Abstract
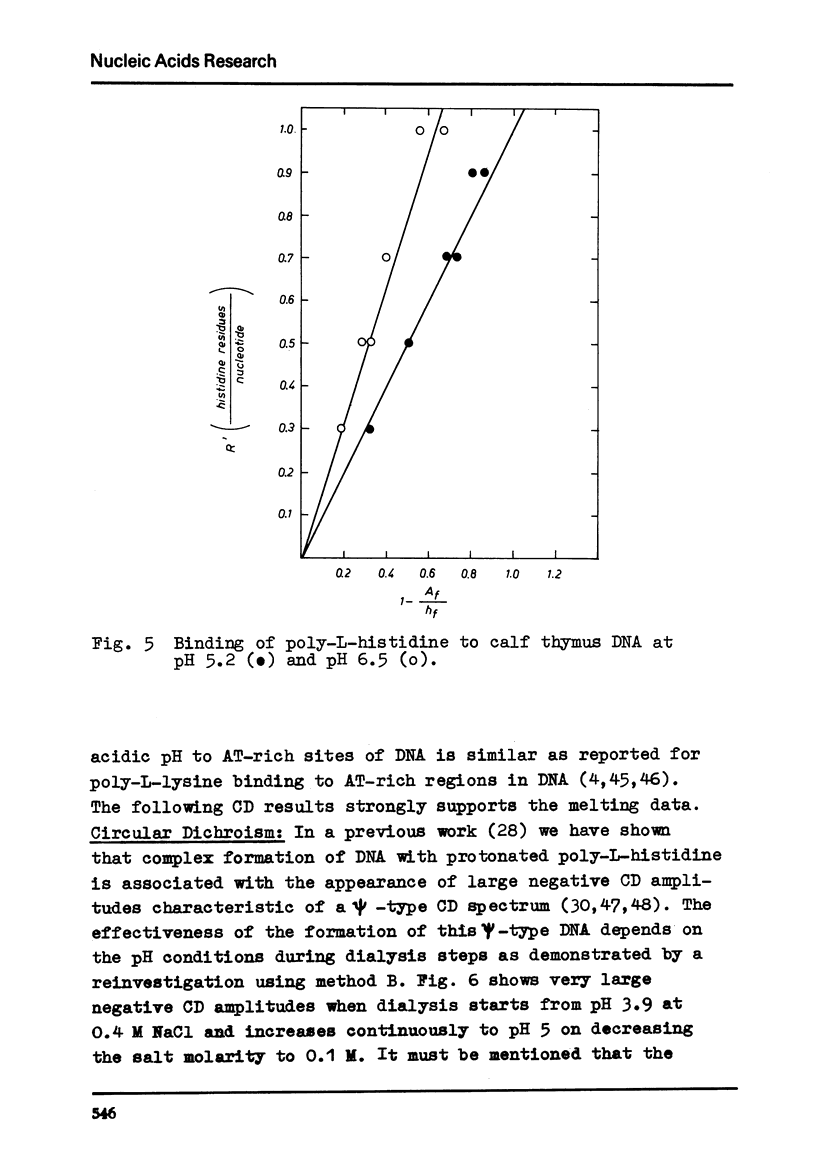
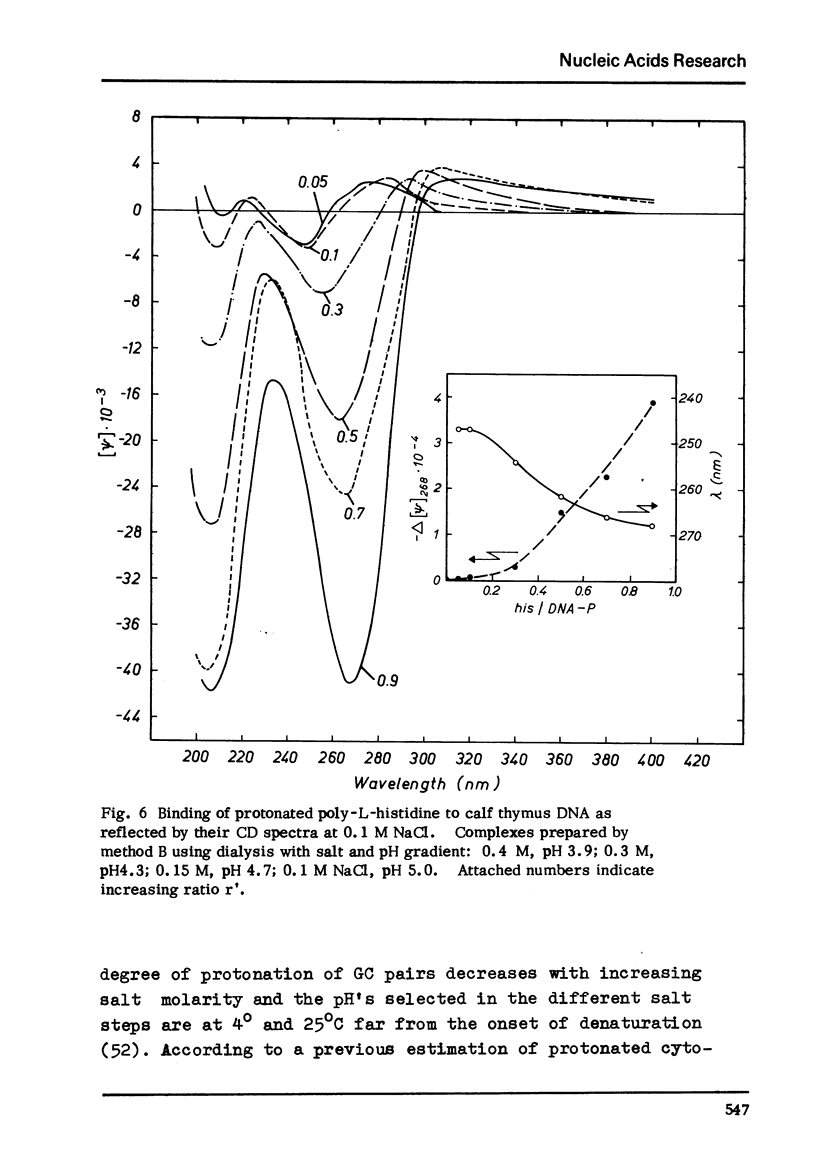
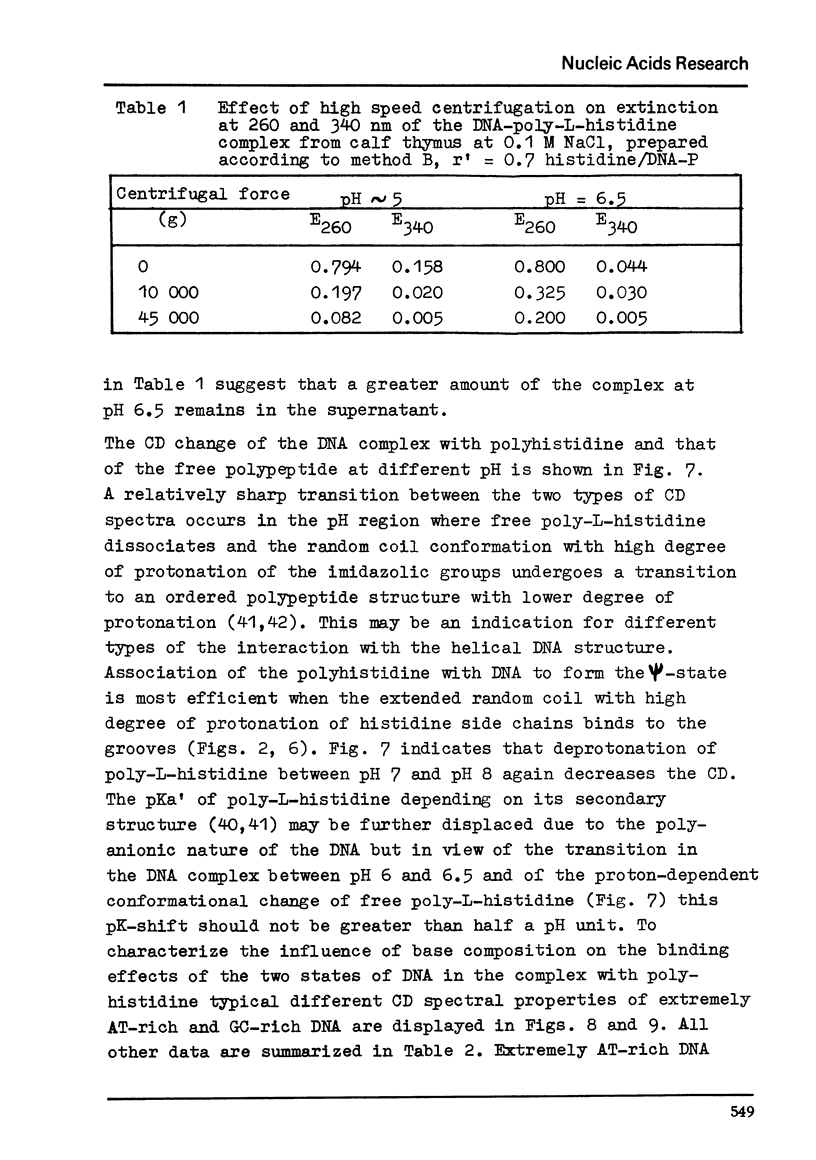
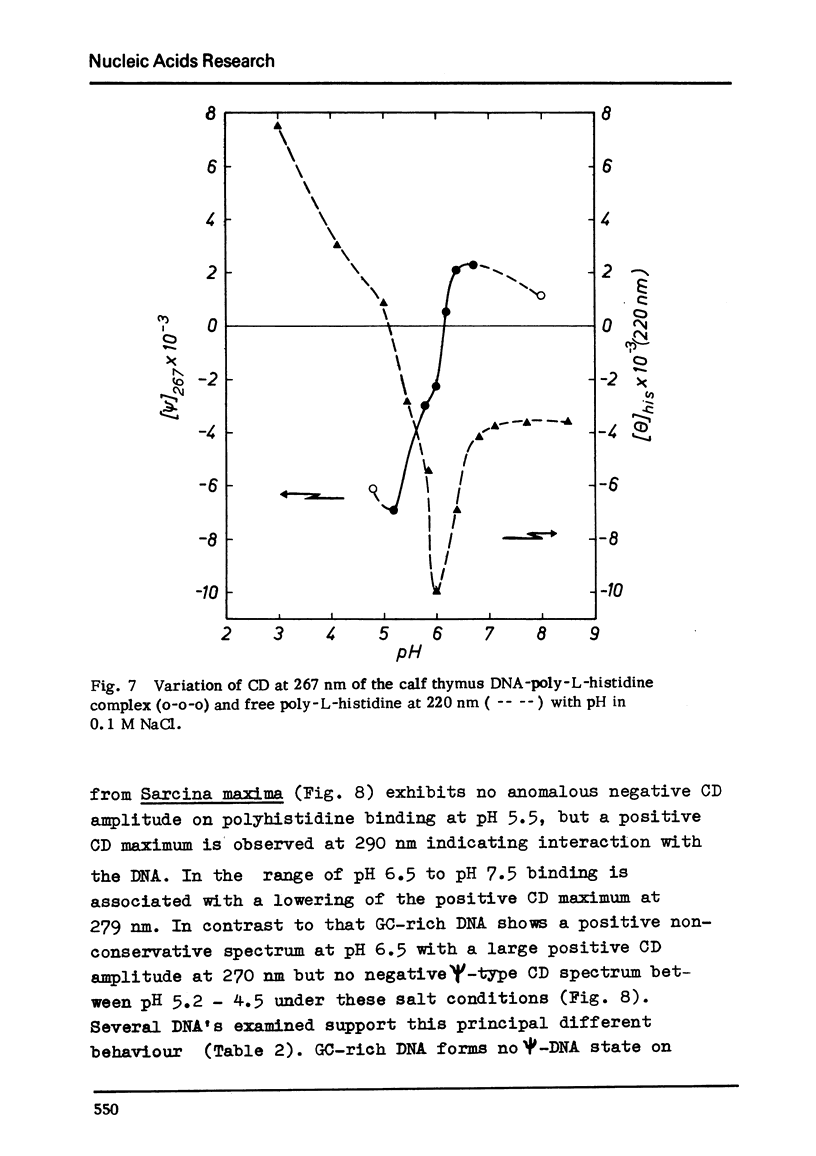
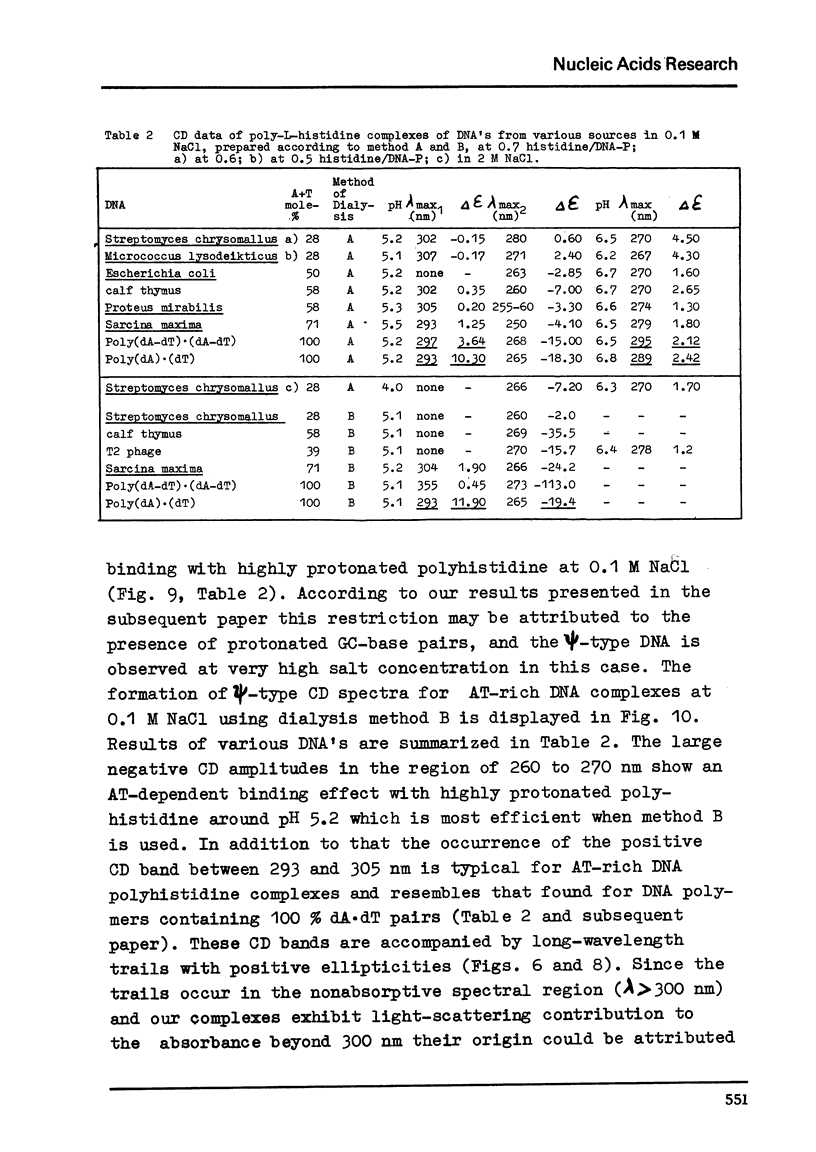
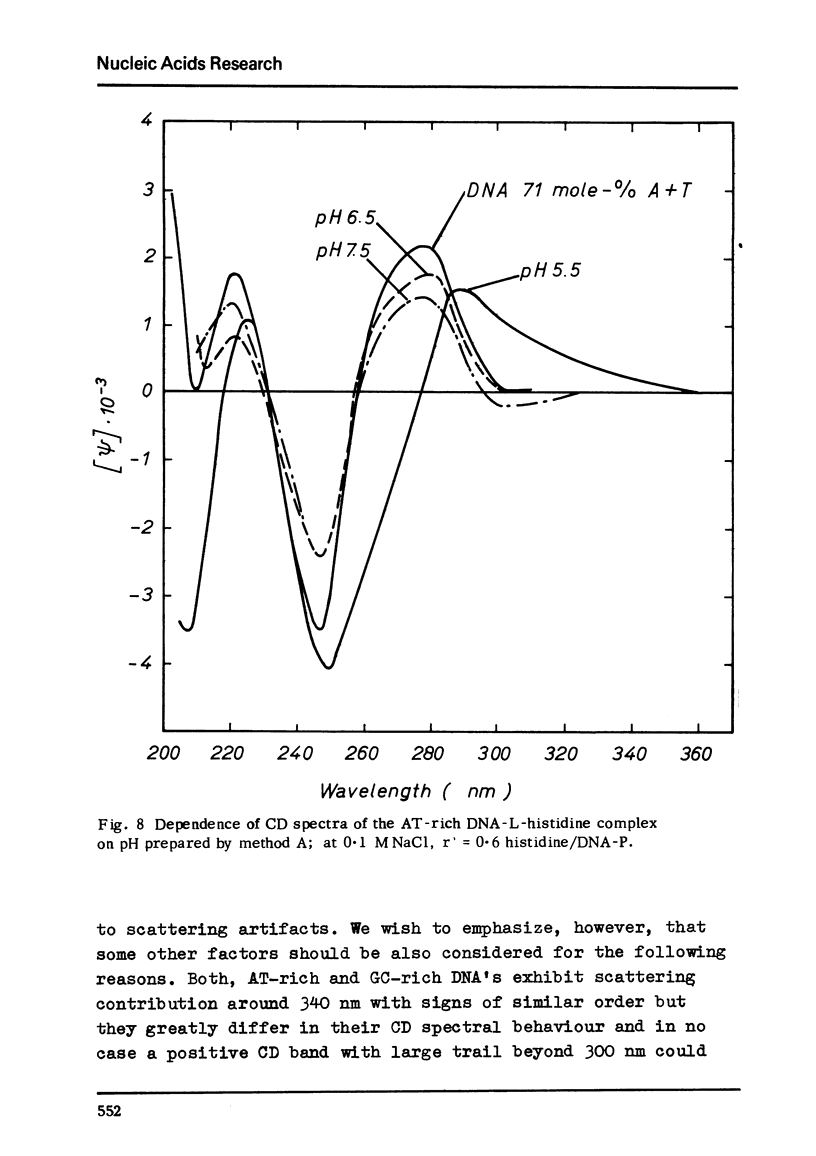
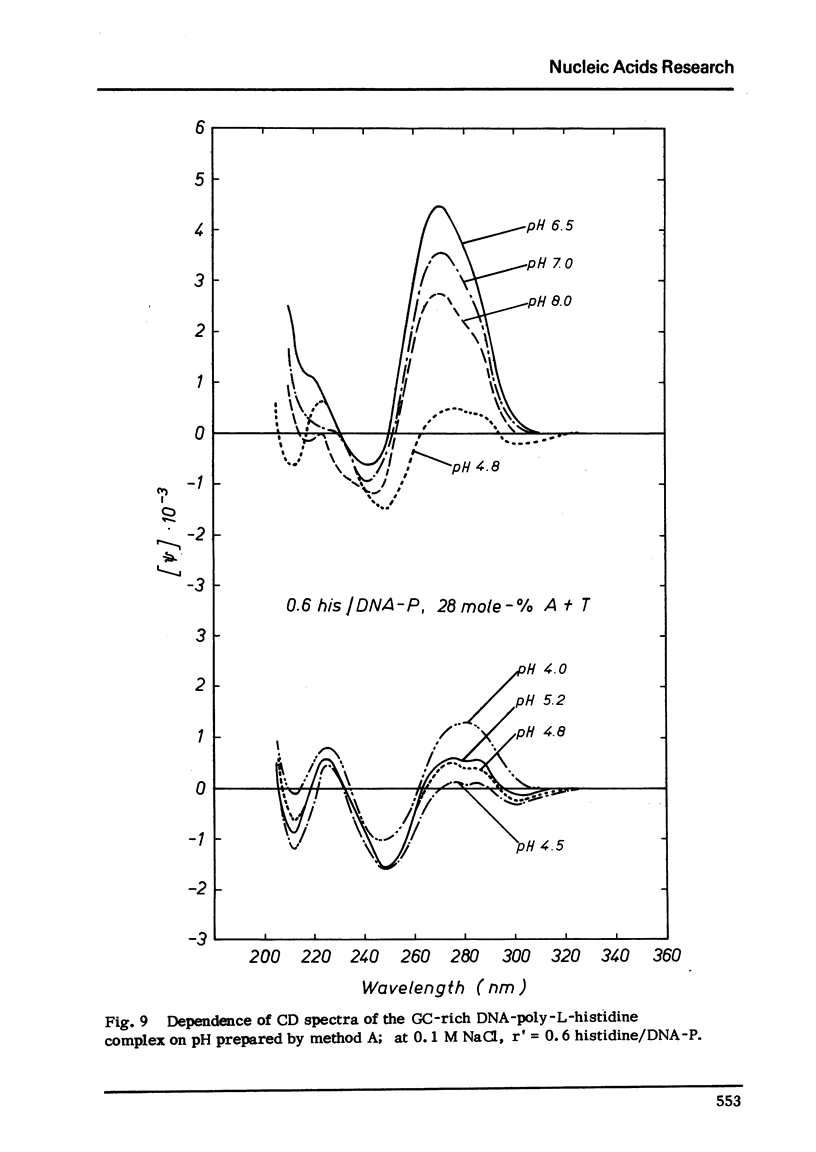
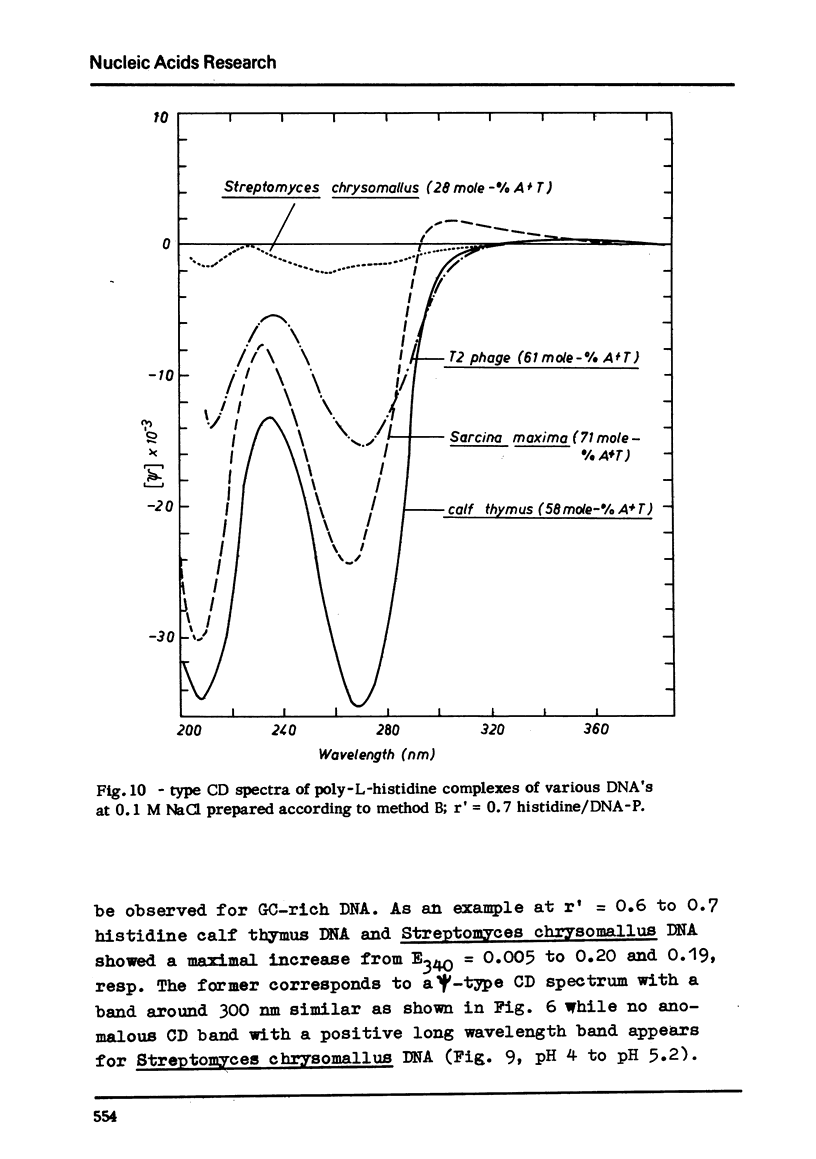
Differences in the interaction of poly-L-histidine with DNA of various base composition have been demonstrated using melting and CD measurements. The two types of complexes formed with DNA at pH values below the pK of 5.9 and in the region of pH 6.5 are very different in their CD spectral properties. The binding effects with highly protonated poly-L-histidine are AT-dependent as reflected by large negative CD spectra indicating the formation of psi-DNA as a condensed state of the double helix. GC-rich DNA may, however, also form psi-DNA structures with poly-L-histidine under certain conditions. At pH 6.5 complex formation with the weakly protonated polypeptide is GC-dependent. From the results it is concluded that protonated poly-L-histidine interacts more specifically at AT base pairs, prabably along the small groove while the weakly protonated poly-L-histidine tends to interact preferentially with GC regions which seems to occur rather in the large groove.
