Abstract
Background
Tumor-associated macrophages (TAMs) have recently been recognized as being important players in the tumoriogenesis of many cancers, including advanced thyroid cancer. However, a role in papillary thyroid carcinoma (PTC), the most prevalent thyroid cancer, has not been established. We hypothesized that TAMs also facilitate tumor progression in PTC.
Methods
We investigated TAMs density in both benign thyroid lesions and PTC tumors by CD68 immunostaining. CD68-positive cell density was further associated with the clinicopathological characteristics of PTC patients. Finally, TAMs were isolated from PTC tumors and phenotyped by cytokine and receptor profiling.
Results
The overall density of TAMs was found to be significantly higher in PTC tumors, compared with thyroid goiter and follicular adenoma. The density of TAMs was positively associated with lymph node metastasis in TNM (tumor–node–metastasis) stages III/VI compared with stages I/II. No association was observed in other common tumor features, including the BRAF mutation. The isolated TAMs presented with high levels of M2-associated cytokine and receptors, making M2 the predominant TAM phenotype.
Conclusions
TAMs may play a functional role in the progression of PTC.
Introduction
The increased incidence of thyroid cancer has made it one of the most common newly diagnosed cancers. PTC, which makes up 80%–90% of cases thyroid cancer, accounts for almost all of this increase, in part due to improved diagnostic procedures (1,2). Although PTC is an indolent disease with a favorable prognosis, significant PTC-related mortality occurs in cases of recurrent and metastatic disease, where surgery and 131I ablation are not treatment options (3). The risk factors for progressive disease include older age at the time of diagnosis, male gender, large tumor size, extrathyroidal invasion, lymph node metastases, and distant metastases (4–6). The presence of neck lymph node metastases, and tumor extension beyond the thyroid capsule, have been identified as independent risk factors for recurrence (7). The most investigated genetic alteration in PTC is the BRAF V600E mutation. This somatic BRAF mutation has been associated with disease extent, such as extrathyroidal extension and lymph node metastases. Combining tests for the presence of a BRAF mutation with conventional clinicopathological risk factors has been suggested as a way of more efficiently evaluating the patient's prognosis (8). However, a poor outcome has also been reported in the absence of a BRAF mutation; so, it is important to identify all prognostic indicators of PTC progression.
The tumor microenvironment has now been recognized as playing a key role in tumor progression and metastases. One of the major components of tumor stroma are tumor-associated macrophages (TAMs). The release of cytokines and growth factors from TAMs is thought to stimulate angiogenesis, enhance tumor cell migration and invasion, and suppress antitumor immunity (9). In thyroid cancer patients, studies show that the density of TAMs is increased if the tumor is advanced and the presence of regions of a high density of TAMs positively correlates with tumor invasion and decreased cancer-related survival (10). Notably, an interconnected cellular network was described in anaplastic thyroid carcinoma (11). However, TAMs have not been well studied in PTC. The aim of this study was to investigate the density and phenotype of TAMs in PTC, and to determine whether there are clinicopathological correlations with features such as tumor invasion and metastatic characteristics.
Methods
Patients and tissue collection
The board of medical ethics of Ruijin Hospital approved the study, and all patients provided their informed written consent. Patient tissue was collected by experienced pathologists, formalin fixed, and paraffin embedded to confirm the histological diagnosis. In addition, the PTC tumor and adjacent normal thyroid tissue were snap frozen and stored in liquid nitrogen until use. In total, we collected 103 cases of PTC, 19 cases of thyroid adenoma, and 16 cases of nodular goiter.
