Abstract
Objectives
S-Adenosyl-l-methionine (SAMe) is a dietary supplement commonly used to treat depression. SAMe facilitates dopamine and norepinephrine synthesis in the central nervous system. This study investigated the efficacy of SAMe for increasing tobacco abstinence among cigarette smokers.
Design
A randomized, blinded, placebo-controlled, three-arm, dose-ranging clinical trial was conducted. Subjects were randomly allocated to receive SAMe 1600 mg or 800 mg by mouth every day or a matching placebo for 8 weeks. All subjects received a behavioral smoking cessation intervention. Self-reported smoking abstinence was biochemically confirmed with exhaled-air carbon monoxide.
Subjects
Subjects in the study comprised 120 adults.
Results
One hundred and twenty (120) subjects with a mean age of 40.0±14.0 (SD) years were enrolled. Participants smoked an average of 19.6±8.6 cigarettes per day for 21±13.2 years. The study dropout rate was high (42.5%). By intention-to-treat analysis, no significant differences were observed in abstinence rates at 8 and 24 weeks between SAMe dose groups and placebo. SAMe did not attenuate withdrawal symptoms among abstinent subjects. Rates of gastrointestinal side-effects were higher with SAMe 1600 mg/d compared to placebo.
Conclusions
SAMe did not increase smoking abstinence rates. Abstinence and tobacco withdrawal data from this clinical trial suggest that SAMe holds little promise for the treatment of tobacco dependence.
Introduction
The prevalence of current smoking among U.S. adults declined from 42% in 1965 to 20.6% in 2009.1 However, the rate of decline may not allow us to meet the Healthy People 2020 national health objectives goal (adult smoking prevalence <12%).2 This may be partially related to the limited efficacy of the available pharmacotherapies associated with an estimated 12-month biochemically confirmed continuous smoking abstinence rate of 12.3%.3 New pharmacotherapeutic options need to be evaluated.
S-Adenosyl-l-methionine (SAMe) is an over-the-counter dietary supplement commonly used to treat depression. SAMe donates a methyl group for the presynaptic synthesis of dopamine, norepinephrine, epinephrine, and serotonin.4–6 SAMe causes elevations in dopamine and norepinephrine levels and increases serotonin turnover.4,7–9 SAMe use may enhance the efficiency of receptor–effector coupling and signal transduction.4 SAMe crosses the blood–brain barrier and affects neurotransmission at the level of nucleus accumbens.10 On neuroimaging studies, the effect of SAMe on the brain seems similar to that of other antidepressants.11,12 A meta-analysis of 47 studies evaluating the efficacy of SAMe for the treatment of depression reported the overall effect size of −0.65 (95% confidence interval −1.05 to −0.25) with SAMe (parenteral or oral) compared to placebo. This corresponded to a difference in Hamilton Rating Scale for Depression (HAMD) of 5–6 points and a clinically significant improvement in depressive symptoms compared to placebo.13
By facilitating the synthesis of dopamine and norepinephrine in the central nervous system, it was hypothesized that SAMe may ameliorate the symptoms of nicotine withdrawal and improve tobacco abstinence rates among smokers trying to stop smoking. In order to test this hypothesis, the authors conducted a randomized, blinded, placebo-controlled, three-arm, parallel-group, dose-ranging clinical trial.
Materials and Methods
The Mayo Clinic Institutional Review Board (IRB) reviewed and approved the study protocol prior to subject recruitment.
Subjects
Individuals interested in stopping smoking were recruited from the community surrounding Mayo Clinic in Rochester, MN. Subjects were eligible to participate if they were (1) at least 18 years of age; (2) smoked ≥10 cigarettes per day for ≥6 months; and (3) willing to make a quit attempt.
Individuals were excluded from study enrollment if they (1) had clinically significant levels of current depression as assessed by the Center for Epidemiologic Studies Depression Scale14 or had a lifetime diagnosis of bipolar disorder, schizophrenia, or dementia; (2) had an unstable medical condition; (3) were currently (past 30 days) using antipsychotics or antidepressants; (4) were currently (past 30 days) using any treatment for tobacco dependence; (5) had recent use (past 30 days) of an investigational drug; (6) had a recent history (past 3 months) of alcohol abuse or dependence; (7) had a recent history (past 3 months) of drug abuse; (8) were pregnant, lactating, or of child-bearing potential, or likely to become pregnant during the medication phase and not willing to use a reliable form of contraception; (9) had a recent cardiovascular event (past 3 months); (10) had a clinically significant acute or chronic progressive or unstable medical condition; (11) were currently on medications interacting with SAMe; (12) had another household member or relative participating in the study; or (13) had an allergy to SAMe.
