Abstract
Background
There is a concern regarding the use of iodinated contrast agents (ICA) for chest and neck computed tomography (CT) to localize metastatases in patients with differentiated thyroid cancer (DTC). This is because the iodine in ICA can compete with 131I and interfere with subsequent whole scans or radioactive iodine treatment. The required period for patients to eliminate the excess iodine is not clear. Therefore, knowing the period for iodine levels to return to baseline after the injection of ICA would permit a more reliable indication of CT for DTC patients. The most widely used marker to assess the plasmatic iodine pool is the urinary iodine (UI) concentration, which can be collected over a period of 24 hours (24U) or as a single-spot urinary sample (sU). As 24U collections are more difficult to perform, sU samples are preferable. It has not been established, however, if the measurement of iodine in sU is accurate for situations of excess iodine.
Methods
We evaluated 25 patients with DTC who received ICA to perform chest or neck CT. They collected 24U and sU urinary samples before the CT scan and 1 week and 1, 2, and 3 months after the test. UI was quantified by a semiautomated colorimetric method.
Results
Baseline median UI levels were 21.8 μg/dL for 24U and 26 μg/dL for sU. One week after ICA, UI median levels were very high for all patients, 800 μg/dL. One month after ICA, however, UI median levels returned to baseline in all patients, 19.0 μg/dL for 24U and 20 μg/dL for sU. Although the values of median UI obtained from sU and 24U samples were signicantly different, we observed a significant correlation between samples collected in 24U and sU in all evaluated periods.
Conclusion
One month is required for UI to return to its baseline value after the use of ICA and for patients (after total thyroidectomy and radioiodine therapy) to eliminate the excess of iodine. In addition, sU samples, although not statistically similar to 24U values, can be used as a good marker to evaluate patients suspected of contamination with iodine.
Introduction
Thyroid carcinoma is the most common malignancy of the endocrine system and differentiated thyroid cancer (DTC), which includes both papillary and follicular cancer, comprises the vast majority (90%) of all thyroid cancers (1,2). Although the prognosis of DTC is frequently quite favorable, sometimes the disease can behave more aggressively, and loco-regional recurrences or distant metastases are present in 20% of cases (3). Radioactive iodine can be used for diagnostic tests or therapeutic purposes in DTC patients. 131I uptake in remnant thyroid tissue and focal metastases is related to a number of factors, which include thyroid-stimulating hormone (TSH) elevation through the withdrawal of levothyroxine or the use of recombinant TSH and depletion of inorganic plasma iodine before 131I administration that, theoretically, could increase the expression of the Na+/I− symporter (4).
DTC, particularly papillary thyroid carcinoma (PTC), involves cervical lymph nodes in 20%–50% of patients, which may be present even when the primary tumor is small and intrathyroidal (5,6). For a cervical lymph node investigation, the combination of ultrasound (US) and computed tomography (CT) may be superior to US alone (7). The lung is another common site of DTC metastases, and the rate of lung metastases ranges from 2% to 20% (8). These metastases usually appear as micronodules (<1 cm), which are undetectable in 50% of chest X-ray examinations (9). Therefore, CT can also be used for patients with high-risk DTC or when lung metastases are suspected.
Most of the CT protocols that are used in daily radiological routines include contrast-enhanced images based on the administration of oral or intravenous iodinated contrast agents (ICA) to improve the delineation of anatomic structures (2,8). Furthermore, ICA can have additional value when characterizing 18F-fluorodeoxyglucose (FDG) positron-emission tomography (PET)–negative tumors (10). However, there is a concern regarding the use of ICA in patients with DTC because the enormous amount of iodine in these agents can compete with 131I and interfere with subsequent diagnostic tests, such as whole-body scans (WBS), and treatments with radioactive iodine (11,12). The available data suggest that ICA increase the total body iodine stores for at least 3 months following contrast exposure and, in some situations, for as long as 2 years (13). However, these data concern patients with an intact thyroid gland, a situation where the iodine stored in the thyroid might adversely influence the radioiodine uptake. None of these data evaluated patients after thyroidectomy, where the elimination of iodine should be different, due to the absence of the thyroid gland.
To assess the plasma iodine pool, the most widely used marker is the urinary iodine (UI) concentration (14–17). Furthermore, Follis et al. (18,19) showed that the percentage of 131I captured by the thyroid gland is inversely proportional to UI levels. Hence, a finding of low UI indicates increased uptake of radioiodine by the thyroid.
