Abstract
Introduction
Although the Bone Mass Measurement Act outlines the indications for central dual energy x-ray absorptiometry (DXA) testing for U.S. Medicare beneficiaries, the specifics regarding the appropriate ICD-9 codes to use for covered indications have not been specified by Medicare and are sometimes ambiguous. We describe the extent to which DXA reimbursement was denied by gender and age of beneficiary, ICD-9 code submitted, time since previous DXA, whether the scan was performed in the physician’s office and local Medicare carrier.
Methods
Using Medicare administrative claims data from 1999–2005, we studied a 5% national sample of beneficiaries age ≥ 65 with part A+B coverage who were not HMO enrollees. We identified central DXA claims and evaluated the relationship between the factors listed above and reimbursement for central DXA (CPT code 76075). Multivariable logistic regression was used to evaluate the independent relationship between DXA reimbursement, ICD-9 diagnosis code and Medicare carrier.
Results
For persons that had no DXA in 1999 or 2000 and who had one in 2001 or 2002, the proportion of DXA claims denied was 5.3% for women and 9.1% for men. For repeat DXAs performed within 23 months, the proportion denied was approximately 19% and did not differ by sex. Reimbursement varied by more than 6-fold according to the ICD-9 diagnosis code submitted. For repeat DXAs performed at < 23 months, the proportion of claims denied ranged from 2% to 43%, depending on Medicare carrier.
Conclusion
Denial of Medicare reimbursement for DXA varies significantly by sex, time since previous DXA, ICD-9 diagnosis code submitted, place of service (office versus facility) and local Medicare carrier. Greater guidance and transparency in coding policies are needed to ensure that DXA as a covered service is reimbursed for Medicare beneficiaries with the appropriate indications.
Keywords: osteoporosis, bone mineral density, epidemiology, dual energy xray absorptiometry, reimbursement
Introduction
The Bone Mass Measurement Act (BMMA) of 1997 provides for reimbursement for central dual energy x-ray absorptiometry (DXA) and other bone mass measurement testing for U.S. Medicare beneficiaries. The BMMA outlines five indications for which bone mass measurement may be performed. These include primary preventive screening for estrogen deficient women, long term glucocorticoid therapy, hyperparathyroidism, radiographic abnormalities of vertebral bodies including osteopenia and fractures, and longitudinal assessment of response to Food and Drug Administration (FDA) approved osteoporosis medications. In 2005, DXA was added as a covered service provided as part of the ‘Welcome to Medicare’ exam. Central DXA may be repeated as a billable service as often as every 23 months; more frequent testing may be covered by Medicare if deemed “medically necessary”. Examples of reasons for “medical necessity” include use of glucocorticoids for three or more months and the need for a confirmatory baseline test if monitoring is to be performed using an alternate technology (e.g. central DXA) than a prior test (e.g. heel ultrasound). Despite the clear intent to allow DXA as a reimbursable service for the covered indications, some DXA claims are denied. This may impose a financial hardship on patients who are responsible for the charge and it may dissuade providers from ordering DXAs in the future, even for appropriate patients.
The purpose of this paper is to evaluate the patterns of Medicare reimbursement denial for central DXA testing associated ICD-9 diagnosis coding among U.S. Medicare beneficiaries from 1999–2005. We tested the hypotheses that denials would vary by ICD-9 diagnosis code submitted with the DXA claim and by local Medicare carrier. We further tested whether these factors would substantially differ for a repeat DXA performed within 23 months of the prior DXA.
There are several reasons to be concerned about Medicare denial rates. First, the translation of the five BMMA indications to specific ICD-9 diagnosis codes to use when submitting a claim for a DXA is often unclear. Unlike for mammography [1, 2], the Centers for Medicare and Medicaid Services (CMS) has not specified which ICD-9 codes should be used to identify the specific diagnostic criteria for qualified individuals and leaves this to the determination of the local Medicare carrier. Second, local Medicare carriers are left to individually define the “medically necessary” conditions under which testing could be performed more frequently than every 23 months. Lack of clear and easily accessible guidance from the local carriers, or failure of providers to follow that guidance when it exists, may substantially affect the likelihood of reimbursement denial. Because of the uncertainty surrounding the appropriate ICD-9 code to use, providers may have DXA claims rejected even for patients who have one of the indications specified in the BMMA.