Procedures
Potential subjects interested in study participation were prescreened for eligibility over the telephone. If subjects passed the phone screening, an appointment was made for a clinic visit. During the clinic visit, subjects signed the informed consent and completed baseline questionnaires.
Eligible subjects were randomized to (1) SAMe 1600 mg/d; (2) SAMe 800 mg/d; or (3) matching placebo by mouth for 8 weeks. SAMe was available in 400-mg tablet strength. Medication was increased over a 2-week period in order to minimize the risk of adverse effects. The target dose was 800 mg twice a day for the first group, 400 mg twice a day for the second group, and two tablets twice a day for the placebo group. All participants were taking an equal number of tablets. All subjects received behavioral counseling using the “Smoke Free and Living It” manual used in our previous smoking-cessation clinical trials.
SAMe
SAMe was supplied by Pharmavite LLC., USA under its Nature Made™ brand (Table 1).
Table 1.
S-Adenosyl-l-Methionine (SAMe) Product Information
| Manufacturer | Gnosis, Italy (Distributed by Pharmavite LLC) |
| Salt | Tosylate |
| Isomers | The minimum observed value for SAMe SS to RS ratio was 65:35 |
| Tablet strength | Enteric-coated tablet with 400-mg SAMe/tablet |
| Storage | Room temperature |
| Batch stability | Over 2 years |
| Certificate of analysis | High-pressure liquid chromatography |
SS, active isomer; RS, inactive isomer.
Assessments
Demographic and tobacco use history information was collected, screening questionnaires were completed, and nicotine dependence was assessed with the Fagerström Test for Nicotine Dependence.15 Readiness to quit smoking was assessed using the Contemplation Ladder.16 During each study visit, vital signs were measured, and side-effects and concomitant medications were recorded.
Tobacco craving and nicotine withdrawal were assessed with a daily diary that contained a tobacco use self-report and the Minnesota Nicotine Withdrawal Scale–Revised.17–19 This measure consisted of the following items: desire or craving to smoke; anger, irritability, or frustration; anxiety or nervousness; difficulty concentrating; impatience; restlessness; increased appetite or hunger; insomnia, sleep problems, or awakening at night; and depressed mood or sadness. Based upon the previous 24 hours, items were rated on a 5-point scale ranging from 0 (not present) to 4 (severe). Daily nicotine withdrawal data were obtained from the information session visit to 2 weeks after the target quit date (TQD). Medication adherence was assessed by conducting pill counts at each weekly visit and by self-reports of missed doses.
Smoking abstinence outcomes
The primary endpoint was the 7-day point-prevalence smoking abstinence rate at end-of-treatment and secondary endpoints were the point-prevalence and prolonged abstinence rates at 6 months.20 The 7-day point-prevalence smoking abstinence was determined by (1) self-reported smoking abstinence for the previous 7 days, and (2) exhaled air carbon monoxide ≤8 ppm. Prolonged smoking abstinence was verified by (1) self-reported smoking abstinence from TQD (initial 2 weeks grace period following TQD was allowed); (2) exhaled air carbon monoxide ≤8 ppm at each visit; and (3) exhaled air carbon monoxide ≤8 ppm at end-of-treatment and 6 months. Subjects reporting use of tobacco products other than cigarettes were considered treatment failures.
Statistical analyses
For smoking abstinence outcomes, each SAMe dose was compared to placebo using a one-sided Fisher's exact test. For this analysis, subjects with missing outcome information were assumed to be smoking. Baseline scores for nicotine withdrawal and craving were calculated using data from the 7 days prior to starting medication. For these endpoints, data from the first 2 weeks following TQD were analyzed as change from baseline using generalized estimating equations (GEE). The explanatory variables for these models were treatment group (placebo versus 800 mg/d versus 1600 mg/d) and time (in days, treated as a continuous variable). The percentage of subjects in each of the SAMe groups experiencing adverse events possibly, probably, or definitely related to study drug were compared to placebo using Fisher's exact test. The sample size for this investigation was determined for the primary aim of obtaining preliminary evidence of the efficacy of SAMe (1600 mg/d or 800 mg/d) for the endpoint of 7-day point-prevalence tobacco abstinence at week 8 (end of treatment). For a randomized phase II trial, a one-sided test with a false-positive (type I error) rate of 0.20 is considered appropriate for the primary comparison to assess whether additional studies of the given regimen are warranted.21,22 The 7-day point-prevalence smoking abstinence rate at end-of-treatment of was hypothesized to be 15% for placebo.23 Based on this assumption, it was determined that a total sample size of 120 subjects (40 in placebo, 40 in 800 mg/d SAMe, and 40 in 1600 mg/d SAMe) would provide statistical power of approximately 80% to detect an end-of-treatment abstinence rate of 30% or greater for an active SAMe group compared to placebo (using a one-sided, α=0.20 level test).
