt null mutant is an inability to convert guanine and xanthine into guanylate nucleotides or a deficit in both guanylate and adenylate nucleotide production. Ongoing investigations to examine the impact of single Δhgprt or Δxprt mutations on parasite fitness in mice should clarify this point. Although the Δhgprt/Δxprt double knockout was profoundly incapacitated in its ability to infect mice, parasites could still be recovered from livers and spleens after four weeks of infection. Whether persistent parasites were quiescent, dividing slowly, or dying is not clear, since studies from our laboratory indicate that purine-starved promastigotes can persist in a ‘quiescent-like’ state for days and even several weeks in culture [39]. The persistence of the Δhgprt/Δxprt parasites in mice may arise from an increase in APRT activity, since APRT protein levels were slightly augmented in these cells - consistent with our previous findings in which APRT overexpression was shown to suppress the Δhgprt/Δxprt mutation in vitro. This is also substantiated by results from macrophage infectivity experiments, where the incapacitating effects of the Δhgprt/Δxprt lesion could be surmounted by the overexpression of APRT, restoring infections to near wild type levels. Thus, the infectivity data recapitulates the in vitro phenotype for HGPRT and XPRT and suggests that combined lesions in these enzymes severely impact the capacity to infect within the mouse model.
Furthermore, the Δhgprt/Δxprt lesions have also been introduced into the Δaah background, and these triple knockout parasites have been tested for their capacity to infect mice and murine macrophages [10]. Although we expected that the Δaah lesion would preserve host-derived adenine for conversion to AMP by APRT (Figures 1 and 2), the Δaah/Δhgprt/Δxprt mutant was even more compromised in the murine model than the Δhgprt/Δxprt strain. In fact, no Δaah/Δhgprt/Δxprt parasites were recovered from livers or spleens from five infected mice or from parallel infections in cultured macrophages. Parasite infectivity was fully restored by an episomal copy of XPRT, demonstrating that loss of infectivity was not due to the Δaah mutation but rather to the HGPRT and XPRT deficiencies [10].
Concluding remarks
Metabolic flux and gene replacement studies suggest that, despite a superficial complexity and convolutedness, the majority of purine flux into nucleotides in Leishmania is limited to two main routes, HGPRT and XPRT. Thus the salvage of exogenous purine nucleosides and nucleobases is simplified by their robust distillation to HGPRT and XPRT substrates. These studies have also highlighted GMPS as a potential Achilles’ heel within the pathway, where due to the robust conversion of guanine to xanthine by GDA, the pharmacological ablation of GMPS activity would effectively pit the metabolism of the parasite against itself to starve them for guanylate nucleotides.
Figure I.
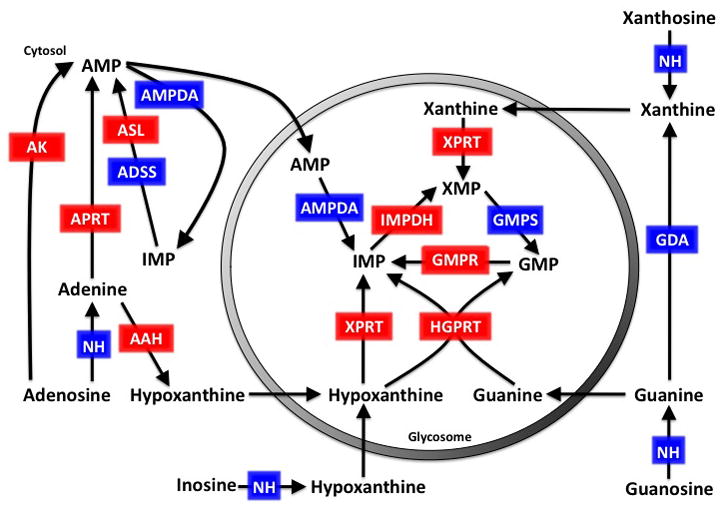
Predicted compartmentalization of the Leishmania purine salvage pathway. The gray shaded circle represents the glycosomal membrane and the cytosolic environment is located outside of the circle. Enzymes within the purine salvage pathway that have been experimentally determined to be in the cytosol or the glycosome are shown in red and those that are based on their in silico predictions are shown in blue. Abbreviations: HGPRT, hypoxanthine-guanine phosphoribosyltransferase; XPRT, xanthine phosphoribosyltransferase; IMPDH, inosine monophosphate dehydrogenase; GMPR, GMP reductase; GMPS, GMP synthase; APRT, adenine phosphoribosyltransferase; AAH, adenine aminohydrolase; AK, adenosine kinase; ADSS, adenylosuccinate synthetase; ASL, adenylosuccinate lyase; AMPDA, AMP deaminase; GDA, guanine deaminase; NH, nucleoside hydrolase.