Immunohistochemistry
Paraffin-embedded tissue blocks of 4 μm were sectioned and heated at 60°C for 3 hours. Slides were deparaffinized and rehydrated. The sections were immersed in sodium citrate buffer adjusted to pH 6.0 and heated in a microwave oven for 5 minutes at 100°C to facilitate antigen retrieval. Treatment with 0.3% H2O2 in methanol for 30 minutes at room temperature was performed to inhibit the activity of peroxidases. To reduce non-specific background staining, the slides were incubated with 2% goat serum for 10 minutes at room temperature. The slides were then incubated with mouse monoclonal antibody against human CD68 (1:200; Dako, Glostrup, Denmark), CD163 (1:100;Vector Laboratories, Burlingame, CA) in a moist chamber overnight at 4°C. The primary antibody binding was demonstrated with the ABC staining system (Santa Cruz Biotechnology, Santa Cruz, CA). Peroxidase activity was detected with diaminobenzidine as the substrate. Finally, sections were weakly counterstained with Harris's hematoxylin and mounted. Negative controls were incubated without the primary antibody.
A single pathologist (Q.W.), who was blinded to the clinical assessments of each case, scored the cases by counting the number of CD68C TAMs in six independent fields under a 400× magnification. CD68+ cell counts were expressed as the mean±standard deviation.
Somatic mutation analysis
DNA was extracted from the frozen tissue using the DNA purification kit (Omega; Biotek, Doraville, GA) and quantitated by the NanoDrop 2000 spectrophotometer (NanoDrop Technologies, Wilmington, DE). To determine BRAF V600E (T1799A) mutation status, the exon was selectively amplified by the polymerase chain reaction (PCR) (primers forward 5′-ACCTAAACTCTTCATAATGCTTGCT-3′; reverse 5′-TGATTTTTGTGAATACTGGGAACT-3′). PCR reactions were performed in 25 μL of buffer containing1.5 mM MgCl2, 200 mM deoxynucleoside triphosphates, 50–100 ng genomic DNA, 0.5 mM each primer, and 2.5U Taq DNA polymerase (Takara, Tokyo, Japan). Thirty-five cycles with annealing temperatures were optimized at 60°C. The PCR product was purified and Sanger sequenced using an ABI PRISM 3730 DNA Analyzer (Applied Biosystems, Foster City, CA).
Isolation of TAMs
TAMs were isolated from solid tumors as previously described (12–14). Briefly, tumor tissue was minced, followed by type 1 collagenase digestion (3 mg/mL; Gibco Invitrogen Corporation, Grand Island, NY) for 2 hours at 37°C. The suspension was filtered through a 70 μm stainless steel wire mesh to generate a single-cell suspension. This suspension was centrifuged, washed twice with phosphate-buffered saline (PBS), and the cells were allowed to adhere to a 3.5 cm culture dish in serum-free RPMI 1640 for 40 minutes. Nonadherent cells were removed by washing. Ninety-five percent of the remaining adherent cells were TAMs as assessed by morphology and CD68 staining.
Blood monocyte preparation
Peripheral blood mononuclear cells (PBMCs) were isolated from fresh blood by Ficoll–Hypaque density gradient centrifugation (density, 1.077±0.001 g/mL; Axis-Shield, Oslo, Norway) at 450 g for 30 minutes at room temperature. The mononuclear cells were washed thrice with PBS and cultured in serum-free RPMI 1640 for 40 minutes before RNA isolation.
Immunofluorescence
TAMs were adhered to four-well Culture Slides (BD Biosciences, Heidelberg, Germany), fixed in 4% paraformaldehyde at room temperature for 20 minutes, washed with PBS twice, incubated with 1% bovine serum albumin at 37°C for 30 minutes to block nonspecific interactions, and then stained with the CD68 antibody (1:100; Dako) at 4°C overnight. After several washes with PBS, the cells were incubated with the appropriate rhodamine-labeled goat anti-mouse secondary antibody purchased from Invitrogen at room temperature for 2 hours. Nuclei were then stained with 4′6-diamidino-2-phenylindole. Images were taken at a 400×magnification on an Olympus-BX51 microscope. Immunofluorescence was repeated in three randomly selected patients.