Results
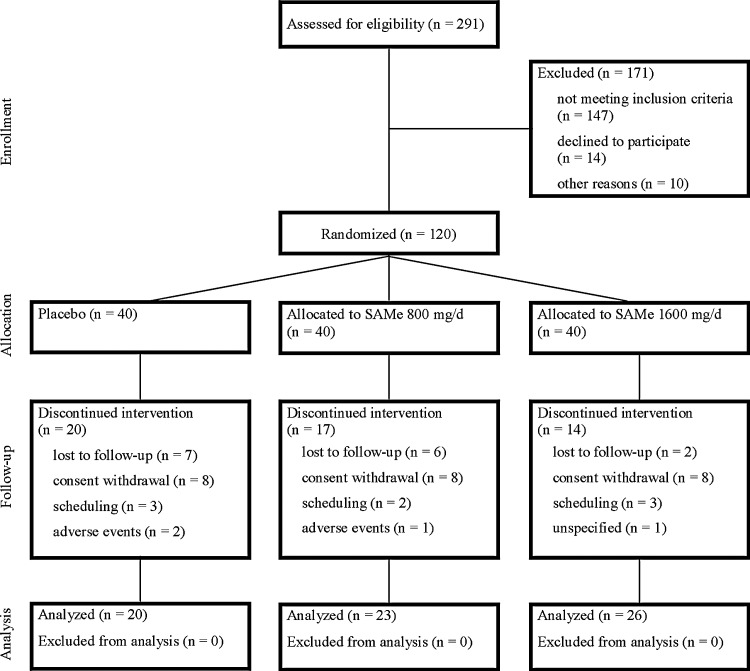
A total of 120 smokers were enrolled (Fig. 1 and Table 2).24 Of the 120 participants, 51 (42.5%) discontinued study participation prior to the end of the medication phase (20, 17, and 14 for placebo, 800 mg/d and 1600 mg/d, respectively). Reasons for discontinuing the study early included consent withdrawal (8, 8, and 8), loss to follow-up (7, 6, and 2), scheduling difficulties (3, 2, and 3), adverse events (2, 1, and 0), and unspecified reasons (0, 0, and 1).
FIG. 1.
Consolidated Standards of Reporting Trials diagram of a clinical trial to assess the efficacy of S-adenosyl-l-methionine (SAMe) versus placebo for smoking abstinence.
Table 2.
Baseline Characteristics
| Characteristic | Placebo (N=40) | 800 mg/d (N=40) | 1600 mg/d (N=40) |
|---|---|---|---|
| Age, years (mean±SD) | 37.0±13.6 | 42.1±16.0 | 40.8±12.3 |
| Gender, N (%) | |||
| Male | 20 (50) | 23 (58) | 20 (50) |
| Female | 20 (50) | 17 (42) | 20 (50) |
| Race, N (%) | |||
| White, non-Hispanic | 40 (100) | 37 (92) | 37 (92) |
| Othera | 0 (0) | 3 (8) | 3 (8) |
| Marital status, N (%) | |||
| Never married | 17 (42) | 11 (28) | 12 (30) |
| Separated/divorced | 9 (22) | 10 (25) | 14 (35) |
| Married/living as married | 14 (35) | 17 (42) | 14 (35) |
| Widowed | 0 (0) | 2 (5) | 0 (0) |
| Level of education, N (%) | |||
| High school graduate or less | 10 (25) | 12 (30) | 19 (48) |
| Some college or technical school | 26 (65) | 22 (55) | 17 (42) |
| 4-year college degree or more | 4 (10) | 6 (15) | 4 (10) |
| Cigarettes per day (mean±SD) | 19.5±8.0 | 19.5±9.3 | 19.9±8.4 |
| Number of years smoked (mean±SD) | 17.6±11.6 | 23.8±15.3 | 22.5±12.7 |
| Fagerström Test for Nicotine Dependence (mean±SD) | 4.7±2.1 | 5.4±2.1 | 5.5±2.2 |
| Previous stop attempts, N (%) | |||
| None | 5 (12) | 7 (18) | 5 (12) |
| 1–2 | 17 (42) | 13 (32) | 18 (45) |
| 3–5 | 16 (40) | 15 (38) | 8 (20) |
| 6 or more | 2 (5) | 5 (12) | 9 (22) |
| Other tobacco users in the household, N (%) | |||
| No | 22 (55) | 23 (58) | 31 (78) |
| Yes | 18 (45) | 17 (42) | 9 (22) |
These include: black/African American (N=3), white Hispanic (N=2), and more than 1 race (N=1).