Table 1.
Accession numbers for all of the known purine salvage and interconversion enzymes of five different Leishmania species
| Gene |
Leishmania species
|
|||||
|---|---|---|---|---|---|---|
| L. donovani | L. infantum | L. major | L. mexicana | L. braziliensis | Refs. | |
| AK | LdBPK_300940.1 | LinJ.30.0940 | LmjF.30.0880 | LmxM.29.0880 | LbrM.30.1010 | [16,17] |
| APRT | LdBPK_260120.1 | LinJ.26.0120 | LmjF.26.0140 | LmxM.26.0140 | LbrM.26.0130 | [16,17] |
| HGPRT | LdBPK_210980.1 | LinJ.21.0980 | LmjF.21.0845 | LmxM.21.0845 | LbrM.21.0990 | [16,17] |
| XPRT | LdBPK_210990.1 | LinJ.21.0990 | LmjF.21.0850 | LmxM.21.0850 | LbrM.21.1000 | [16,17] |
| AAH | LdBPK_352200.1 | LinJ.35.2200 | LmjF.35.2160 | LmxM.34.2160 | LbrM.34.2090 | [16,17] |
| ADSS | LdBPK_131090.1 | LinJ.13.1090 | LmjF.13.1190 | LmxM.13.1190 | LbrM.13.0970 | [16,17] |
| ASL | LdBPK_040440.1 | LinJ.04.0440 | LmjF.04.0460 | LmxM.04.0460 | LbrM.04.0500 | [16,17] |
| GDA | LdBPK_290920.1 | LinJ.29.0920 | LmjF.29.0867 | LmxM.08_29.0867 | LbrM.29.0910 | [16,17] |
| GMPS | LdBPK_220013.1 | LinJ.22.0013 | LmjF.22.0110 | LmxM.22.0110 | LbrM.22.0110 | [16,17] |
| GMPR | LdBPK_170870.1 | LinJ.17.0870 | LmjF.17.0725 | LmxM.17.0725 | LbrM.17.0790 | [16,17] |
| IMPDH | LdBPK_191590.1 | LinJ.19.1590 | LmjF.19.1560 | LmxM.19.1560 | LbrM.19.1820 | [16,17] |
| IAGNH | LdBPK_292910.1 | LinJ.29.2910 | LmjF.29.2800 | LmxM.08_29.2800 | LbrM.29.2850 | [16,17] |
| IGNH | LdBPK_140130.1 | LinJ.14.0130 | LmjF.14.0130 | LmxM.14.0130 | LbrM.14.0130 | [16,17] |
| IUNH | LdBPK_181570.1 | LinJ.18.1570 | LmjF.18.1580 | LmxM.18.1580. | LbrM.18.1610 | [16,17] |
| AMPDA | LdBPK_040270.1 | LinJ.04.0270 | LmjF.04.0280 | LmxM.04.0280 | LbrM.04.0320 | [16,17] |
| AMPDA | LdBPK_130870.1 | LinJ.13.0870 | LmjF.13.0980 | LmxM.13.0980 | LbrM.13.0790 | [16,17] |
| AMPDA | LdBPK_322690.1 | LinJ.32.2690 | LmjF.32.2550 | LmxM.31.2550 | LbrM.32.2780 | [16,17] |
| AMPDA | LdBPK_354860.1 | LinJ.35.4860 | LmjF.35.4800 | LmxM.34.4800 | LbrM.34.4760 | [16,17] |
Abbreviations: AK, adenosine kinase; APRT, adenine phosphoribosyltransferase; HGPRT, hypoxanthine-guanine phosphoribosyltransferase; XPRT, xanthine phosphoribosyltransferase; AAH, adenine aminohydrolase; ADSS, adenylosuccinate synthetase; ASL, adenylosuccinate lyase; GDA, guanine deaminase; GMPS, GMP synthase; GMPR, GMP reductase; IMPDH, inosine monophosphate dehydrogenase; IAGNH, inosine, adenosine, guanosine nucleoside hydrolase; IGNH, inosine-guanosine nucleoside hydrolase; IUNH, inosine uridine nucleoside hydrolase; AMPDA, AMP deaminase
Acknowledgments
The research detailed in this article was supported in part by grants AI023682, AI044138, and NS065405 from the National Institutes of Health, and a Discovery grant from the Natural Science and Engineering Research Council of Canada.