Full text
PDF


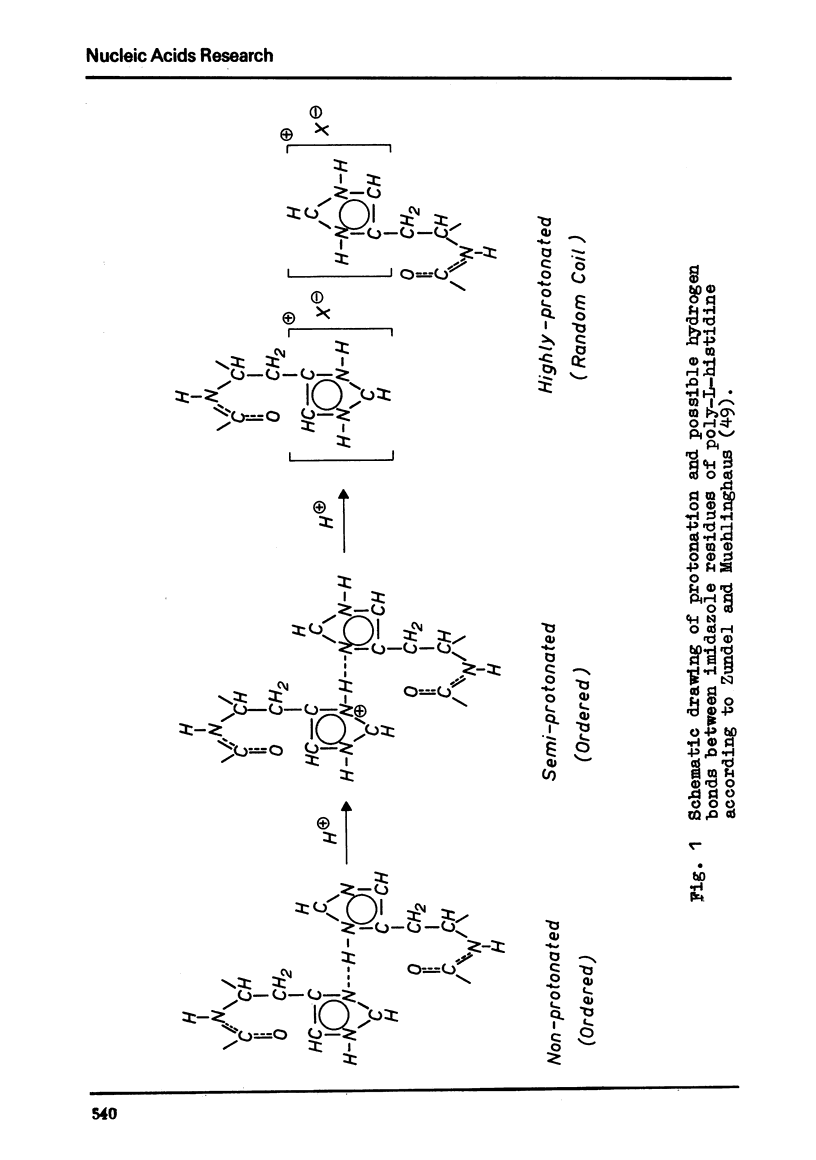

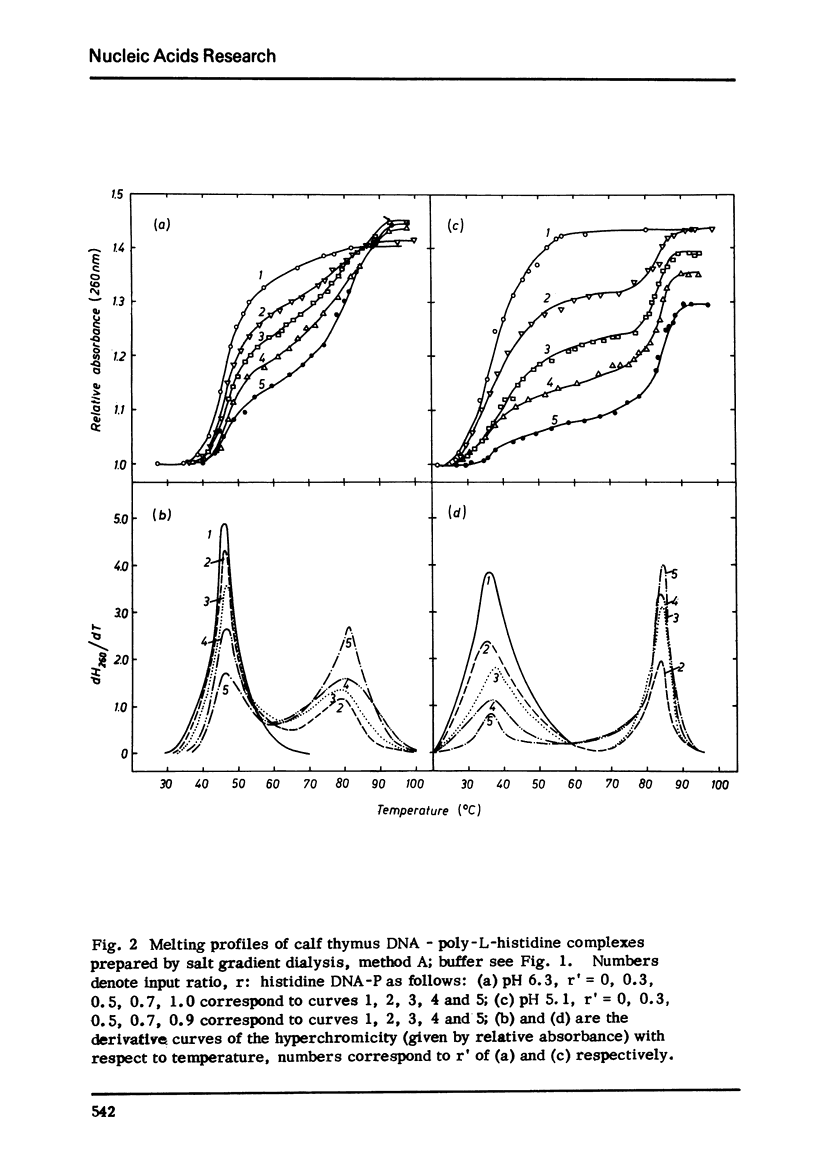
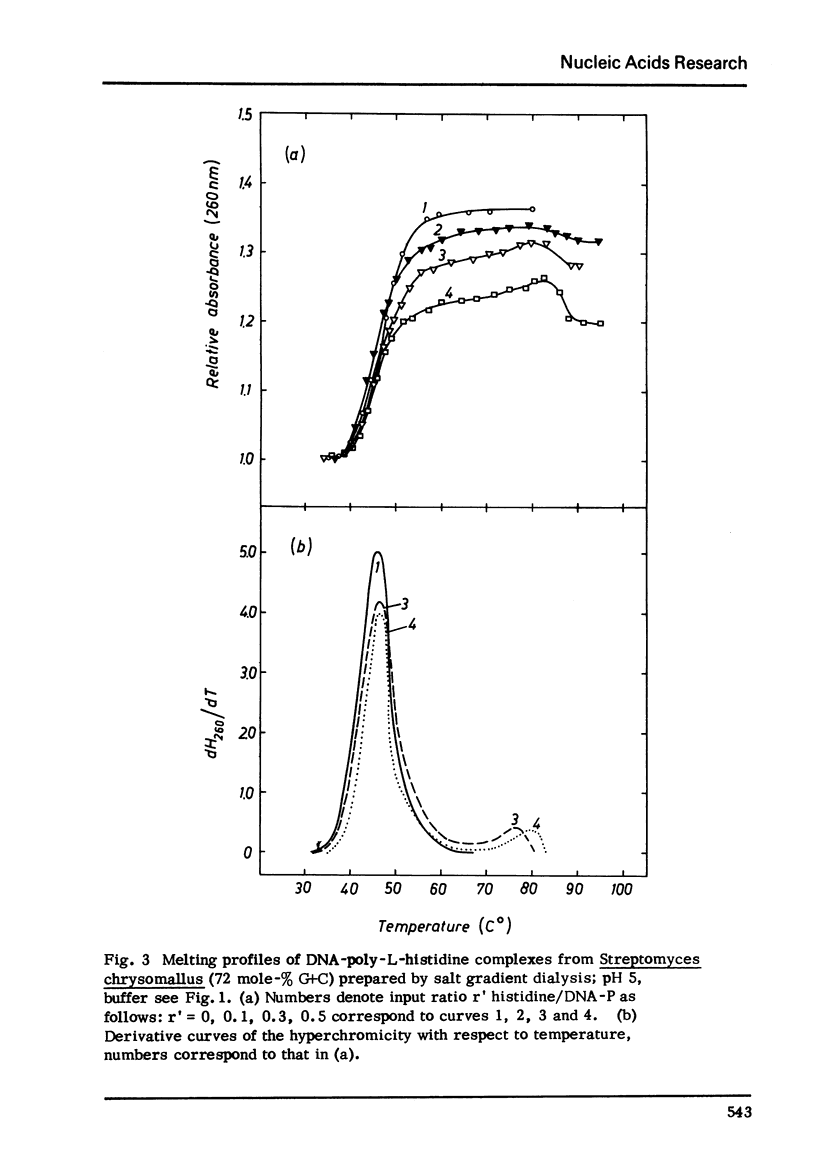
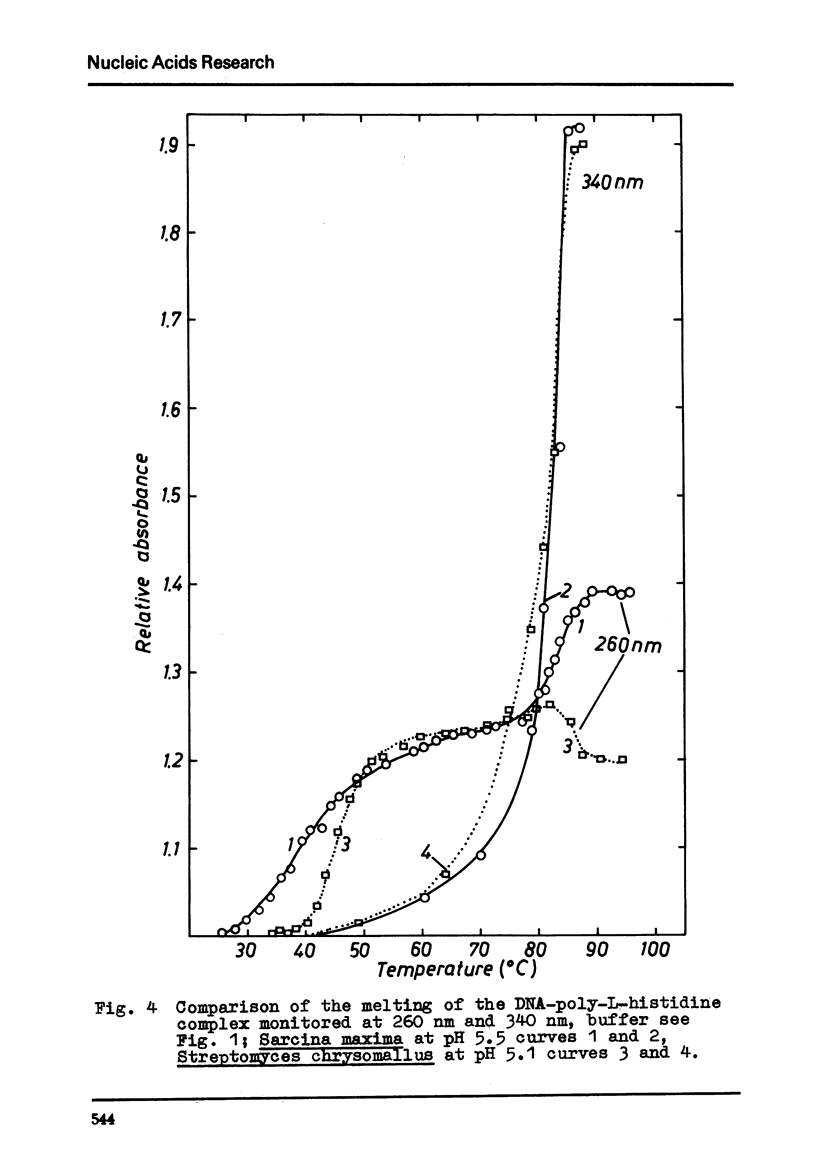






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adawadkar P., Wilson W. D., Brey W., Gabbay E. J. Letter: Stereospecific interaction of dipeptide amides with DNA. Evidence for partial intercalation and bending of the helix. J Am Chem Soc. 1975 Apr 2;97(7):1959–1961. doi: 10.1021/ja00840a062. [DOI] [PubMed] [Google Scholar]
- Beychok S., Pflumm M. N., Lehmann J. E. Sense of helix of poly-L-histidine. J Am Chem Soc. 1965 Sep 5;87(17):3990–3991. doi: 10.1021/ja01095a042. [DOI] [PubMed] [Google Scholar]
- Brunner W. C., Maestre M. F. Circular dichroism of films of polynucleotides. Biopolymers. 1974;13(2):345–357. doi: 10.1002/bip.1974.360130210. [DOI] [PubMed] [Google Scholar]
- Burckhardt G., Zimmer Ch, Luck G. Conformation and reactivity of DNA V. pH-dependent conformational changes of DNA in complexes with poly-L-histidine: Transitions from B- to A-form and to a condensed state. FEBS Lett. 1973 Feb 15;30(1):35–39. doi: 10.1016/0014-5793(73)80613-1. [DOI] [PubMed] [Google Scholar]
- CHIH R., HUANG C., BONNER J., MURRAY K. PHYSICAL AND BIOLOGICAL PROPERTIES OF SOLUBLE NUCLEOHISTONES. J Mol Biol. 1964 Jan;8:54–64. doi: 10.1016/s0022-2836(64)80148-0. [DOI] [PubMed] [Google Scholar]
- Carroll D. Optical properties of deoxyribonucleic acid--polylysine complexes. Biochemistry. 1972 Feb 1;11(3):421–426. doi: 10.1021/bi00753a019. [DOI] [PubMed] [Google Scholar]
- Chang C., Weiskopf M., Li H. J. Conformational studies of nucleoprotein. Circular dichroism of deoxyribonucleic acid base pairs bound by polylysine. Biochemistry. 1973 Jul 31;12(16):3028–3032. doi: 10.1021/bi00740a013. [DOI] [PubMed] [Google Scholar]
- Cheng S. M., Mohr S. C. Condensed states of nucleic acids. II. Effects of molecular size, base composition, and presence of intercalating agents on the psi transition of DNA. Biopolymers. 1975 Mar;14(3):663–674. doi: 10.1002/bip.1975.360140318. [DOI] [PubMed] [Google Scholar]
- Cheng S. M., Mohr S. C. The thermal transition of 'psi' DNA monitored by circular dichroism. FEBS Lett. 1974 Dec 1;49(1):37–42. doi: 10.1016/0014-5793(74)80626-5. [DOI] [PubMed] [Google Scholar]
- DeLange R. J., Smith E. L. Histones: structure and function. Annu Rev Biochem. 1971;40:279–314. doi: 10.1146/annurev.bi.40.070171.001431. [DOI] [PubMed] [Google Scholar]
