Abstract
Background
Smokers in the general population have lower thyrotropin (TSH) and higher free triiodothyronine (fT3) and free thyroxine (fT4) concentrations, but the results in pregnant population vary from no effect to a decrease in TSH and fT4 concentrations and an increase in fT3 levels. Our objective was to further evaluate the question of whether there is an association between smoking, before and during pregnancy, with maternal thyroid function during pregnancy and with the risk for subsequent hypothyroidism.
Methods
Our study population was a prospective population-based cohort (N=9362), the Northern Finland Birth Cohort 1986, with extensive data throughout gestation. The mothers underwent serum sampling in early pregnancy. The samples were assayed for TSH, fT3, fT4, thyroid-peroxidase antibodies (TPO-Ab), and thyroglobulin antibodies (TG-Abs) (n=5805). Mothers with thyroid dysfunction diagnosed before or during pregnancy were excluded, leaving 4837 euthyroid mothers. The smoking status of mothers and fathers were requested by questionnaires during pregnancy. Subsequent maternal morbidity relating to hypothyroidism 20 years after the index pregnancy was evaluated using national registers.
Results
Euthyroid mothers who smoked before, or continued smoking during first trimester of pregnancy, had higher serum fT3 (p<0.001) and lower fT4 (p=0.023) concentrations than nonsmokers. Smoking in the second trimester was associated with higher fT3 (p<0.001) concentrations, but no difference in fT4 concentrations compared with nonsmokers. TG-Abs were less common among smoking than nonsmoking mothers (2.5% vs. 4.7%, p<0.001), but the prevalence of TPO-Ab was similar. Paternal smoking had no independent effect on maternal early pregnancy thyroid hormone or antibody concentrations. The risk of subsequent maternal hypothyroidism after follow-up of 20 years was similar among prepregnancy smokers and nonsmokers.
Conclusions
In euthyroid women, smoking during pregnancy was associated with higher fT3 levels and lower fT4 levels; possibly reflecting smoking-induced changes in peripheral metabolism of thyroid hormones. No differences were found in TSH concentrations between smokers and nonsmokers. Our results differ from those of the general population, which usually have shown smoking-induced thyroidal stimulation. This is possibly due to pregnancy-induced changes in thyroid function. Decreases in fT4 levels among smokers might predispose to hypothyroidism or hypothyroxinemia during pregnancy. Despite these changes in thyroid function, smoking did not increase the woman's risk of subsequent hypothyroidism.
Introduction
One in five working-aged women smoke daily and up to 15% of women smoke during pregnancy (1) despite the well-known harmful effects of smoking on fetal growth and pregnancy outcomes (2). Smoking is also known to affect thyroid function, but the magnitude and direction of the effect varies greatly with different studies. Studies in nonpregnant populations have shown an increase in free triiodothyronine (fT3) and free thyroxine (fT4) concentrations (3–6) and a decrease in thyrotropin (TSH) concentrations among smokers (3–13). However, one study of nonpregnant female smokers with subclinical hypothyroidism showed higher TSH levels, and smokers with overt hypothyroidism had poorer metabolic health markers, indicating greater degree of hypothyroidism (14). A lower prevalence of thyroid autoantibodies, especially thyroglobulin antibodies (TG-Abs) has been found in nonpregnant populations of smokers (12,13,15), as well as an increase in the risk for developing thyroid peroxidase antibodies and/or thyroglobulin antibodies with smoking cessation (16).
Previous studies among pregnant women have shown that smokers have higher fT3 (17). The results regarding TSH and fT4 concentrations are more heterogenous, with some showing no association with TSH (18,19) or fT4 (17,20) and others having lower TSH (17,20) and fT4 levels among smokers (18,19). The possibility of an association of smoking with alterations in thyroid function among pregnant women is, however, of importance. Hypothyroidism and hypothyroxinemia affect up to 5% of pregnant women and have been associated with several adverse pregnancy and perinatal outcomes (21,22).