Glossary
- AAH
adenine aminohydrolase
- ADSS
adenylosuccinate synthetase
- AK
adenosine kinase
- AMPDA
AMP deaminase
- APRT
adenine phosphoribosyltransferase
- ASL
adenylosuccinate lyase
- GDA
guanine deaminase
- GMPR
GMP reductase
- GMPS
GMP synthase
- HGPRT
hypoxanthine-guanine phosphoribosyltransferase
- IMP
inosine monophosphate
- IAGNH
inosine-adenosine-guanosine nucleoside hydrolase
- IGNH
inosine-guanosine nucleoside hydrolase
- IMPDH
inosine monophosphate dehydrogenase
- IUNH
inosine-uridine (or non-specific) nucleoside hydrolase
- LdNT
Leishmania donovani Nucleoside or Nucleobase Transporter
- PRPP
phosphoribosylpyrophosphate
- XMP
xanthine monophosphate
- XPRT
xanthine phosphoribosyltransferase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marr JJ, et al. Purine metabolism in Leishmania donovani and Leishmania braziliensis. Biochim Biophys Acta. 1978;544:360–371. doi: 10.1016/0304-4165(78)90104-6. [DOI] [PubMed] [Google Scholar]
- 2.LaFon SW, et al. Purine and pyrimidine salvage pathways in Leishmania donovani. Biochem Pharmacol. 1982;31:231–238. doi: 10.1016/0006-2952(82)90216-7. [DOI] [PubMed] [Google Scholar]
- 3.Looker DL, et al. Purine metabolism in Leishmania donovani amastigotes and promastigotes. Mol Biochem Parasitol. 1983;9:15–28. doi: 10.1016/0166-6851(83)90053-1. [DOI] [PubMed] [Google Scholar]
- 4.Berens RL, Krug Edward C, Marr Joseph J. Purine and Pyrimidine Metabolism. In: Marr JJ, Muller M, editors. Biochemistry and Molecular Biology of Parasites. Academic Press Ltd; 1995. pp. 89–117. [Google Scholar]
- 5.Tuttle JV, Krenitsky TA. Purine phosphoribosyltransferases from Leishmania donovani. J Biol Chem. 1980;255:909–916. [PubMed] [Google Scholar]
- 6.Allen T, et al. Cloning and expression of the adenine phosphoribosyltransferase gene from Leishmania donovani. Mol Biochem Parasitol. 1995;74:99–103. doi: 10.1016/0166-6851(95)02475-1. [DOI] [PubMed] [Google Scholar]
- 7.Allen TE, et al. Cloning and expression of the hypoxanthine-guanine phosphoribosyltransferase from Leishmania donovani. Mol Biochem Parasitol. 1995;73:133–143. doi: 10.1016/0166-6851(94)00105-v. [DOI] [PubMed] [Google Scholar]
- 8.Hwang HY, et al. Creation of homozygous mutants of Leishmania donovani with single targeting constructs. J Biol Chem. 1996;271:30840–30846. doi: 10.1074/jbc.271.48.30840. [DOI] [PubMed] [Google Scholar]
- 9.Hwang HY, Ullman B. Genetic analysis of purine metabolism in Leishmania donovani. J Biol Chem. 1997;272:19488–19496. doi: 10.1074/jbc.272.31.19488. [DOI] [PubMed] [Google Scholar]
- 10.Boitz JM, et al. Adenine aminohydrolase from Leishmania donovani: a unique enzyme in parasite purine metabolism. J Biol Chem. 2012 doi: 10.1074/jbc.M111.307884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boitz JM, Ullman B. A conditional mutant deficient in hypoxanthine-guanine phosphoribosyltransferase and xanthine phosphoribosyltransferase validates the purine salvage pathway of Leishmania donovani. J Biol Chem. 2006;281:16084–16089. doi: 10.1074/jbc.M600188200. [DOI] [PubMed] [Google Scholar]
- 12.Boitz JM, Ullman B. Leishmania donovani singly deficient in HGPRT, APRT or XPRT are viable in vitro and within mammalian macrophages. Mol Biochem Parasitol. 2006;148:24–30. doi: 10.1016/j.molbiopara.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 13.Fulwiler AL, et al. A rapid, efficient and economical method for generating leishmanial gene targeting constructs. Mol Biochem Parasitol. 2011;175:209–212. doi: 10.1016/j.molbiopara.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jardim A, et al. Xanthine phosphoribosyltransferase from Leishmania donovani. Molecular cloning, biochemical characterization, and genetic analysis. J Biol Chem. 1999;274:34403–34410. doi: 10.1074/jbc.274.48.34403. [DOI] [PubMed] [Google Scholar]