A third concern is the notable omission in the BMMA indications of primary preventive screening for men. Although the National Osteoporosis Foundation (NOF) and the International Society for Clinical Densitometry (ISCD) recommend screening for men beginning at age 70 [3, 4], and a recent cost-effectiveness analysis suggests that screening older men may indeed be cost-effective [5], the BMMA does not include this provision. For this reason, a man undergoing DXA testing for one of the covered BMMA indications must have the claim coded accurately to maximize the likelihood of reimbursement. For example, if DXA is used to evaluate a man at high risk for fracture (e.g. a long term glucocorticoid user), an ICD-9 diagnostic code that indicates this condition is needed since a screening code will likely be rejected. In some circumstances, more than one code may be appropriate, e.g. a DXA for a postmenopausal woman on a prescription drug therapy for osteoporosis that is being monitored longitudinally. If DXA was repeated for monitoring purposes for this individual at less than 23 months, an ICD-9 code for postmenopausal osteoporosis might be rejected but one for long-term monitoring might be reimbursed.
Methods
Medicare Data Source and Eligible Population
After approval of the study protocol by the University of Alabama at Birmingham institutional review board and CMS, we obtained the Chronic Conditions Warehouse (CCW) claims data from Medicare for the years 1999 through 2005. These files provide data on a 5% random sample of Medicare beneficiaries based upon the beneficiary’s identification number. Thus, the files provide all Medicare inpatient, outpatient and physician claims for this 5% sample, over time and linked across files by the beneficiary’s unique identification number. Results based on the 5% sample were multiplied by 20 to obtain estimates for the entire Medicare Fee-for-Service population. In accordance with Medicare policies, use of the Medicare data was governed by a Data Use Agreement, and all results were reviewed by CMS prior to public release.
Identification of Central DXA Tests
To identify claims submitted to Medicare for bone mass measurement for the years 1999–2005 we used Healthcare Current Procedure Classification System (HCPCS) codes for central DXA 76075 in claims from the Medicare carrier and outpatient files. DXA procedures are typically billed either as a single claim, indicating that the billing provider (e.g. a physician) both performed and interpreted the test in an office-based (non-facility) setting, or alternatively, as two claims, one for the technical charge for the test and another for interpretation. In the latter circumstance, a testing facility (e.g. the outpatient department of a hospital) usually bills for the technical charge, and a physician bills for the interpretation. Because claims for the technical charge and the interpretation are often not submitted by the same provider or on the same day, the use of HCPCS modifiers –TC (technical component only) and −26 (professional component only) were examined to identify facility claims and link these separate components. Claims for DXA occurring within 15 days of one another were aggregated together as a single unit to permit such linkage. DXA tests for which Medicare was not the primary payer were excluded from analysis (2.5 % of total).
Eligibility Criteria
Subjects were Medicare beneficiaries ≥ 65 years of age as of 1/1/1999 that were living in the 50 United States or the District of Columbia. Medicare beneficiaries younger than 65 are covered by Medicare mainly due to disability or end stage renal disease. We excluded these people because their DXA utilization is likely to differ from that of the general population of Medicare beneficiaries. Eligible subjects must have had 12 months of fee-for-service (FFS) Medicare part A and part B and not be enrolled in a health maintenance organization (HMO) from 1999–2005. Persons receiving care from a Medicare HMO for part or all of a year were excluded because Medicare data do not include all of their outpatient claims. Because we were interested in payments for initial and repeat DXAs, we also required that eligible individuals not have undergone central DXA in either 1999 or 2000. Thus, we examined DXA tests where the initial DXA was performed in 2001 or 2002. DXAs first performed in 2003 or later were not included in the analysis of initial DXAs since their pattern of repeat DXA testing might have been skewed by inadequate follow-up time. For the initial DXAs performed in 2001–2002, we then evaluated repeat (i.e. second) DXAs, where repeat tests were performed in any year through 2005 inclusive. Third and subsequent DXAs were not included in any analyses.