- Dimicoli J. L., Hélène C. Interactions of aromatic residues of proteins with nucleic acid. II. Proton magnetic resonance studies of the binding of tyramine and tyrosine-containing peptides to poly(adenylic acid) and deoxyribonucleic acid. Biochemistry. 1974 Feb 12;13(4):724–730. doi: 10.1021/bi00701a014. [DOI] [PubMed] [Google Scholar]
- Dimicoli J. L., Hélène C. Interactions of aromatic residues of proteins with nucleic acids. I. Proton magnetic resonance studies of the binding of tryptophan-containing peptides to poly(adenylic acid) and deoxyribonucleic acid. Biochemistry. 1974 Feb 12;13(4):714–723. doi: 10.1021/bi00701a013. [DOI] [PubMed] [Google Scholar]
- Evdokimov Y. M., Platonov A. L., Tikhonenko A. S., Varshavsky Y. M. A compact form of double-stranded DNA in solution. FEBS Lett. 1972 Jun 15;23(2):180–184. doi: 10.1016/0014-5793(72)80335-1. [DOI] [PubMed] [Google Scholar]
- Friedman S., Ts'o P. O. Interaction of poly-L-tyrosine with nucleic acids. I. Formation of complexes. Biochemistry. 1971 Aug 3;10(16):3099–3104. doi: 10.1021/bi00792a018. [DOI] [PubMed] [Google Scholar]
- Gabbay E. J., Sanford K., Baxter C. S. Specific interaction of peptides with nucleic acids. Biochemistry. 1972 Aug 29;11(18):3429–3435. doi: 10.1021/bi00768a016. [DOI] [PubMed] [Google Scholar]
- Hearst J. E., Botchan M. The eukaryotic chromosome. Annu Rev Biochem. 1970;39:151–182. doi: 10.1146/annurev.bi.39.070170.001055. [DOI] [PubMed] [Google Scholar]
- Holzwarth G., Gordon D. G., McGinness J. E., Dorman B. P., Maestre M. F. Mie scattering contributions to the optical density and circular dichroism of T2 bacteriophage. Biochemistry. 1974 Jan 1;13(1):126–132. doi: 10.1021/bi00698a020. [DOI] [PubMed] [Google Scholar]
- Hélène C., Dimicoli J. L., Brun F. Binding of tryptamine and 5-hydroxytryptamine (serotonin) to nucleic acids. Fluorescence and proton magnetic resonance studies. Biochemistry. 1971 Sep 28;10(20):3802–3809. doi: 10.1021/bi00796a025. [DOI] [PubMed] [Google Scholar]
- Hélène C. Role of aromatic amino-acid residues in the binding of enzymes and proteins to nucleic acids. Nat New Biol. 1971 Nov 24;234(47):120–121. doi: 10.1038/newbio234120a0. [DOI] [PubMed] [Google Scholar]