- 15.Kidder GW, Nolan LL. Adenine aminohydrolase: occurrence and possible significance in trypanosomid flagellates. Proc Natl Acad Sci U S A. 1979;76:3670–3672. doi: 10.1073/pnas.76.8.3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aslett M, et al. TriTrypDB: a functional genomic resource for the Trypanosomatidae. Nucleic Acids Res. 2010;38:D457–462. doi: 10.1093/nar/gkp851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Logan-Klumpler FJ, et al. GeneDB--an annotation database for pathogens. Nucleic Acids Res. 2012;40:D98–108. doi: 10.1093/nar/gkr1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ivens AC, et al. The genome of the kinetoplastid parasite, Leishmania major. Science. 2005;309:436–442. doi: 10.1126/science.1112680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peacock CS, et al. Comparative genomic analysis of three Leishmania species that cause diverse human disease. Nat Genet. 2007;39:839–847. doi: 10.1038/ng2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iovannisci DM, Ullman B. Characterization of a mutant Leishmania donovani deficient in adenosine kinase activity. Mol Biochem Parasitol. 1984;12:139–151. doi: 10.1016/0166-6851(84)90131-2. [DOI] [PubMed] [Google Scholar]
- 21.Datta AK, et al. Isolation and characterization of adenosine kinase from Leishmania donovani. J Biol Chem. 1987;262:5515–5521. [PubMed] [Google Scholar]
- 22.Koszalka GW, Krenitsky TA. Nucleosidases from Leishmania donovani. Pyrimidine ribonucleosidase, purine ribonucleosidase, and a novel purine 2′-deoxyribonucleosidase. J Biol Chem. 1979;254:8185–8193. [PubMed] [Google Scholar]
- 23.Cui L, et al. A nonspecific nucleoside hydrolase from Leishmania donovani: implications for purine salvage by the parasite. Gene. 2001;280:153–162. doi: 10.1016/s0378-1119(01)00768-5. [DOI] [PubMed] [Google Scholar]
- 24.Mauricio IL, et al. Towards multilocus sequence typing of the Leishmania donovani complex: resolving genotypes and haplotypes for five polymorphic metabolic enzymes (ASAT, GPI, NH1, NH2, PGD) Int J Parasitol. 2006;36:757–769. doi: 10.1016/j.ijpara.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Downing T, et al. Whole genome sequencing of multiple Leishmania donovani clinical isolates provides insights into population structure and mechanisms of drug resistance. Genome Res. 2011;21:2143–2156. doi: 10.1101/gr.123430.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi W, et al. Nucleoside hydrolase from Leishmania major. Cloning, expression, catalytic properties, transition state inhibitors, and the 2.5-a crystal structure. J Biol Chem. 1999;274:21114–21120. doi: 10.1074/jbc.274.30.21114. [DOI] [PubMed] [Google Scholar]
- 27.Debrabant A, et al. A unique surface membrane anchored purine-salvage enzyme is conserved among a group of primitive eukaryotic human pathogens. Mol Cell Biochem. 2001;220:109–116. doi: 10.1023/a:1010809420104. [DOI] [PubMed] [Google Scholar]
- 28.Gottlieb M. The surface membrane 3′-nucleotidase/nuclease of trypanosomatid protozoa. Parasitol Today. 1989;5:257–260. doi: 10.1016/0169-4758(89)90259-7. [DOI] [PubMed] [Google Scholar]
- 29.Debrabant A, et al. Isolation and characterization of the gene encoding the surface membrane 3′-nucleotidase/nuclease of Leishmania donovani. Mol Biochem Parasitol. 1995;71:51–63. doi: 10.1016/0166-6851(95)00035-y. [DOI] [PubMed] [Google Scholar]
- 30.Gottlieb M, Dwyer DM. Protozoan parasite of humans: surface membrane with externally disposed acid phosphatase. Science. 1981;212:939–941. doi: 10.1126/science.7233189. [DOI] [PubMed] [Google Scholar]