Comparative population studies evaluating areas with iodine deficiency have revealed a strong correlation between the values obtained by randomly collecting urine samples (sU) versus collecting urine samples over 24 hours (24U) (14,16,17,20). Whether or not the UI collection should be done using 24U or sU in a situation of iodine excess, as after ICA administration, remains to be determined. Therefore, this study was designed to evaluate the period required for UI levels to return to baseline values and to compare UI samples collected by 24U or sU in the follow-up of DTC patients (treated with total thyroidectomy and radioiodine) who have employed CT using ICA for evaluation of metastases.
Methods
Subjects and procedures
We evaluated 25 patients between 17 and 63 years of age who were followed by a single team of physicians at the associated Thyroid Diseases Centers at the Division of Endocrinology, Department of Medicine, Escola Paulista de Medicina, Universidade Federal de São Paulo, and the Instituto Israelita de Ensino e Pesquisa Albert Einstein (both in São Paulo, Brazil) (Table 1). All of the diagnostic procedures were performed in accordance with the regulations of the local Ethics Committee. Written informed consent was obtained from each patient.
Table 1.
Characteristics of Patients Included in the Study
| Gender | Age | Variant | TNM staging | Presentation of metastatic lesions | Imaging tests performed |
|---|---|---|---|---|---|
| M | 56 | Diffuse sclerosis | T3N1M0 | Mediastinum | Cervical/chest CT |
| M | 20 | Follicular | T3mN1bM1 | Lung | Chest CT |
| F | 51 | Follicular | T3N1M0 | Mediastinal lymphonode | Cervical CT |
| F | 35 | Follicular | T1N1M1 | Mediastinal lymphonode | Cervical CT |
| F | 34 | Classic | T4bN1bM0 | Mediastinal lymphonode | Cervical CT |
| F | 37 | Trabecular | T2mN1M0 | Cervical lymphonode | Cervical CT |
| F | 17 | Classic | T3mN1M0 | Cervical lymphonode | Cervical CT |
| F | 32 | Classic | T3mN1Mx | Cervical lymphonode | Cervical CT |
| F | 57 | Classic | T2N1bM1 | Lung | Chest CT |
| F | 26 | Follicular | T1mN1bM0 | Cervical lymphonode | Cervical CT |
| F | 28 | Follicular | T3N1M0 | Lung | Chest CT |
| F | 42 | Classic | T1mN1M1 | Cervical lymphonode | Cervical CT |
| M | 50 | Follicular | T2N1M0 | Cervical lymphonode | Cervical CT |
| F | 25 | Classic | T2N1bM0 | Cervical lymphonode | Cervical CT |
| F | 22 | Follicular | T2mN1bM1 | Lung | Chest CT |
| F | 28 | Classic | T3N1M0 | Cervical lymphonode | Cervical CT |
| M | 47 | Classic | T3N1bM1 | Cervical/mediastinal lymphonode | Cervical/chest CT |
| F | 34 | Classic | T3N1M0 | Cervical lymphonode | Cervical CT |
| M | 25 | Classic | T4N1M1 | Lung | Cervical/chest CT |
| M | 38 | Classic | T1N1bMx | Cervical lymphonode | Cervical CT |
| F | 63 | Follicular | T2N1M1 | Lung | Chest CT |
| F | 57 | Classic | T2N1bM1 | Lung | Chest CT |
| F | 28 | Classic | T3N1M0 | Lung | Chest CT |
| F | 24 | Follicular | T3N1bM1 | Lung | Chest CT |
| F | 55 | Classic | T3N1bM1 | Lung and bone | Cervical/spine/chest CT |
Variant, papillary thyroid carcinoma variant on surgical pathology diagnosis; CT, computed tomography.
The selected patients had a history of PTC, which was treated with total thyroidectomy and radioiodine therapy, and they all were advised to receive chest and/or neck CT using ICA to investigate the possibility of metastases during their follow-up. The exclusion criteria were: renal failure, any patients who were on an iodine-rich medication, and the presence of elevated basal UI levels, in values ≥100 μg/dL, which was considered to be related to previous iodine contamination.
To perform the CT, all patients received the nonionic, water-soluble, iodinated, low-osmolarity contrast agent Henetix 300, which has an iodine concentration of 658 mg/mL iobitridol (equivalent to 300 mg iodine/mL contrast agent). The contrast dose received by the patients ranged from 50 to 100 mL (1 mL/kg), with the amount of iodine received by each patient ranging from 15,000 to 30,000 mg per test performed.