Real-time PCR
Total RNA was isolated from the TAMs of six PTCs and PBMC from six normal controls using TRIzol (Invitrogen, Carlsbad, CA) as described by the manufacturer's protocol. cDNA was prepared from total RNA (1 μg) using AMV reverse transcriptase (Promega, Madison, WI). Real-time PCR (RT-PCR) was performed by an SYBRgreen assay, which used an Applied Biosystems 7300 Real-Time PCR System (Applied Biosystems). The primers for quantitative RT-PCR were shown in Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/thy). The amplification protocol consisted of an initial denaturation step for 10 minutes at 95°C, followed by 40 cycles of PCR at 95°C for 15 seconds, and at 60°C for 60 seconds. Each sample was assayed in triplicate. The comparative cycle threshold (CT) method (ΔΔCT method) was used to determine the quantity of the target sequences in TAMs relative both to PBMC and to β-actin (an endogenous control). Relative expression levels were presented as the relative fold change and calculated using the following formula: 2−ΔΔCT=2−(ΔCT(TAM)−ΔCT(PBMC)), where each ΔCT=ΔCT(target)−ΔCT (β-actin).
Statistical analysis
Statistical analysis software (Prism 5.0; GraphPad Software Inc, La Jolla, CA and SPSS Version 13.0 software; SPSS, Inc., Chicago, IL) was used to perform the analyses. Data are expressed as mean±standard deviation. Differences between different thyroid lesions were determined using the one-way analysis of variance and the Student–Newman–Keuls test. The Mann–Whitney test was used to compare the expression levels of each gene by RT-PCR between TAMs and PBMC. The correlation between average CD68 counts and clinicopathologic factors was analyzed by the independent-samples T test. A p-value<0.05 was accepted as a significant difference.
Results
Increased TAMs density in PTC compared with benign thyroid disease
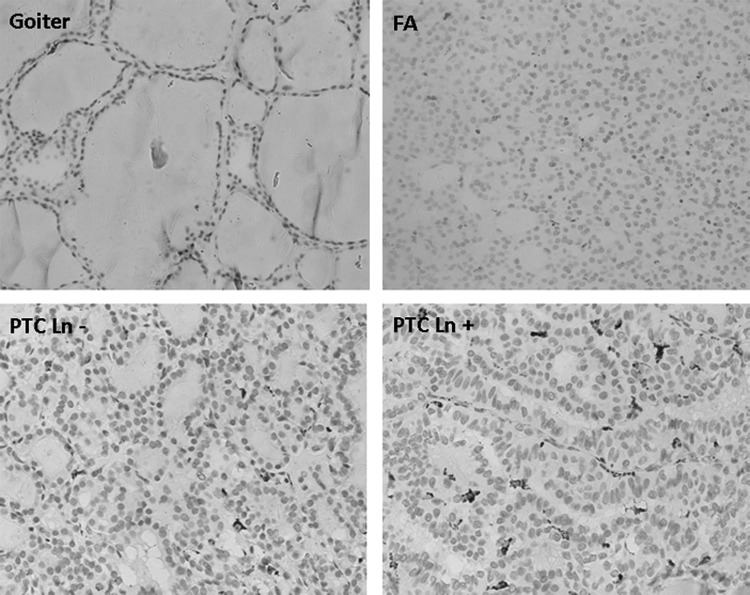
To identify and quantify the amounts of TAMs in thyroid lesions, we examined macrophage-CD68 antigen expression by immunostaining. CD68 showed dense cytoplasmic staining of mononuclear or multinucleated giant-type cells that morphologically resembled macrophages, as shown in Figure 1. In PTC, we found that positively labeled cells were mainly distributed in the lumen of the follicles or interspersed between the tumor cells, but unevenly distributed. To avoid evaluation bias, we counted the CD68-positive cells in six independent high power fields for each single slide. The average density was 26±19 in PTC tumors. However, for benign thyroid lesions, we found a significantly lower density of CD68-positive cells (2±1 for goiter and 7±4 for follicular adenoma, p<0.001 when compared with PTC, Table 1).