SD, standard deviation.
Smoking abstinence
No differences in smoking abstinence rates were observed between the groups at end-of-treatment (week 8) and week 24 (Table 3). With the exception of 1 subject in the 800 mg/d group, all of the subjects who were biochemically confirmed abstinent for the last 7 days at end-of-medication and 6 months also met the criteria for prolonged abstinence.
Table 3.
Biochemically Confirmed 7-day Point-Prevalence Abstinencea
| Time/group | Abstinent N (%) | p-Valueb |
|---|---|---|
| End of medication (week 8) | ||
| Placebo (N=40) | 7 (17.5) | |
| 800 mg/d (N=40) | 7 (17.5) | 0.615 |
| 1600 mg/d (N=40) | 5 (12.5) | 0.826 |
| Six months | ||
| Placebo (N=40) | 5 (12.5) | |
| 800 mg/d (N=40) | 3 (7.5) | 0.868 |
| 1600 mg/d (N=40) | 4 (10.0) | 0.759 |
Self-reported 7-day point-prevalence abstinence confirmed by expired CO<8 ppm.
One-tailed Fisher's exact test comparing the biochemically confirmed abstinence rate for the given group versus placebo.
Nicotine withdrawal and tobacco craving
In all cases, neither SAMe group differed significantly from placebo using GEE analyses for the composite withdrawal score nor the individual diary item assessing craving for the first 14 days following the TQD (Table 4).
Table 4.
Symptoms of Nicotine Withdrawal and Tobacco Craving in a Clinical Trial to Assess the Efficacy of S-Adenosyl-l-Methionine Versus Placebo for Smoking Abstinence
| Parameter estimate | SEa | p-Value | |
|---|---|---|---|
| Nicotine withdrawal | |||
| Placebo | Referent | ||
| 800 mg/d | −0.11 | 0.12 | 0.35 |
| 1600 mg/d | −0.04 | 0.12 | 0.72 |
| Tobacco craving | |||
| Placebo | Referent | ||
| 800 mg/d | −0.08 | 0.26 | 0.76 |
| 1600 mg/d | −0.25 | 0.29 | 0.39 |
SE, standard error.
Adverse events
Overall, 25 subjects (6, 7, and 12 in placebo, 800 mg/d and 1600 mg/d, respectively) reported one or more adverse events considered possibly, probably, or definitely related to the study drug. No significant differences were observed in the rates of adverse events, although the rates of gastrointestinal side-effects were higher in the SAMe 1600 mg/d group for abdominal pain (12.5% versus 2.5% for placebo) and diarrhea (7.5% versus 0% in placebo) (Table 5).
Table 5.
Adverse Eventsa in a Clinical Trial to Assess the Efficacy of S-Adenosyl-l-Methionine Versus Placebo for Smoking Abstinence
| Event | Placebo (N=40) | 800 mg/d (N=40) | 1600 mg/d (N=40) |
|---|---|---|---|
| Abdominal pain | 1 (2.5) | 2 (5.0) | 5 (12.5) |
| Nausea | 2 (5.0) | 2 (5.0) | 2 (5.0) |
| Headache | 2 (5.0) | 2 (5.0) | 0 (0.0) |
| Diarrhea | 0 (0.0) | 0 (0.0) | 3 (7.5) |
| Insomnia | 1 (2.5) | 0 (0.0) | 1 (2.5) |
| Anorexia | 0 (0.0) | 0 (0.0) | 1 (2.5) |
| Confusion | 0 (0.0) | 0 (0.0) | 1 (2.5) |
| Constipation | 0 (0.0) | 1 (2.5) | 0 (0.0) |
| Dizziness | 1 (2.5) | 0 (0.0) | 0 (0.0) |
| Drowsiness | 0 (0.0) | 1 (2.5) | 0 (0.0) |
| Fatigue | 0 (0.0) | 1 (2.5) | 0 (0.0) |
| Flatulence | 0 (0.0) | 0 (0.0) | 1 (2.5) |
| Vivid dreams | 1 (2.5) | 0 (0.0) | 0 (0.0) |
| Vomiting | 0 (0.0) | 0 (0.0) | 1 (2.5) |
Adverse events considered to be possibly, probably, or definitely related to study drug are summarized according to the treatment group.