Data Analysis
Our primary interest was to determine how patterns of testing and associated payments varied by sex, diagnosis submitted on the DXA claim, and local Medicare carrier. Results for reimbursement were compared among subgroups of interest using descriptive statistics and chi-square tests. We were particularly interested in patterns of reimbursement for DXAs performed within 23 months of the prior DXA and so stratified all analyses on this factor. A Pearson correlation coefficient was used to evaluate the relationship between reimbursement for initial DXAs and reimbursement for repeat DXAs performed at < 23 months. Multivariable logistic regression was used to evaluate the association between payment for DXA and Medicare carrier adjusting for several factors of interest that were included in these models based upon clinical interest (e.g. sex, urban / rural testing location, facility vs. non-facility testing center). All analyses were conducted using SAS 9.1 (SAS Institute, Cary, NC).
Results
The characteristics of eligible Medicare beneficiaries, stratified by whether they underwent only one versus more than one central DXA study are shown in Table 1. Overall, 53.7% of people who had a DXA in 2001 or 2002 were not re-tested through 2005. African Americans, men, and those living in rural areas were less likely to be re-tested.
Table 1.
Characteristics of Eligible* Medicare Beneficiaries with only 1 or > 1 claim submitted for 76075 (central DXA) in 2001 – 2005
| Beneficiaries (%) | |||
|---|---|---|---|
| Underwent Only 1 DXA |
Underwent > 1 DXA |
P value | |
| Total | 853,000 (53.7) | 736,200 (46.3) | |
| Race/ethnicity | < 0.0001 | ||
| African American | 36,420 (58.8) | 25,540 (41.2) | |
| Caucasian | 790,500 (53.5) | 687,540 (46.5) | |
| Other | 26,080 (53.0) | 23,120 (47.0) | |
| Sex | < 0.0001 | ||
| Women | 765,280 (52.6) | 688,720 (47.4) | |
| Men | 87,720 (64.9) | 47,480 (35.1) | |
| Age, yrs | < 0.0001 | ||
| 65–69 | 147,780 (48.2) | 159,040 (51.8) | |
| 70–74 | 268,940 (50.1) | 267,800 (49.9) | |
| 75–79 | 233,580 (54.7) | 193,500 (45.3) | |
| 80+ | 202,700 (63.6) | 115,860 (36.4) | |
| Geographic Region | < 0.0001 | ||
| Northeast | 163,040 (53.4) | 142,440 (46.6) | |
| Midwest | 244,600 (55.9) | 193,180 (44.1) | |
| West | 127,940 (54.4) | 107,080 (45.6) | |
| South | 317,420 (52.0) | 293,500 (48.0) | |
| Location | < 0.0001 | ||
| Urban Metropolitan | 531,900 (52.2) | 485,160 (47.8) | |
| Rural | 321,100 (56.3) | 251,040 (43.7) | |
eligible persons must have had full year Medicare Part A + Part B for 1999–2005, not have had a central DXA study done in 1999 or 2000, and be age ≥ 65 on 1/1/1999. These estimates reflect data from the Medicare national 5% sample.
For initial DXA tests, the proportion of DXA claims denied was higher for men (9.1%) than women (5.3%), a 1.7-fold difference (Table 2). For central DXA studies repeated within 23 months of the prior test, the proportion of claim denials was much higher (about 19%) and did not differ significantly by sex. There were no significant differences in the proportion of DXA claims denied among men older than 70 years compared to men younger than 70 years.
Table 2.