All of the selected patients were instructed to collect 24U and sU samples (between 8 and 11 am) before the CT scan and 1 week and 1, 2, and 3 months after receiving the iodine contrast agent.
UI was quantified by a semiautomated colorimetric method developed and validated in our laboratory in extensive surveys (17). The method follows recommendations from the International Council for Control of Iodine Deficiency Disorders (ICCIDD) and is based on the indirect iodine detection through the reduction of cerium (17,21,22). This method has a sensitivity of 1 μg/dL, and coefficient of variation of 12.6% for a sample of 8.9 μg/dL, 2.3% for a sample of 27.7 μg/dL, and 10.5% for a sample of 262 μg/dL.
Statistical analysis
We used nonparametric methods based on ranks. The correlation analysis was done by the Spearman rank correlation test and the group analysis by the Kruskal–Wallis statistic. All the data were described as median and by 95% confidence interval (23). All data were analyzed using the PRISM software (Version X). A p-value of 0.05 was considered significant.
Results
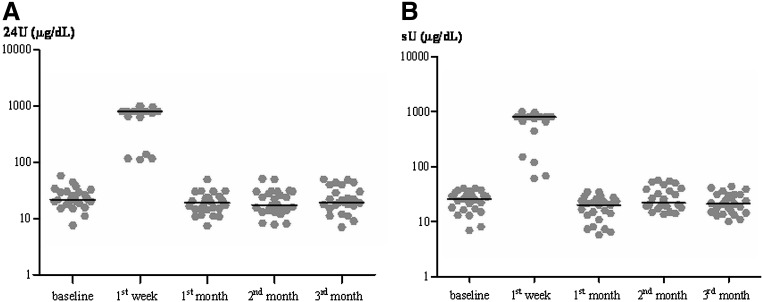
The results are shown in Figure 1 and Table 2. Figure 1 shows the individual UI results of all patients, demonstrating remarkable similarity in their return to baseline values one month or perhaps earlier after contrast agent administration. The median UI level at baseline was 21.8 μg/dL for the 24U samples and 26 μg/dL for sU samples. As expected, one week after the examination, the median UI level was very high, having a median of 800 μg/dL for both the 24U samples and sU samples and demonstrating a significant increase after the injection of the ICA (Fig. 1 and Table 2). In contrast, the UI levels were similar to baseline by 4 weeks and perhaps earlier after ICA administration, and the median values of the 24U and sU samples were 19.0 and 20 μg/dL, respectively (Table 2). There were no significant differences between the UI results found at baseline versus those obtained 1, 2, and 3 months after ICA administration. There was a significant difference between the UI results at the baseline and those found at the first week (p<0.01). The medians of the UI results from both samples (24U and sU) in the first month are similar to those found in the second and third months (Fig. 1 and Table 2).
FIG. 1.
(A) The results of urinary iodide in all 25 patients collected in 24 hours (24U) at the baseline, first week, and first, second, and third month after iodinated contrast agents (ICA) (in logarithmic scale). (B) The results of urinary iodide in all 25 patients collected in spot sample (sU) at the baseline, first week, and first, second, and third month after ICA (in logarithmic scale).
Table 2.
Median and Ranges of 24-Hour and Spot Urinary Iodide Samples After Use of Iodinated Contrast Agents
| Baseline | First week | First month | Second month | Third month | |
|---|---|---|---|---|---|
| 24U [median (range)] | 21.8 (7.6–58) | 800 (112–1000) | 19 (7.4–49.5) | 17.5 (7.8–51) | 19.2 (7.0–50) |
| sU [median (range)] | 26 (6.9–40.5) | 800 (61–1000) | 20 (5.7–35) | 21.7 (13.5–56.5) | 21 (10–44) |
| ρ† (p value) | 0.70 (p: 0.0005) | 0.50 (p: 0.01) | 0.73 (p: 0.0003) | 0.74 (p: 0.0003) | 0.42 (p: 0.03) |
24U and sU values are in μg/dL. ρ† (Spearman's rho) represents the correlation coefficient between 24U and sU results.
24U, 24-hour urinary iodide sample; sU, spot urinary iodide sample.
Although the UI values in the 24U and sU were in the same direction, showing significant correlation (ρ†) in all evaluated periods (Table 2), they were significantly different at all time points.