Discussion
In this randomized, blinded study, SAMe did not significantly increase tobacco abstinence rates nor decrease nicotine withdrawal compared to placebo. Study dropout was high and no differences in abstinence rates were observed.
Several reasons may account for the lack of efficacy of SAMe for smoking abstinence in this study. First, SAMe may lack efficacy for tobacco abstinence. Second, the dose of medication used may not have been high enough to observe an effect. In a meta-analysis of 47 clinical trials, however, all of the studies evaluating oral preparations of SAME used 1600 mg/d.13 Among the three placebo-controlled studies using this dose, SAMe was superior to placebo in two and similar in one.25
The major limitation of this study was a high dropout rate (42.5%). This observed dropout rate is consistent with the dropout rate observed in previous tobacco cessation studies in placebo groups.26,27 A high dropout rate in the present study could be related to significant patient burden with taking numerous pills. In this study, SAMe was used in 400-mg formulation and participants had to take up to four tablets a day, which likely adversely affected treatment adherence. The high dropout rate may also relate to the lack of efficacy of SAMe for smoking abstinence perceived by the subjects. However, the low adherence rate to SAMe suggests that, even if efficacious, low adherence to SAMe in the clinical setting would translate into an ineffective intervention.
Conclusions
In summary, it was observed that SAMe did not increase smoking abstinence rates and did not decrease tobacco withdrawal symptoms. Given the lack of apparent efficacy at an appropriately justified dose and low adherence to this medication, further testing of SAMe for tobacco cessation is not warranted.
Acknowledgments
The authors acknowledge the excellent support of the staff at the Mayo Clinic Nicotine Research Program and the subjects who participated in this research project. The project described was supported by R21 DA 21247 (Principal Investigator: AS) from the National Institutes of Health. The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health.
Disclosure Statement
No competing financial interests exist.
References
- 1.Centers for Disease Control and Prevention. Vital Signs: Current Cigarette Smoking Among Adults aged ≥18 years—United States, 2009. MMWR Morb Mortal Wkly Rep. 2010;59:1135–1140. [PubMed] [Google Scholar]
- 2.U.S. Department of Health and Human Services. Healthy People 2020—Improving the Health of Americans. 2020 Topics & Objectives. 2010. www.healthypeople.gov/2020/default.aspx. [Mar 15;2011 ]. www.healthypeople.gov/2020/default.aspx
- 3.Hoogendoorn M. Feenstra TL. Hoogenveen RT. Rutten-van Molken MP. Long-term effectiveness and cost-effectiveness of smoking cessation interventions in patients with COPD. Thorax. 2010;65:711–718. doi: 10.1136/thx.2009.131631. [DOI] [PubMed] [Google Scholar]
- 4.Bottiglieri T. S-Adenosyl-L-methionine (SAMe): From the bench to the bedside. Molecular basis of a pleiotrophic molecule. Am J Clin Nutr. 2002;76:1151S–1157S. doi: 10.1093/ajcn/76/5.1151S. [DOI] [PubMed] [Google Scholar]
- 5.Otero-Losada ME. Rubio MC. Acute changes in 5-HT metabolism after S-adenosyl-L-methionine administration. Gen Pharmacol. 1989;20:403–406. doi: 10.1016/0306-3623(89)90186-9. [DOI] [PubMed] [Google Scholar]
- 6.Losada ME. Rubio MC. Acute effects of S-adenosyl-L-methionine on catecholaminergic central function. Eur J Pharmacol. 1989;163:353–356. doi: 10.1016/0014-2999(89)90205-7. [DOI] [PubMed] [Google Scholar]
- 7.Rosenbaum JF. Fava M. Falk WE, et al. The antidepressant potential of oral S-adenosyl-l-methionine. Acta Psychiatr Scand. 1990;81:432–436. doi: 10.1111/j.1600-0447.1990.tb05476.x. [DOI] [PubMed] [Google Scholar]
- 8.Bottiglieri T. Hyland K. Reynolds EH. The clinical potential of ademetionine (S-adenosylmethionine) in neurological disorders. Drugs. 1994;48:137–152. doi: 10.2165/00003495-199448020-00002. [DOI] [PubMed] [Google Scholar]