FIG. 1.
Immunostaining of CD68 in different thyroid lesions. Papillary thyroid carcinoma (PTC) showed a significantly higher density of tumor associated macrophage (TAM) compared with benign thyroid lesion (goiter and follicular adenoma [FA]). PTCs with lymph node metastasis are associated with a higher TAM density.
Table 1.
Increased Tumor-Associated Macrophage Density in Papillary Thyroid Carcinoma
| Tissue type | Number of cases | Mean number of TAMs | p-Value |
|---|---|---|---|
| Goiter | 16 | 16±2 | 0.00a |
| FA | 19 | 7±4 | 0.00a |
| PTC | 103 | 26±18 |
Compared with PTC.
TAM, tumor-associated macrophages; FA, follicular adenoma; PTC, papillary thyroid carcinoma.
Correlation of TAMs density with clinicopathological characters in patients with PTC
Histological-grade and the BRAF V600E mutation are important determinants of tumor behavior and the prognosis of patients with PTC. We, therefore, investigated the correlations between the density of TAMs and clinicopathological characteristics and the tumor genotype. As shown in Table 2, the density of TAMs density was most significantly associated with lymph node metastasis (13±6 vs. 40±17, lymph node negative vs. lymph node positive, p<0.001) and TNM (tumor–node–metastasis) stage (22±16 vs. 37±20, stage I–II vs. stage III–IV, p=0.001). We did not observe a significant association between TAMs density and other tumor features, such as age, gender, size, Hashimoto background, or multifocal disease. The BRAF mutation was detected in 70% of the PTC tumors in our cohort. We did not find a significant association between BRAF mutations and clinicopathological features such as lymph node metastasis. The density of TAMs (see Methods) was 28±20 in PTC with the BRAF V600E mutation and 22±17 in PTC with the negative BRAF V600E mutation (p=0.122).
Table 2.
Association Study of Tumor-Associated Macrophage Density with Clinicopathogenic Characteristics in Papillary Thyroid Carcinoma
| Number of patients (%) | CD68+ counta | p-Value | |
|---|---|---|---|
| Age (years) | |||
| <45 | 48 (46.6) | 27.73±18.30 | |
| ≥45 | 55 (53.4) | 24.69±19.50 | 0.28 |
| Gender | |||
| Male | 38 (36.9) | 23.89±19.09 | |
| Female | 65 (63.1) | 27.40±18.85 | 0.211 |
| Microcarcinoma | |||
| Yes | 28 (27.2) | 22.03±12.43 | 0.594 |
| No | 75 (72.8) | 27.63±20.70 | |
| Hashimoto's thyroiditis | |||
| Yes | 27 (26.2) | 34.47±24.00 | 0.076 |
| No | 76 (73.8) | 23.13±15.91 | |
| Multifocal | |||
| Yes | 27 (26.2) | 32.89±24.05 | 0.331 |
| No | 76 (73.8) | 23.70±16.25 | |
| LN metastasis | |||
| Yes | 48 (46.6) | 40.99±17.63 | <0.000 |
| No | 55 (53.4) | 13.16±6.07 | |
| Stage | |||
| I and II | 76 (73.8) | 22.22±16.94 | 0.001 |
| III and IV | 27 (26.2) | 37.04±20.22 | |
| FVPTC | |||
| Yes | 8 (7.8) | 21.38±7.22 | |
| No | 95 (92.2) | 26.51±19.56 | 0.885 |
| BRAF mutation | |||
| Yes | 56 (63.6) | 22.63±17.00 | |
| No | 32 (36.4) | 28.96±20.43 | 0.690 |
See Methods.
LN, lymph node; FVPTC, follicular variant of PTC.