Discussion
The lymph nodes and lungs are common sites of DTC metastases and CT examinations of the neck and lung are important tests for investigating them (2,8,24,25). On some occasions, the use of an iodine contrast agent can be advantageous, and, according to the literature, there are some advantages that justify the use of these agents. They can improve the delineation of anatomic structures, increase the sensitivity for detecting pathological lesions, and improve the characterization of lesions (2,8). Furthermore, CT contrast agents can have additional value when characterizing 18F-FDG PET–negative tumors (10). For example, in cases of confirmed lung metastases and normal chest X-rays, CT tests can provide images of diffuse micronodules in 50%–70% of patients (24,25). Moreover, in patients with high thyroglobulin levels and a negative diagnostic WBS, CT is considered to be the most sensitive method for detecting lung micrometastases, being even superior to a PET scan (2).
The problem with using contrast agents in patients with DTC is that it exposes them to high levels of free iodine as the amount of iodine administered by a contrast CT is very high (0.01%–0.15% of the amount of organically bound iodine administered) (11,15,26). Although most of the iodine from the contrast agent is strongly bound to organic compounds and, therefore is not accessible for uptake by the thyroid, the resulting amount of free iodide is much higher than the quantity of iodine ingested in a standard or regular diet (11,15). For example, a 200-mL dose of a contrast medium containing 35 μg/mL iodine, provides 7000 μg of free iodide, which is equivalent to 45× the recommended daily intake (11).
Furthermore, after exposure to an iodine contrast agent, the plasma concentrations of free iodine remains elevated for a long period (26,27), since the body's iodine stores are expanded in the interstitial fluids, in the colloid within the thyroid, and in virtually every organ in the body (13). The available data in the literature suggest that, in a individual with an intact thyroid gland, contrast agents increase the body's total iodine stores for at least 3 months, and in some situations for as long as 2 years (13). Currently, however, water-soluble iodinated contrast materials are usually used for CT. These are quickly excreted by the kidneys in contrast to fat-soluble contrast material that was used previously to image the hepatobiliary tract; in addition, it is well known that agents excreted by the hepatobiliary route take a longer time to be excreted and therefore a longer time for the iodine stores of the body to be depleted (11). Furthermore, all these studies of iodine contrast agents were conducted in patients with an intact thyroid gland. In contrast, our patients were studied after total thyroidectomy and radioiodine therapy.
The mechanism by which ICA inhibits RI uptake has not been clearly demonstrated, but it is thought that the excess of free inorganic iodides have primary importance (26). Research conducted by Follis et al. showed that the percentage of 131I uptake by the thyroid gland is inversely proportional to the UI excretion (16,17), indicating that the UI level is one of the predictors of thyroid iodine uptake.
In our studies, we observed that the required period for the UI values to return to baseline after patients had been exposed to water-soluble ICA was no more than 4 weeks, and perhaps earlier. Certainly, in patients with a thyroid gland, the period could be longer because of the presence of colloid and more iodine retention due to the existence of more tissue uptake.
Considering that the UI is a good marker for evaluating the plasma pool of iodine (renal excretion accounts for more than 90% of the losses and is equivalent to the nutritional intake) (2,13,28), and that 24U collection is difficult and inconvenient for patients, some researchers have evaluated the value of sU samples for this analysis. However, most of these studies were performed in populations with iodine deficiency. Therefore, an important contribution of our study is the confirmation of a significant correlation between UI measurements using 24U and sU samples in situations where the iodine plasma pool is high and thus, contamination must be excluded. In addition, these data showed the possibility of evaluating patients suspected of iodine contamination (e.g., by medications containing iodine) through sU samples. In particular, it is valuable for thyroid cancer patients because it allows them to be assessed by sU collection before performing a WBS or radioiodine treatment. The advantage of sU collection is that it is simpler, and, therefore, the risk for errors during collection is smaller.
In conclusion, this study showed that 4 weeks and perhaps earlier is the required time for UI to return to its baseline value after the use of water soluable ICA and for patients to eliminate the excess iodine. In addition, we found a good correlation between the results of 24U and sU samples, indicating that spot urine samples can be used instead of 24U to evaluate patients suspected of contamination with iodine.
Acknowledgments
This work was supported by the São Paulo State Research Foundation (FAPESP) Grants 09/50573-1 (to R.M.B.M.) and 09/50574-8 (Research-Fellowship Grant to R.P.P.) and by a grant from the Brazilian Ministry of Health (25000.168513/2008-11). R.M.B.M. is an investigator of the Brazilian Research Council and of the Fleury Group; R.P.M.B. is an investigator of the Fleury Group.