Number of Central DXA Studies Performed and Proportion Denied, by Sex and Testing Interval
| Women | Men | P value* | |||
|---|---|---|---|---|---|
| Number Performed |
Proportion Denied (%) |
Number Performed |
Proportion Denied (%) |
||
| Initial DXA | 1,454,000 | 5.3 | 135,200 | 9.1 | < 0.0001 |
|
Repeat DXA Interval < 23 months Interval ≥ 23 months |
154,980 533,740 |
18.5 3.3 |
16,520 30,960 |
19 4.8 |
0.75 0.002 |
comparing the proportion of central DXA studies not paid between women and men
Table 3 shows how the proportion of DXA claim denials varied by ICD-9 diagnosis code submitted. For the initial claim submitted, the proportion not reimbursed by the local Medicare carrier varied from 1.8 – 18.0%, with the mean proportion not paid of 5.7%. In contrast, for DXA studies repeated within 23 months, the proportion not reimbursed by the local Medicare carrier varied from 8.1 to 33.1%, with a mean of 18.6%. For DXAs repeated at > 23 months, the proportion not paid was much lower (3.4%).
Table 3.
Number of Central DXA Studies Performed and Proportion of Claims Denied Reimbursement, by Diagnosis and Testing Interval
| Initial DXA | Repeat DXA | ||||||
|---|---|---|---|---|---|---|---|
| < 23 months | ≥ 23 months | ||||||
| ICD9 | Number | Proportion | Number | Proportion | Number | Proportion | |
| Diagnosis | Code | Performed | Not Paid (%) | Performed | Not Paid (%) | Performed | Not Paid (%) |
| Osteoporosis NOS | 733 | 493,220 | 3.4 | 67,020 | 14.5 | 181,680 | 2.1 |
| Disorder Bone & Cartilage NOS (i.e. Osteopenia) | 733.9 | 240,900 | 3.4 | 30,400 | 22.8 | 126,160 | 2.1 |
| Other Diagnosis Codes | All Others | 215,880 | 14.5 | 17,020 | 21.4 | 55,580 | 9.6 |
| Symptomatic Menopausal State | 627.2 | 179,460 | 2.8 | 9,460 | 28.3 | 46,200 | 2.1 |
| Senile Osteoporosis | 733.01 | 125,220 | 2.6 | 16,200 | 18.8 | 45,000 | 2 |
| Screening-Osteoporosis | V82.81 | 74,680 | 18 | 5,680 | 21.8 | 21,800 | 11.2 |
| Ovarian Failure NEC | 256.39 | 33,560 | 4.1 | 2,520 | 25.4 | 16,500 | 1.6 |
| Menopausal Disorder NOS | 627.9 | 43,220 | 4 | 2,360 | 33.1 | 12,320 | 3.4 |
| Screen Mammogram NEC | V76.12 | 35,580 | 7.3 | 5,180 | 8.1 | 26,260 | 5.8 |
| Postmenopausal Status | V49.81 | 34,800 | 6.3 | 2,780 | 32.4 | 20,220 | 2.8 |
| Postablative Ovarian Failure | 256.2 | 22,060 | 1.8 | In “Other” | - | In “Other” | - |
| Osteoporosis NEC (e.g. drug-induced) | 733.09 | 19,400 | 5.6 | 3,320 | 18.1 | 6,220 | 2.9 |
| Long Term Use Other Medications | V58.69 | 17,100 | 2.9 | 3,060 | 9.8 | In “Other” | - |
| Hyperparathyroidism | 252.XX | 3,980 | 1.0 | 640 | 25 | 940 | 6.4 |
| Follow-up Exam | V67.59 | 3,540 | 4.0 | 3,840 | 13.6 | 4,820 | 2.1 |
| Adrenal Steroid Adverse Events | E932.0 | 440 | 45.5 | ≪ 1% | 0 | ≪ 1% | 20.0 |
| Cushings | 255.0 | 260 | 0 | ≪ 1% | 0 | ≪ 1% | 11.1 |
| Long Term Steroid Use | V58.65 | 0 | - | ≪ 1% | 0 | 720 | 0 |
| Total | 1,589,200 | 5.7 | 171,500 | 18.6 | 564,700 | 3.4 | |
| P value for column | - | < 0.0001 | - | < 0.0001 | - | < 0.0001 | |
“≪ 1%” indicates there there were fewer than 200 DXAs performed with these diagnoses codes
NOS = Not Otherwise Specified; NEC = Not Elsewhere Classified
Data are sorted in decreasing order by the number of initial DXAs performed for each diagnosis code
Separate rows are shown for all diagnoses that comprise ≥ 1% of column total except for the last 5 rows which were included based upon high clinical interest. The remainder of the diagnoses appear in the ‘Other Diagnosis Codes’ row.