Macrophage phenotype determination of TAMs isolated from PTC
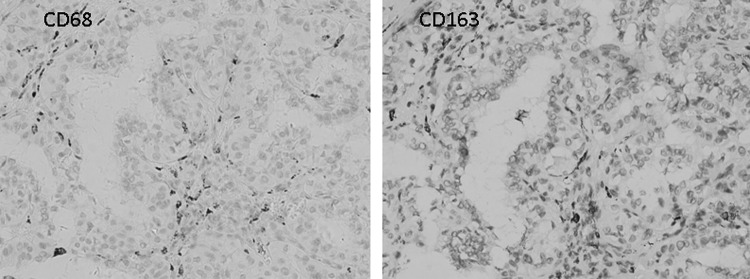
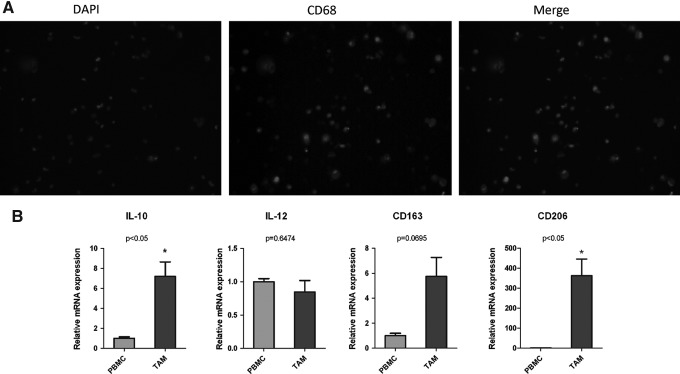
It has been shown that TAMs typically presents as a M2 phenotype in cancer. We first determined the phenotype of TAMs in PTC by a comparison of CD163 and CD68 immunostaining. We observed a similar pattern of positive CD68 and CD163 staining in adjacent PTC sections (Fig. 2). In isolated macrophages from six independent fresh PTC tumors, CD68 immunofluorescent staining was positive in the cytoplasma of most of the attached cells (Fig. 3A). Furthermore, we profiled macrophage-associated cytokines and receptors in the isolated TAMs by RT-PCR. Significantly, we found a significant increase of interleukin (IL)-10 and CD206 expression level in PTC-associated TAMs compared with peripheral blood monocytes (Fig. 3B).
FIG. 2.
Representative CD68 and CD163 staining in adjacent sections of PTC.
FIG. 3.
(A) Immunofluorescence assay of CD168 in TAM isolated from PTC tumors. (B) The ratio of a panel of macrophage-related cytokine and receptor mRNA in TAMs from PTC (n=6) versus peripheral blood mononuclear cells (PBMCs) from the normal control (n=6). *p<0.05.
Discussion
In this study, we observed a significant infiltration of TAMs in PTC compared with non-cancerous thyroid disease. The evaluation of surface marker and cytokine profiling suggests a M2 phenotype. The density of TAM was associated with lymph node metastasis and TNM stage. These findings suggest a functional role of TAMs, facilitating the progression of PTC.
The association of TAMs and cancer has been well studied in a variety of cancers. The majority of clinical studies make a strong case that TAMs promote tumorigenesis in cancer, including lung, lymphoma, and thyroid (15,16). However, exceptions exist; high macrophage densities are correlated with an increased survival in pancreatic cancer (17). Both positive and negative associations have been reported for thyroid cancer. Increased TAMs in high-grade thyroid cancers were associated with invasive cancers and decreased cancer-related survival (10). However, due to the overall benign prognosis of the well-differentiated thyroid cancer (WDTC), the role in PTC was not directly examined. A negative correlation of neoplastic cell phagocytosis with vascular invasion and distance metastasis has been reported in WDTC (18). However, this study mainly focused on macrophage phagocytosis, the anti-tumor aspects of the macrophage. In our study, we asked whether there was an association between TAM density and prognostic factors for PTC. Our findings suggest that TAMs may facilitate tumor progression in PTC patients.