- 9.Papakostas GI. Mischoulon D. Shyu I, et al. S-adenosyl methionine (SAMe) augmentation of serotonin reuptake inhibitors for antidepressant nonresponders with major depressive disorder: A double-blind, randomized clinical trial. Am J Psychiatry. 2010;167:942–948. doi: 10.1176/appi.ajp.2009.09081198. [DOI] [PubMed] [Google Scholar]
- 10.Genedani S. Saltini S. Benelli A, et al. Influence of SAMe on the modifications of brain polyamine levels in an animal model of depression. Neuroreport. 2001;12:3939–3942. doi: 10.1097/00001756-200112210-00017. [DOI] [PubMed] [Google Scholar]
- 11.Saletu B. Anderer P. Di Padova C, et al. Electrophysiological neuroimaging of the central effects of S-adenosyl-L-methionine by mapping of electroencephalograms and event-related potentials and low-resolution brain electromagnetic tomography. Am J Clin Nutr. 2002;76:1162S–1171S. doi: 10.1093/ajcn/76.5.1162S. [DOI] [PubMed] [Google Scholar]
- 12.Arnold O. Saletu B. Anderer P, et al. Double-blind, placebo-controlled pharmacodynamic studies with a nutraceutical and a pharmaceutical dose of ademetionine (SAMe) in elderly subjects, utilizing EEG mapping and psychometry. Eur Neuropsychopharmacol. 2005;15:533–543. doi: 10.1016/j.euroneuro.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Hardy M. Coulter I. Favreau J, et al. S-Adenosyl-L-Methionine for Treatment of Depression, Osteoarthritis, and Liver Disease. Rockville, MD: Agency for Healthcare Research and Quality; 2002. [Google Scholar]
- 14.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 15.Heatherton TF. Kozlowski LT. Frecker RC. Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: A revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 16.Biener L. Abrams DB. The Contemplation Ladder: Validation of a measure of readiness to consider smoking cessation. Health Psychol. 1991;10:360–365. doi: 10.1037//0278-6133.10.5.360. [DOI] [PubMed] [Google Scholar]
- 17.Hughes J. Minnesota Nicotine Withdrawal Scale—Revised. 2007. www.uvm.edu/∼hbpl/?Page=minnesota/default.html. [Mar 1;2011 ]. www.uvm.edu/∼hbpl/?Page=minnesota/default.html
- 18.Hughes JR. Effects of abstinence from tobacco: Valid symptoms and time course. Nicotine Tob Res. 2007;9:315–327. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- 19.Hughes J. Hatsukami DK. Errors in using tobacco withdrawal scale. Tob Control. 1998;7:92–93. doi: 10.1136/tc.7.1.92a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hughes JR. Keely JP. Niaura RS, et al. Measures of abstinence in clinical trials: Issues and recommendations. Nicotine Tob Res. 2003;5:13–25. [PubMed] [Google Scholar]
- 21.Ratain MJ. Sargent DJ. Optimising the design of phase II oncology trials: The importance of randomisation. Eur J Cancer. 2009;45:275–280. doi: 10.1016/j.ejca.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 22.Rubinstein LV. Korn EL. Freidlin B, et al. Design issues of randomized phase II trials and a proposal for phase II screening trials. J Clin Oncol. 2005;23:7199–7206. doi: 10.1200/JCO.2005.01.149. [DOI] [PubMed] [Google Scholar]
- 23.Hays JT. Ebbert JO. Bupropion sustained release for treatment of tobacco dependence. Mayo Clin Proc. 2003;78:1020–1024. doi: 10.4065/78.8.1020. quiz 1024. [DOI] [PubMed] [Google Scholar]
- 24.Moher D. Hopewell S. Schulz KF, et al. CONSORT 2010 Explanation and Elaboration: Updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol. 2010;63:e1–37. doi: 10.1016/j.jclinepi.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Papakostas GI. Alpert JE. Fava M. S-adenosyl-methionine in depression: A comprehensive review of the literature. Curr Psychiatry Rep. 2003;5:460–466. doi: 10.1007/s11920-003-0085-2. [DOI] [PubMed] [Google Scholar]
- 26.Jorenby DE. Hays JT. Rigotti NA, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: A randomized controlled trial. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- 27.Gonzales D. Rennard SI. Nides M, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: A randomized controlled trial. JAMA. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]