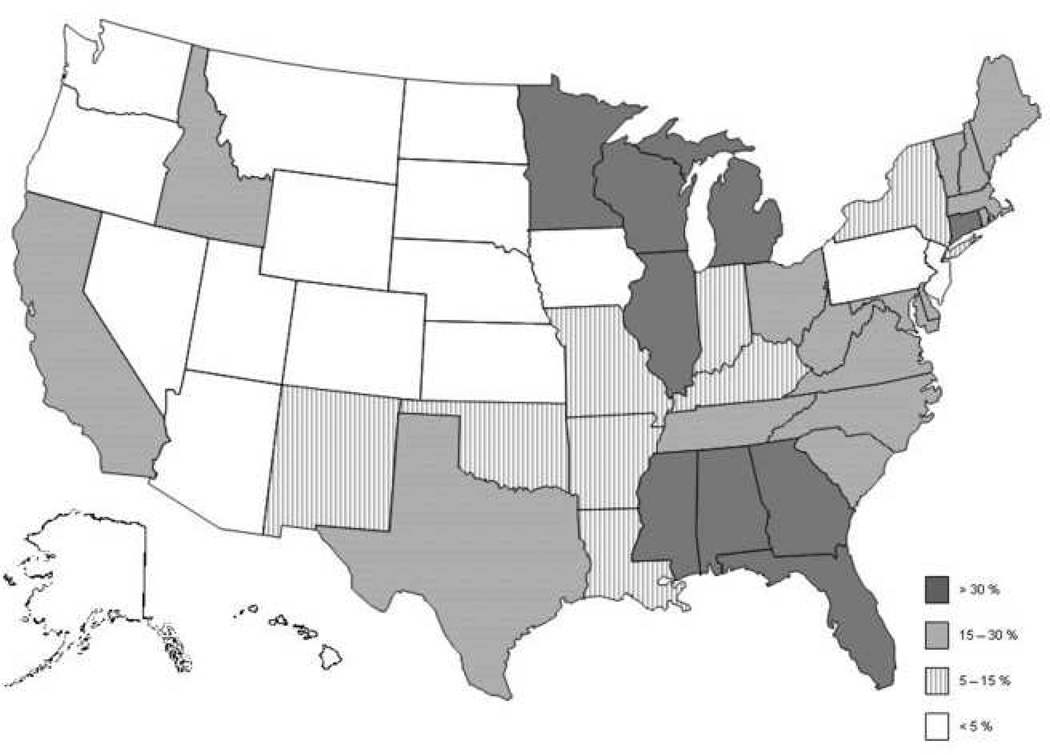
For initial DXA studies and those repeated at ≥ 23 month intervals, there were only small differences among carriers in the proportion of claims not paid (Table 4). In contrast, for DXA studies repeated at less than 23 months, the differences between Medicare carriers in the proportion not paid was striking, ranging from 1.9 to 42.5% (Figure 1). The correlation between the rejection rates for initial DXA and repeat DXAs performed at < 23 months by carrier was 0.31. The transition from higher to lower proportions of claims not being paid occurred gradually between 101 – 104 weeks since the prior test, and there was some variability among carriers (data not shown). Table 5 demonstrates that even after adjustment for age, sex, and characteristics of the sites performing DXAs (i.e. rural/urban, facility vs. non-facility), there remained substantial variability among Medicare carriers in the likelihood that repeat DXAs performed at < 23 months would be reimbursed.
Table 4.