Plasticity and functional polarization are hallmarks of the mononuclear phagocyte system. It was reported that macrophages in tumors were biased away from the activated (M1) type to the immune-regulatory type (M2) (19, 20). In humans, CD68 is a major marker for macrophages, while CD163 is a marker for the M2 phenotype (21). We used CD68 staining to evaluate the density of TAMs in PTC and found overlap staining for both CD68 and CD163, indicating an M2 phenotype of the TAMs in PTC. In addition, M1 showed a high level of IL-12 production, while M2-polarized macrophages are characterized by a predominated high level of IL-10 production (22). We isolated TAMs from PTC tumors and evaluated the expression level of IL-10, IL-12, and receptor hallmarks. As expected, we found a high-level expression of IL-10, CD163, and CD206. Considering these data together provides strong evidence for an M2 phenotype of PTC-related TAMs.
It is clear that macrophages are a critical component of a tumor microenvironment, with implicated roles in tumor initiation, progression, and malignance (9,23,24). TAMs facilitate tumor growth, angiogenesis, invasion, and immune regulation by producing growth factors, cytokines, and chemokines (25,26). Not only did we find TAMs to be associated with malignant thyroid lesions, but also the density of TAMs was significantly and positively associated with lymph node metastasis. When these findings are considered along with the previously reported observation that increased TAMs contribute to the decreased survival of advanced thyroid cancer (10), the results suggest that TAMs function in the progression stages of thyroid cancer, including PTC. The specific molecular mechanisms by which TAM favors the thyroid cancer cell still require further investigation.
Macrophage recruitment and differentiation are regulated by growth factors such as colony stimulating factor-1 (CSF-1), granulocyte-macrophage-CSF, IL-3, and chemokines such as CCL-2 (27). PTC-related genetic alterations, including RET rearrangement, BRAF and RAS mutations, have been shown to induce a transcription program that is characterized by the up-regulation of CCL-2, which could possibly recruit macrophages to the tumor site (28). We, therefore, investigated the correlation between the BRAF mutation and the TAM density in our study. Interestingly, we did not find a correlation between the BRAF mutation and the TAMs density, indicating that other molecular mechanisms should play a role in the observed effects or associations of TAMs.
To our knowledge, this is the first study that investigates the density of TAMs and phenotypes in PTC. We found a remarkable high density of TAMs in PTC compared with benign thyroid lesions; furthermore, the density of TAMs was positively associated with lymph node metastasis and the TNM stage. The limitation of our study is the lack of stage III and IV patients to evaluate advanced PTC, and also lack of follow-up data which evaluate long-term outcome. However, our findings suggest a functional role of TAMs in the progression of PTC.
Supplementary Material
Acknowledgments
This study was supported by the grants from the Chinese National Natural Science Foundation (30900701), the Shanghai Rising-Star Program (10QA1404700), the Key Project of Science and Technology of Shanghai (10411951100), and the Innovation Program of Shanghai Municipal Education Commission (11YZ45).
Disclosure Statement
The authors declare that no competing financial interests exist.