- 31.Shakarian AM, et al. Molecular dissection of the functional domains of a unique, tartrate-resistant, surface membrane acid phosphatase in the primitive human pathogen Leishmania donovani. J Biol Chem. 2002;277:17994–18001. doi: 10.1074/jbc.M200114200. [DOI] [PubMed] [Google Scholar]
- 32.Wiese M, et al. Gene cloning and cellular localization of a membrane-bound acid phosphatase of Leishmania mexicana. Mol Biochem Parasitol. 1996;82:153–165. doi: 10.1016/0166-6851(96)02729-6. [DOI] [PubMed] [Google Scholar]
- 33.Carter NS, et al. Purine and pyrimidine metabolism in Leishmania. Adv Exp Med Biol. 2008;625:141–154. doi: 10.1007/978-0-387-77570-8_12. [DOI] [PubMed] [Google Scholar]
- 34.Landfear SM, et al. Nucleoside and nucleobase transporters in parasitic protozoa. Eukaryot Cell. 2004;3:245–254. doi: 10.1128/EC.3.2.245-254.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carter NS, et al. Nucleoside transporters of parasitic protozoa. Trends Parasitol. 2001;17:142–145. doi: 10.1016/s1471-4922(00)01806-7. [DOI] [PubMed] [Google Scholar]
- 36.Liu W, et al. Functional characterization of nucleoside transporter gene replacements in Leishmania donovani. Mol Biochem Parasitol. 2006;150:300–307. doi: 10.1016/j.molbiopara.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arastu-Kapur S, et al. Functional analysis of an inosine-guanosine transporter from Leishmania donovani. The role of conserved residues, aspartate 389 and arginine 393. J Biol Chem. 2003;278:33327–33333. doi: 10.1074/jbc.M305141200. [DOI] [PubMed] [Google Scholar]
- 38.Carter NS, et al. Cloning of a novel inosine-guanosine transporter gene from Leishmania donovani by functional rescue of a transport-deficient mutant. J Biol Chem. 2000;275:20935–20941. doi: 10.1074/jbc.M002418200. [DOI] [PubMed] [Google Scholar]
- 39.Carter NS, et al. Adaptive responses to purine starvation in Leishmania donovani. Mol Microbiol. 2010;78:92–107. doi: 10.1111/j.1365-2958.2010.07327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanchez MA, et al. Functional expression and characterization of a purine nucleobase transporter gene from Leishmania major. Mol Membr Biol. 2004;21:11–18. doi: 10.1080/0968768031000140845. [DOI] [PubMed] [Google Scholar]
- 41.Ortiz D, et al. An acid-activated nucleobase transporter from Leishmania major. J Biol Chem. 2009;284:16164–16169. doi: 10.1074/jbc.M109.006718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hart DT, Opperdoes FR. The occurrence of glycosomes (microbodies) in the promastigote stage of four major Leishmania species. Mol Biochem Parasitol. 1984;13:159–172. doi: 10.1016/0166-6851(84)90110-5. [DOI] [PubMed] [Google Scholar]
- 43.Opperdoes FR. Compartmentation of carbohydrate metabolism in trypanosomes. Annu Rev Microbiol. 1987;41:127–151. doi: 10.1146/annurev.mi.41.100187.001015. [DOI] [PubMed] [Google Scholar]
- 44.Parsons M, et al. Biogenesis and function of peroxisomes and glycosomes. Mol Biochem Parasitol. 2001;115:19–28. doi: 10.1016/s0166-6851(01)00261-4. [DOI] [PubMed] [Google Scholar]
- 45.Opperdoes FR, Szikora JP. In silico prediction of the glycosomal enzymes of Leishmania major and trypanosomes. Mol Biochem Parasitol. 2006;147:193–206. doi: 10.1016/j.molbiopara.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 46.Gualdron-Lopez M, et al. When, how and why glycolysis became compartmentalised in the Kinetoplastea. A new look at an ancient organelle. Int J Parasitol. 2011;42:1–20. doi: 10.1016/j.ijpara.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 47.Shih S, et al. Localization and targeting of the Leishmania donovani hypoxanthine-guanine phosphoribosyltransferase to the glycosome. J Biol Chem. 1998;273:1534–1541. doi: 10.1074/jbc.273.3.1534. [DOI] [PubMed] [Google Scholar]