Proportion of Central DXA Claims Denied Reimbursement, by State Medicare Carrier and Testing Interval
| Medicare Carrier and Associated States Covered* | Initial DXA | Repeat DXAM |
|
|---|---|---|---|
| < 23 months | >= 23 months | ||
| Proportion Denied (%) |
Proportion Denied (%) |
Proportion Denied (%) |
|
| Wisconsin Physician Services (WI, IL, MI) | 5.8 | 42.5 | 3.4 |
| Cahaba GBA (AL, GA, MS) | 6.3 | 41.6 | 3.0 |
| Physician Services (MN) | 6.1 | 38.3 | 4.2 |
| First Coast Service (FL, CT) | 6.5 | 32.0 | 3.2 |
| Cigna (NC, TN, ID) | 7.1 | 29.7 | 3.1 |
| BC/BS of RI (RI) | 4.4 | 28.6 | 2.0 |
| Palmetto (OH, WV, SC) | 6.3 | 27.7 | 3.9 |
| NHIC (CA, ME, NH, MA, VT) | 5.2 | 19.1 | 2.9 |
| Trailblazer (TX, VA, MD, DE, DC) | 7.0 | 18.6 | 5.2 |
| New York*** | 4.7 | 14.6 | 4.0 |
| Adminastar (IN, KY) | 6.3 | 6.7 | 2.3 |
| BC/BS of Ark. (NM, OK, AR, LA, MO) | 4.1 | 5.3 | 3.3 |
| BC/BS of Utah (UT) | 6.0 | 4.3 | 0.0 |
| Empire (NJ) | 4.7 | 4.0 | 3.3 |
| Noridian (CO, WY, IA, ND, HI, NV, SD, OR, WA, AZ, AK, MT) | 4.3 | 3.8 | 2.3 |
| HGSA Admin (PA) | 4.5 | 3.7 | 2.8 |
| BC/BS of Kansas (KS, NE) | 4.2 | 1.9 | 4.2 |
| Total Proportion Not Paid* | 5.7 | 18.6 | 3.4 |
| P value for column | < 0.0001 | < 0.0001 | < 0.0001 |
Data are sorted by the proportion of DXAs repeated at < 23 months that were denied
Total number of DXAs performed is the same as shown in Table 3
New York has multiple carriers
Figure 1.
Proportion of DXA Studies Repeated at Less than 2 years Whose Submitted Claims Were Not Reimbursed, by State
Table 5.
Multivariable-adjusted Relationship between Medicare Carrier and Payment for DXAs repeated < 23 months since prior DXA
| Factor or Medicare Carrier | Odds Ratio (95% CI) |
|---|---|
| Demographics | |
| Age | 1.01 (1.00 – 1.03) |
| Women | 0.98 (0.80 – 1.20) |
| Repeated at < 12 months (vs. 12 – 23 months) | 0.46 (0.39 – 0.55) |
| Characteristics of Facility in which DXA was performed | |
| Urban location (vs. rural) | 0.94 (0.81 – 1.08) |
| Performed in physician office (vs. a facility) | 0.12 (0.10 – 0.14) |
| Medicare Carrier (referent to Noridian) | |
| Wisconsin (WI, IL, MI) | 0.03 (0.02 – 0.04) |
| Cahaba GBA (AL, GA, MS) | 0.04 (0.03 – 0.06) |
| Physician Service (MN) | 0.05 (0.03 – 0.08) |
| First Coast Service (FL, CT) | 0.08 (0.06 – 0.12) |
| Cigna (NC, TN, ID) | 0.08 (0.05 – 0.12) |
| BC/BS of RI (RI) | 0.09 (0.04 – 0.19) |
| Palmetto (OH, WV, SC) | 0.06 (0.04 – 0.09) |
| NHIC (CA, ME, NH, MA, VT) | 0.16 (0.11 – 0.23) |
| Trailblazer (TX, VA, MD, DE, DC) | 0.18 (0.13 – 0.26) |
| New York* | 0.25 (0.16 – 0.38) |
| Adminastar (IN, KY) | 0.55 (0.32 – 0.95) |
| BC/BS of Ark. (NM, OK, AR, LA, MO) | 0.69 (0.43 – 1.10) |
| BC/BS of Utah (UT) | 0.82 (0.24 – 2.80) |
| Empire (NJ) | 1.10 (0.59 – 2.05) |
| HGSA Admin (PA) | 0.67 (0.34 – 1.32) |
| BC/BS of Kansas (KS, NE) | 2.06 (0.80 – 5.33) |
The c statistic for this model was 0.82.