References
- 1.Davies L. Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 2.Elisei R. Molinaro E. Agate L. Bottici V. Masserini L. Ceccarelli C. Lippi F. Grasso L. Basolo F. Bevilacqua G. Miccoli P. Di Coscio G. Vitti P. Pacini F. Pinchera A. Are the clinical and pathological features of differentiated thyroid carcinoma really changed over the last 35 years? Study on 4187 patients from a single Italian institution to answer this question. J Clin Endocrinol Metab. 2010;95:1516–1527. doi: 10.1210/jc.2009-1536. [DOI] [PubMed] [Google Scholar]
- 3.Mazzaferri EL. Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97:418–428. doi: 10.1016/0002-9343(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 4.DeGroot LJ. Kaplan EL. McCormick M. Straus FH. Natural history, treatment, and course of papillary thyroid carcinoma. J Clin Endocrinol Metab. 1990;71:414–424. doi: 10.1210/jcem-71-2-414. [DOI] [PubMed] [Google Scholar]
- 5.Mazzaferri EL. Kloos RT. Clinical review 128: current approaches to primary therapy for papillary and follicular thyroid cancer. J Clin Endocrinol Metab. 2001;86:1447–1463. doi: 10.1210/jcem.86.4.7407. [DOI] [PubMed] [Google Scholar]
- 6.Sherman SI. Brierley JD. Sperling M. Ain KB. Bigos ST. Cooper DS. Haugen BR. Ho M. Klein I. Ladenson PW. Robbins J. Ross DS. Specker B. Taylor T. Maxon HR., III Prospective multicenter study of thyroid carcinoma treatment: initial analysis of staging and outcome. National Thyroid Cancer Treatment Cooperative Study Registry Group. Cancer. 1998;83:1012–1021. doi: 10.1002/(sici)1097-0142(19980901)83:5<1012::aid-cncr28>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 7.Leboulleux S. Rubino C. Baudin E. Caillou B. Hartl DM. Bidart JM. Travagli JP. Schlumberger M. Prognostic factors for persistent or recurrent disease of papillary thyroid carcinoma with neck lymph node metastases and/or tumor extension beyond the thyroid capsule at initial diagnosis. J Clin Endocrinol Metab. 2005;90:5723–5729. doi: 10.1210/jc.2005-0285. [DOI] [PubMed] [Google Scholar]
- 8.Xing M. Prognostic utility of BRAF mutation in papillary thyroid cancer. Mol Cell Endocrinol. 2010;321:86–93. doi: 10.1016/j.mce.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qian BZ. Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryder M. Ghossein RA. Ricarte-Filho JC. Knauf JA. Fagin JA. Increased density of tumor-associated macrophages is associated with decreased survival in advanced thyroid cancer. Endocr Relat Cancer. 2008;15:1069–1074. doi: 10.1677/ERC-08-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caillou B. Talbot M. Weyemi U. Pioche-Durieu C. Al Ghuzlan A. Bidart JM. Chouaib S. Schlumberger M. Dupuy C. Tumor-associated macrophages (TAMs) form an interconnected cellular supportive network in anaplastic thyroid carcinoma. PLoS One. 2011;6:e22567. doi: 10.1371/journal.pone.0022567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solinas G. Schiarea S. Liguori M. Fabbri M. Pesce S. Zammataro L. Pasqualini F. Nebuloni M. Chiabrando C. Mantovani A. Allavena P. Tumor-conditioned macrophages secrete migration-stimulating factor: a new marker for M2-polarization, influencing tumor cell motility. J Immunol. 2010;185:642–652. doi: 10.4049/jimmunol.1000413. [DOI] [PubMed] [Google Scholar]
- 13.Sierra JR. Corso S. Caione L. Cepero V. Conrotto P. Cignetti A. Piacibello W. Kumanogoh A. Kikutani H. Comoglio PM. Tamagnone L. Giordano S. Tumor angiogenesis and progression are enhanced by Sema4D produced by tumor-associated macrophages. J Exp Med. 2008;205:1673–1685. doi: 10.1084/jem.20072602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duff MD. Mestre J. Maddali S. Yan ZP. Stapleton P. Daly JM. Analysis of gene expression in the tumor-associated macrophage. J Surg Res. 2007;142:119–128. doi: 10.1016/j.jss.2006.12.542. [DOI] [PubMed] [Google Scholar]