Disclosure Statement
The authors have nothing to disclose.
References
- 1.Edwards BK. Howe HL. Ries LAG. Thun MJ. Rosenberg HM. Yancik R. Wingo PA. Jemal A. Feigal EG. Annual report to the nation on the status of cancer, 1973–1999, featuring implications of age and aging on US cancer burden. Cancer. 2002;94:2766–2792. doi: 10.1002/cncr.10593. [DOI] [PubMed] [Google Scholar]
- 2.Cooper DS. Doherty GM. Haugen BR. Kloos RT. Lee SL. Mandel SJ. Mazzaferri EL. McIver B. Sherman SI. Tuttle RM. Revised American Thyroid Association Management Guidelines for Patients with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 3.Rouxel A. Hejblum G. Bernirer MO. Boelle PY. Menegaux F. Mansour G. Hoang C. Aurengo A. Leenhardt L. Prognostic factors associated with the survival of patients developing loco-regional recurrences of differentiated thyroid carcinomas. J Clin Endocrinol Metab. 2004;89:5362–5368. doi: 10.1210/jc.2003-032004. [DOI] [PubMed] [Google Scholar]
- 4.Chung JK. Sodium iodide symporter: its role in nuclear medicine. J Nucl Med. 2004;43:1188–2000. [PubMed] [Google Scholar]
- 5.Nam-Goong IS. Kim HY. Gong G. Lee HK. Hong SJ. Kim WB. Shong YK. Ultrasonography-guided fine-needle aspiration of thyroid incidentaloma: correlation with pathological findings. Clin Endocrinol (Oxf) 2004;60:21–28. doi: 10.1046/j.1365-2265.2003.01912.x. [DOI] [PubMed] [Google Scholar]
- 6.Hay ID. Grant CS. Van Heerden JA. Goellner JR. Ebersold JR. Bergstralh EJ. Papillary thyroid microcarcinoma: a study of 535 cases observed in a 50-year period. Surgery. 1992;112:1139–1146. [PubMed] [Google Scholar]
- 7.Ward LS. Maciel RMB. Camargo RY. Teixeira GV. Tincani AJ. Kulcsar MAV. Carvalho GA. Graf H. Tomimori E. Maia AL. Kimura ET. Vaisman M. Hojaij FC. Araújo PPC. Miyahara L. Pereira SAM. Pereira EM. Marone M. Brandão RC. Soares J., Jr Andrada NC. Câncer Diferenciado de tiroide: Diagnostico., Projeto diretrizes. 2011. www.projetodiretrizes.org.br www.projetodiretrizes.org.br
- 8.Maia AL. Ward LS. Carvalho GA. Graf H. Maciel RMB. Rosario PW. Vaisman M. Thyroid nodules and differentiated thyroid câncer: Brazilian Consensus 2007. Arq Bras Endocrinol Metab. 2007;51:867–893. doi: 10.1590/s0004-27302007000500027. [DOI] [PubMed] [Google Scholar]
- 9.Lorenzen J. Beese M. Mester J. Brumma K. Beyer W. Clausen Chester M. X-ray: routine indication in the follow up of differentiated thyroid cancer? Nuklearmedizin. 1998;37:208–212. [PubMed] [Google Scholar]
- 10.Antoch G. Freudenberg LS. Beyer T. Bockisch A. Debatin JF. To enhance or not enhance? 18 FDG and CT Contrast agent in Dual-Modality 18 FDG PET/CT. J Nucl Med. 2004;45:56S–65S. [PubMed] [Google Scholar]
- 11.Van der Molen AJ. Thomsen HS. Morcos SK. Contrast Media Safety Committee, European Society of Urogenital Radiology (ESUR). Effects of iodinated contrast media on thyroid function in adults. Eur Radiol. 2004;14:902–907. doi: 10.1007/s00330-004-2238-z. [DOI] [PubMed] [Google Scholar]
- 12.Thomsen HS. Guidelines for Contrast Media from the European Society of Urogenital. Radiology. 2003;181:1463–1471. doi: 10.2214/ajr.181.6.1811463. [DOI] [PubMed] [Google Scholar]
- 13.Amdur RJ. Mazzaferri EL. Intravenous iodinated contrast effects iodine uptake for months. In: Amdur RJ, editor; Mazzaferri EL, editor. Essentials of Thyroid Cancer Management. 1st. Springer; New York: 2005. pp. 211–213. [Google Scholar]