CI = Confidence Interval
New York is served by more than 1 local Medicare carrier
Discussion
Using longitudinal claims data for Medicare beneficiaries from 1999–2005, we observed marked heterogeneity by ICD-9 diagnosis code submitted and local Medicare carrier in the proportion of central DXA claims that were denied. This was especially notable for repeat DXA studies performed within 23 months of the prior test. Additionally, in men the proportion of claims denied for the initial DXA study was approximately 1.7-fold greater than for women. Assuming that DXA studies are performed for male and female Medicare beneficiaries with one of the five indications specified in the BMMA, the overall 5–9% rejection rate for the claim submitted for the initial DXA study suggests that providers are unclear as to which ICD-9 diagnosis code to use.
In the face of a federally legislated mandate to provide DXA testing to appropriately qualified individuals, why does regional variation in DXA reimbursement denial exist? The indications for DXA testing seem clear in the BMMA, yet these indications do not intuitively map to specific ICD-9 diagnosis codes. Indeed, DXA tests with a diagnosis code of “Screening for osteoporosis (V82.81)” had an 18% denial rate. However, this diagnosis was relatively common and accounted for 5% of diagnoses submitted with DXA tests. One might assume that simply substituting one of the diagnosis codes associated with menopausal status (≤ 4% rejection rate) would significantly reduce the likelihood that the DXA would be denied. We suspect that the failure of Medicare carriers to provide specific ICD-9 codes for the categories of “qualified DXA individuals” contributed, at least in part, to these differences in reimbursement rates for initial DXA studies. Alternatively, or perhaps conjointly, information on which ICD-9 codes should be used for which qualified conditions may exist for certain carriers [6], but our results suggest that providers were not aware of it.
Denial rates were much higher for claims submitted on repeat DXA studies performed within 23 months than for the initial DXA study or DXA studies performed at ≥ 23 months. One indication for a repeat bone mass measurement test, to establish a baseline measurement if a different technology will be used to monitor longitudinal change, has no specific ICD-9 code. Other indications for repeat testing that could qualify as “medically necessary” include long term glucocorticoid use and hyperparathyroidism. The ICD-9 codes that could be used for DXAs related to long term glucocorticoid use (although not necessarily specified for this condition) include E932.0 [adrenal steroid adverse events], V58.65 [long term steroid use], V58.69 [long term use of other medications], 255.0 [cushings] and V67.59 [followup examination]. Rejection rates for these diagnosis codes were low, but their use was relatively uncommon. Hyperparathyroidism diagnoses also were uncommon and appeared on < 1% of DXA claims. It is possible that providers are miscoding the diagnosis of people who have these indications for repeat testing, with the result that they are not reimbursed but could have been if they were coded appropriately. It is also possible that physicians are ordering repeat DXA tests for individuals who do not meet one of the specified ‘medically necessary’ indications or that local Medicare carriers interpret “medical necessity” differently when evaluating whether to reimburse a claim submitted for a repeat DXA study within 23 months.
In addition to not providing specific ICD-9 codes for each category of beneficiaries qualified to receive DXA, no specific screening code for women was ever approved by CMS. This contrasts with other nationally mandated preventive services, including mammography and colonoscopy, which have screening codes. With mammograms, for example, all regional Medicare providers are required by federal mandate to accept two simple screening codes for asymptomatic women, with frequency of coverage dependent on date of birth [1, 2]. In contrast, regional variation in reimbursement for less common services (e.g. deep brain stimulation, toenail debridement) has been previously documented [7]. An age-appropriate screening code for DXA in female Medicare beneficiaries could potentially decrease the variation in DXA reimbursement identified in our study. Greater transparency or even standardization between local Medicare carriers in specifying which diagnosis codes should be used for persons meeting the various indications outlined in the BMMA, including screening, would likely impart greater certainty that providers will be reimbursed for appropriate testing.