- Jordan C. F., Lerman L. S., Venable J. H. Structure and circular dichroism of DNA in concentrated polymer solutions. Nat New Biol. 1972 Mar 22;236(64):67–70. doi: 10.1038/newbio236067a0. [DOI] [PubMed] [Google Scholar]
- Leng M., Felsenfeld G. The preferential interactions of polylysine and polyarginine with specific base sequences in DNA. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1325–1332. doi: 10.1073/pnas.56.4.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. J., Brand B., Rotter A. Thermal denaturation of calf thymus DNA: existence of a GC-richer fraction. Nucleic Acids Res. 1974 Feb;1(2):257–265. doi: 10.1093/nar/1.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. J., Chang C., Weiskopf M., Brand B., Rotter A. Helix-coil transition in nucleoprotein: renaturation of polylysine-DNA and polylysine-nucleohistone complexes. Biopolymers. 1974 Apr;13(4):649–667. doi: 10.1002/bip.1974.360130402. [DOI] [PubMed] [Google Scholar]
- Li H. J., Chang C., Weiskopf M. Helix-coil transition in nucleoprotein-chromatin structure. Biochemistry. 1973 Apr 24;12(9):1763–1772. doi: 10.1021/bi00733a016. [DOI] [PubMed] [Google Scholar]
- Muehlinghaus J., Zundel G. Infrared investigation of poly-L-histidine structure dependent on protonation. Biopolymers. 1971;10(4):711–719. doi: 10.1002/bip.360100409. [DOI] [PubMed] [Google Scholar]
- Myer Y. P., Barnard E. A. Structure-reactivity relations of imidazole in polypeptides. II. Structural transitions and a -structure in poly-L-histidine solutions. Arch Biochem Biophys. 1971 Mar;143(1):116–122. doi: 10.1016/0003-9861(71)90190-1. [DOI] [PubMed] [Google Scholar]
- Novak R. L., Dohnal J. 'DNA snapback' peptides. Nucleic Acids Res. 1974 Jun;1(6):753–759. doi: 10.1093/nar/1.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak R. L., Dohnal J. Tyrosyl peptide models for acidic protein-DNA interactions. Nat New Biol. 1973 May 30;243(126):155–157. doi: 10.1038/newbio243155a0. [DOI] [PubMed] [Google Scholar]
- Olins D. E., Olins A. L., Von Hippel P. H. Model nucleoprotein complexes: studies on the interaction of cationic homopolypeptides with DNA. J Mol Biol. 1967 Mar 14;24(2):157–176. doi: 10.1016/0022-2836(67)90324-5. [DOI] [PubMed] [Google Scholar]
- Santella R. M., Li H. J. Studies on poly(L-lysine50, L-tyrosine50)-DNA interaction. Biopolymers. 1974;13(9):1909–1926. doi: 10.1002/bip.1974.360130919. [DOI] [PubMed] [Google Scholar]