- 14.Vejbjerg P. Knudsen N. Perrild H. Lauerberg P. Andersen S. Rasmussen LB. Ovessen L. Jorgensen T. Estimation of iodine intake from various urinary iodine measurements in population studies. Thyroid. 2009;19:1281–1286. doi: 10.1089/thy.2009.0094. [DOI] [PubMed] [Google Scholar]
- 15.Andersen S. Karmisholt J. Pedersen KM. Laurberg P. Reliability of studies of iodine intake and recommendations for number of samples in groups and in individuals. Br J Nutr. 2008;99:813–818. doi: 10.1017/S0007114507842292. [DOI] [PubMed] [Google Scholar]
- 16.Iodine Status Worldwide. In: de Benoist B, editor; Andersson M, editor; Egli I, editor; Takkouche B, editor; Allen H, editor. WHO Global Database on Iodine Deficiency. World Health Organization; Geneva: 2004. [Google Scholar]
- 17.Esteves RZ. Kasamatsu TS. Kunii IS. Furuzawa GK. Vieira JGH. Maciel RMB. Development of a semi-automated method for measuring urinary iodine and its application in epidemiological studies in Brazilian schoolchildren. Arq Bras Endocrinol Metab. 2007;51:1477–1484. doi: 10.1590/s0004-27302007000900010. [DOI] [PubMed] [Google Scholar]
- 18.Follis RH., Jr Vanprapa K. Damrongsakdi D. Studies on iodine nutrition in Thailand. J Nutr. 1962;76:159–173. doi: 10.1093/jn/76.2.159. [DOI] [PubMed] [Google Scholar]
- 19.Follis RH., Jr Patterns of urinary iodine excretion in goitrous and nongoitrous areas. Am J Clin Nutr. 1964;14:253–268. doi: 10.1093/ajcn/14.5.253. [DOI] [PubMed] [Google Scholar]
- 20.Pretell EA. Delange F. Hostalek U. Corigliano S. Barreda L. Higa AM. Altschule N. Barragán D. Cevallos JL. Gonzales O. Jara JA. Medeiros-Neto G. Montes JA. Muzzo S. Pacheco VM. Cordero L. Iodine nutrition improves in Latin America. Thyroid. 2004;14:590–599. doi: 10.1089/1050725041692909. [DOI] [PubMed] [Google Scholar]
- 21.Dunn JT. Crutchfield HE. Gutekunst R. Dunn AD. Two simple methods for measuring iodine in urine. Thyroid. 1993;3:119–123. doi: 10.1089/thy.1993.3.119. [DOI] [PubMed] [Google Scholar]
- 22.Pino S. Fang S-L. Braverman LE. Ammonium persulfate: a safe alternative oxidizing reagent for measuring urinary iodine. Clin Chem. 1996;42:239–243. [PubMed] [Google Scholar]
- 23.Glantz SA. Primer of Biostatistics. 3rd. McGraw-Hill, Inc.; New York: 1992. [Google Scholar]
- 24.Grebe SK. Hay ID. Thyroid cancer nodal metastases: biologic significance and therapeutic considerations. Surg Oncol Clin North Am. 1996;5:43–63. [PubMed] [Google Scholar]
- 25.Ilgan S. Karacalioglu AO. Pabuscu Y. Atac GK. Arslan N. Ozturk E. Gunalp B. Ozguven MA. Iodine-131 treatment and high-resolution CT: results in patients with lung metastasis from differentiated thyroid carcinoma. Eur J Nucl Med Mol Imaging. 2004;31:825–830. doi: 10.1007/s00259-004-1460-x. [DOI] [PubMed] [Google Scholar]
- 26.Laurie AJ. Lyon SG. Lasser EC. Contrast material iodides: potential effects on radioactive iodine thyroid uptake. J Nucl Med. 1992;33:237–238. [PubMed] [Google Scholar]
- 27.Burman KD. Wortofsky L. Iodine effects on the thyroid gland: biochemical and clinical aspects. Rev Endocr Metab Disord. 2000;1:19–25. doi: 10.1023/a:1010004218052. [DOI] [PubMed] [Google Scholar]
- 28.Piekarski JD. Schlumberger M. Leclere J. Couanet D. Masselot J. Parmentier C. Chest computed tomography (CT) in patients with micronodular lung metastasis of differentiated thyroid carcinoma. Int J Radiat Oncol Biol Phys. 1985;11:1023–1027. doi: 10.1016/0360-3016(85)90126-9. [DOI] [PubMed] [Google Scholar]