By intent (Federal Register, vol 71, no 231, December 1, 2006, p. 6965), CMS has not provided specific ICD-9 diagnostic codes for the other categories of qualified individuals under the Bone Mass Measurement Act, which has resulted in different ICD-9 codes being accepted by different local Medicare carriers for the same diagnostic criteria. For example, for patients who are on glucocorticoids, E932.0, V58.65, V58.69 or 255.0 may be required depending on the local Medicare carrier. Monitoring response to medical therapy requires the ICD-9 code V67.59 or v58.69, but this is also often carrier dependent. Even codes for estrogen deficiency vary with specific carriers; for example, 627.2 (symptomatic menopausal or female climacteric states) can be used with Palmetto GBA the Medicare carrier in OH, WV and SC, but is not accepted by CIGNA, the carrier in NC, TN and ID. Standardization by CMS of the various ICD-9 diagnostic codes that could be used when submitting claims to the local Medicare carrier would likely impart greater certainty that providers would be reimbursed for appropriate testing.
The strengths of our study include use of data that allow us to generalize our results to the U.S. Medicare fee-for-service population and that provide diagnosis and state-specific detail regarding reimbursement rates. However, our results may not be generalizable to older persons with Medicare managed care coverage (i.e. Medicare Advantage). As another potential limitation, reimbursement determinations were evaluated after DXA claims had been fully resolved; initial rejection rates may have been higher but could have been successfully appealed by providers. An additional limitation is our inability to identify what “should be” the appropriate diagnostic codes for reimbursement as we only have access to claims data, not medical records. As a result, we do not necessarily know the true medical reason for which the DXA was ordered. Finally, consolidation among local Medicare carriers is expected, such that the 17 carriers currently represented in this analysis may be reduced to a smaller number in the future. This consolidation may alter the reimbursement patterns that we observed.
In conclusion, although most central DXA claims were reimbursed for women and when the interval between scans was greater than 23 months, rates of denial were far higher for men and were highly variable when serial testing was ordered at intervals less than 23 months. Greater guidance and transparency in coding policies, as well as enhanced provider awareness of those policies, are needed to improve access to DXA as a covered service for qualified Medicare beneficiaries.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Services DoHH, editor. The Guide to Medicare preventive Services for Physicians, Providers, Suppliers, and Other Health Care Professionals Second ed, vol 2008: The Medicare Learning Network (MLN) 2007. Screening Mammography; pp. 73–86. [Google Scholar]
- 2.Services CfMM . vol 2008: U.S. Department of Health & Human Services. 2007. Mammography. [Google Scholar]
- 3.Leib E, Lewiecki E, NC B, Hamdy NA. Official Positions of the International Society for Clinical Densitometry. Journal of Clinical Densitometry. 2004;7(1):1–6. doi: 10.1385/jcd:7:1:1. [DOI] [PubMed] [Google Scholar]
- 4.National Osteoporosis Foundation: Clinician's Guide to Prevention and Treatment of Osteoporosis. 2008 [Google Scholar]
- 5.Schousboe JT, Taylor BC, Fink HA, et al. Cost-effectiveness of bone densitometry followed by treatment of osteoporosis in older men. Jama. 2007;298(6):629–637. doi: 10.1001/jama.298.6.629. [DOI] [PubMed] [Google Scholar]
- 6.Watts NB. Understanding the Bone Mass Measurement Act. J Clin Densitom. 1999;2(3):211–217. doi: 10.1385/jcd:2:3:211. [DOI] [PubMed] [Google Scholar]
- 7.Foote SB, Halpern R, Wholey DR. Variation in Medicare's local coverage policies: content analysis of local medical review policies. Am J Manag Care. 2005;11(3):181–187. [PubMed] [Google Scholar]