- Shapiro J. T., Leng M., Felsenfeld G. Deoxyribonucleic acid-polylysine complexes. Structure and nucleotide specificity. Biochemistry. 1969 Aug;8(8):3219–3232. doi: 10.1021/bi00836a014. [DOI] [PubMed] [Google Scholar]
- Shih T. Y., Bonner J. Thermal denaturation and template properties of DNA complexes with purified histone fractions. J Mol Biol. 1970 Mar;48(3):469–487. doi: 10.1016/0022-2836(70)90059-8. [DOI] [PubMed] [Google Scholar]
- Sponar J., Fric I., Bláha K. Basic polypeptides as histone models: circular dichroism of complexes of model polypeptides with DNA. Biophys Chem. 1975 Jul;3(3):255–262. doi: 10.1016/0301-4622(75)80018-4. [DOI] [PubMed] [Google Scholar]
- Sponar J., Fric I. Complexes of histone F1 with DNA in 0.15M NaCl. Circular dichroism and structure of the complexes. Biopolymers. 1972;11(11):2317–2330. doi: 10.1002/bip.1972.360111110. [DOI] [PubMed] [Google Scholar]
- Tsuboi M., Matsuo K., Ts'o P. O. Interaction of poly-L-lysine and nucleic acids. J Mol Biol. 1966 Jan;15(1):256–267. doi: 10.1016/s0022-2836(66)80225-5. [DOI] [PubMed] [Google Scholar]
- Yu S. S., Li H. J. Helix-coil transition and conformational studies of protamine-DNA complexes. Biopolymers. 1973 Dec;12(12):2777–2788. doi: 10.1002/bip.1973.360121211. [DOI] [PubMed] [Google Scholar]
- Zama M., Ichimura S. Difference between polylysine and polyarginine in changing DNA structure upon complex formation. Biochem Biophys Res Commun. 1971 Aug 20;44(4):936–942. doi: 10.1016/0006-291x(71)90802-3. [DOI] [PubMed] [Google Scholar]
- Zama M., Ichimura S. The study of the DNA structure in DNA-polylysine and DNA-polyarginine complexes: induced optical activities of bound dyes. Biochim Biophys Acta. 1973 Jan 19;294(2):214–226. [PubMed] [Google Scholar]
- Zama M. Structure and circular dichroism of DNA--polylysine--polyarginine complex. Biochim Biophys Acta. 1974 Oct 11;366(2):124–134. [PubMed] [Google Scholar]
- Zimmer C., Luck G., Thrum H., Pitra C. Binding of analogues of the antibiotics distamycin A and netropsin to native DNA. Effect of chomophore systems and basic residues of the oligopeptides on thermal stability, conformation and template activity of the DNA complexes. Eur J Biochem. 1972 Mar 15;26(1):81–89. doi: 10.1111/j.1432-1033.1972.tb01742.x. [DOI] [PubMed] [Google Scholar]
- Zimmer C., Venner H. Protonation of cytosine in DNA. Biopolymers. 1966 Dec;4(10):1073–1079. doi: 10.1002/bip.1966.360041004. [DOI] [PubMed] [Google Scholar]