- 15.Chen JJ. Lin YC. Yao PL. Yuan A. Chen HY. Shun CT. Tsai MF. Chen CH. Yang PC. Tumor-associated macrophages: the double-edged sword in cancer progression. J Clin Oncol. 2005;23:953–964. doi: 10.1200/JCO.2005.12.172. [DOI] [PubMed] [Google Scholar]
- 16.Zhu XD. Zhang JB. Zhuang PY. Zhu HG. Zhang W. Xiong YQ. Wu WZ. Wang L. Tang ZY. Sun HC. High expression of macrophage colony-stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. J Clin Oncol. 2008;26:2707–2716. doi: 10.1200/JCO.2007.15.6521. [DOI] [PubMed] [Google Scholar]
- 17.Kim DW. Min HS. Lee KH. Kim YJ. Oh DY. Jeon YK. Lee SH. Im SA. Chung DH. Kim YT. Kim TY. Bang YJ. Sung SW. Kim JH. Heo DS. High tumour islet macrophage infiltration correlates with improved patient survival but not with EGFR mutations, gene copy number or protein expression in resected non-small cell lung cancer. Br J Cancer. 2008;98:1118–1124. doi: 10.1038/sj.bjc.6604256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fiumara A. Belfiore A. Russo G. Salomone E. Santonocito GM. Ippolito O. Vigneri R. Gangemi P. In situ evidence of neoplastic cell phagocytosis by macrophages in papillary thyroid cancer. J Clin Endocrinol Metab. 1997;82:1615–1620. doi: 10.1210/jcem.82.5.3909. [DOI] [PubMed] [Google Scholar]
- 19.Mantovani A. Sozzani S. Locati M. Allavena P. Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 20.Mantovani A. Sica A. Sozzani S. Allavena P. Vecchi A. Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 21.Buechler C. Ritter M. Orsó E. Langmann T. Klucken J. Schmitz G. Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. J Leukoc Biol. 2000;67:97–103. [PubMed] [Google Scholar]
- 22.Sica A. Saccani A. Bottazzi B. Polentarutti N. Vecchi A. van Damme J. Mantovani A. Autocrine production of IL-10 mediates defective IL-12 production and NF-kappa B activation in tumor-associated macrophages. J Immunol. 2000;164:762–767. doi: 10.4049/jimmunol.164.2.762. [DOI] [PubMed] [Google Scholar]
- 23.Mantovani A. Schioppa T. Porta C. Allavena P. Sica A. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev. 2006;25:315–322. doi: 10.1007/s10555-006-9001-7. [DOI] [PubMed] [Google Scholar]
- 24.Mukhtar RA. Moore AP. Nseyo O. Baehner FL. Au A. Moore DH. Twomey P. Campbell MJ. Esserman LJ. Elevated PCNA+ tumor-associated macrophages in breast cancer are associated with early recurrence and non-Caucasian ethnicity. Breast Cancer Res Treat. 2011;130:635–644. doi: 10.1007/s10549-011-1646-4. [DOI] [PubMed] [Google Scholar]
- 25.Lewis CE. Leek R. Harris A. McGee JO. Cytokine regulation of angiogenesis in breast cancer: the role of tumor-associated macrophages. J Leukoc Biol. 1995;57:747–751. doi: 10.1002/jlb.57.5.747. [DOI] [PubMed] [Google Scholar]
- 26.Biswas SK. Gangi L. Paul S. Schioppa T. Saccani A. Sironi M. Bottazzi B. Doni A. Vincenzo B. Pasqualini F. Vago L. Nebuloni M. Mantovani A. Sica A. A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-kappaB and enhanced IRF-3/STAT1 activation) Blood. 2006;107:2112–2122. doi: 10.1182/blood-2005-01-0428. [DOI] [PubMed] [Google Scholar]
- 27.Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol. 2009;9:259–270. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melillo RM. Castellone MD. Guarino V. De Falco V. Cirafici AM. Salvatore G. Caiazzo F. Basolo F. Giannini R. Kruhoffer M. Orntoft T. Fusco A. Santoro M. The RET/PTC-RAS-BRAF linear signaling cascade mediates the motile and mitogenic phenotype of thyroid cancer cells. J Clin Invest. 2005;115:1068–1081. doi: 10.1172/JCI22758. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.