- 48.Zarella-Boitz JM, et al. Subcellular localization of adenine and xanthine phosphoribosyltransferases in Leishmania donovani. Mol Biochem Parasitol. 2004;134:43–51. doi: 10.1016/j.molbiopara.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 49.Dobie F, et al. Kinetic characterization of inosine monophosphate dehydrogenase of Leishmania donovani. Mol Biochem Parasitol. 2007;152:11–21. doi: 10.1016/j.molbiopara.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 50.Cruz A, Beverley SM. Gene replacement in parasitic protozoa. Nature. 1990;348:171–173. doi: 10.1038/348171a0. [DOI] [PubMed] [Google Scholar]
- 51.Cruz A, et al. Double targeted gene replacement for creating null mutants. Proc Natl Acad Sci U S A. 1991;88:7170–7174. doi: 10.1073/pnas.88.16.7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Allen T, et al. Purification and characterization of the adenine phosphoribosyltransferase and hypoxanthine-guanine phosphoribosyltransferase activities from Leishmania donovani. Mol Biochem Parasitol. 1989;33:273–281. doi: 10.1016/0166-6851(89)90089-3. [DOI] [PubMed] [Google Scholar]
- 53.Iovannisci DM, et al. Genetic analysis of adenine metabolism in Leishmania donovani promastigotes. Evidence for diploidy at the adenine phosphoribosyltransferase locus. J Biol Chem. 1984;259:14617–14623. [PubMed] [Google Scholar]
- 54.Freedman DJ, Beverley SM. Two more independent selectable markers for stable transfection of Leishmania. Mol Biochem Parasitol. 1993;62:37–44. doi: 10.1016/0166-6851(93)90175-w. [DOI] [PubMed] [Google Scholar]
- 55.Goyard S, Beverley SM. Blasticidin resistance: a new independent marker for stable transfection of Leishmania. Mol Biochem Parasitol. 2000;108:249–252. doi: 10.1016/s0166-6851(00)00210-3. [DOI] [PubMed] [Google Scholar]
- 56.Beverley SM. Protozomics: trypanosomatid parasite genetics comes of age. Nat Rev Genet. 2003;4:11–19. doi: 10.1038/nrg980. [DOI] [PubMed] [Google Scholar]
- 57.Boitz JM, Ullman B. Amplification of adenine phosphoribosyltransferase suppresses the conditionally lethal growth and virulence phenotype of Leishmania donovani mutants lacking both hypoxanthine-guanine and xanthine phosphoribosyltransferases. J Biol Chem. 2010;285:18555–18564. doi: 10.1074/jbc.M110.125393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fulwiler AL, et al. IMP dehydrogenase deficiency in Leishmania donovani causes a restrictive growth phenotype in promastigotes but is not essential for infection in mice. Mol Biochem Parasitol. 2011;180:123–126. doi: 10.1016/j.molbiopara.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Iovannisci DM, Ullman B. Single cell cloning of Leishmania parasites in purine-defined medium: isolation of drug-resistant variants. Adv Exp Med Biol. 1984;165(Pt A):239–243. doi: 10.1007/978-1-4684-4553-4_46. [DOI] [PubMed] [Google Scholar]
- 60.Stauber LA. Characterization of strains of Leishmania donovani. Exp Parasitol. 1966;18:1–11. doi: 10.1016/0014-4894(66)90002-6. [DOI] [PubMed] [Google Scholar]
- 61.Dwyer DM. Leishmania donovani: surface membrane carbohydrates of promastigotes. Exp Parasitol. 1977;41:341–358. doi: 10.1016/0014-4894(77)90107-2. [DOI] [PubMed] [Google Scholar]
- 62.Debrabant A, et al. Generation of Leishmania donovani axenic amastigotes: their growth and biological characteristics. Int J Parasitol. 2004;34:205–217. doi: 10.1016/j.ijpara.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 63.Goyard S, et al. An in vitro system for developmental and genetic studies of Leishmania donovani phosphoglycans. Mol Biochem Parasitol. 2003;130:31–42. doi: 10.1016/s0166-6851(03)00142-7. [DOI] [PubMed] [Google Scholar]
- 64.Parsons M. Glycosomes: parasites and the divergence of peroxisomal purpose. Mol Microbiol. 2004;53:717–724. doi: 10.1111/j.1365-2958.2004.04203.x. [DOI] [PubMed] [Google Scholar]