Smoking is known to increase the risk of Graves' disease (23) and it is also associated with goiters (3,6,9,23) in the general population, although iodine intake seems to affect this association (6). The results regarding hypothyroidism are less consistent, as some have shown an increase in the degree of hypothyroidism among nonpregnant female smokers (14), but others have shown that smokers have a lower prevalence of current hypothyroidism (8,12,24). The risk of Hashimoto's thyroiditis and postpartum thyroiditis is higher among smokers (23,25). Despite these risks, overt hypothyroidism has not been associated with smoking, although the studies have been heterogeneous (23). To our knowledge, there are no studies evaluating if there is an association between smoking and the development of hypothyroidism during long-term follow-up.
The aim of this study was to determine if there is an association between smoking and serum TSH, fT4, and fT3 as well as thyroid autoantibody concentrations in euthyroid pregnant Finnish women. In addition, the effect of smoking on subsequent morbidity relating to hypothyroidism was evaluated, with a follow-up period of over 20 years.
Subjects and Methods
Northern Finland Birth Cohort 1986
The prospectively collected Northern Finland Birth Cohort 1986 (NFBC 1986) comprises 99% of all births with the calculated term between July 1, 1985 and June 30, 1986 drawn from the two northernmost provinces of Finland (N=9362 mothers, N=9479 children). In this study, only singleton pregnancies were included (n=9247). The cohort has been followed since the 12th week of gestation. Data were collected during routine visits to communal maternity welfare clinics (free-of-charge service for all pregnant women; participation rate 99.8%) as well as via questionnaires. The first questionnaire, on demographic, biologic, and socioeconomic characteristics of the mothers/families, covered the period up to week 24 of gestation, when the mothers were recruited to the study if still pregnant. The second questionnaire contained items about the mother's health during pregnancy. The nurses in the communal maternity clinics helped the mothers to fill in the questionnaire and ensured that all questions were answered. The third questionnaire, which contained items about the pregnancy, hospital care, delivery, and neonatal outcome, was completed in the maternity hospitals by the attending midwives. All births in this area during this time period took place in public hospitals (26,27).
Maternal smoking habits were also evaluated via questionnaires. The questionnaires included questions on maternal smoking before pregnancy and during pregnancy (in the first and second trimesters separately), duration of smoking (years), number of cigarettes smoked per day, change of smoking habit during pregnancy (quit, reduced, increased, and no change), number of cigarettes smoked after the change, and if the change occurred before serum sampling. Paternal smoking habits were asked about separately (smokes/does not smoke). Based on the reported duration of smoking and number of cigarettes smoked per day, the maternal smoking history was recorded as pack-years (one pack-year=20 cigarettes smoked per day for one year). Data concerning the other maternal background factors (age, body mass index [BMI], parity, obstetric history, and previous diseases) were obtained through the questionnaires. The Ethics Committees of the Northern Ostrobothnia Hospital District and the National Institute of Health and Welfare approved this study. Informed consent was obtained from all subjects.
Serum samples and laboratory assays
The biochemical data were obtained via the Finnish Maternity Cohort, which is a biobank consisting of serum samples collected from all pregnant women in Finland and approved under the Finnish law. The law allows use of the samples in studies promoting public health. The samples were stored at −25°C and thawed for the first time for the analyses in this study in 2006. The effects of freezing, thawing, and frozen storage on thyroid laboratory parameters have been reported previously (28). Quantitative analyses of TSH, fT3, fT4, and autoantibodies associated with autoimmune thyroiditis (thyroid-peroxidase antibodies [TPO-Ab] and TG-Ab) were performed by way of chemiluminescent microparticle immunoassays, using an Architect i2000 automatic analyzer (Abbott Diagnostics, Abbott Park, IL). The lower limits of detection, and intra- and interassay coefficients of variation were 0.0025 mU/L, 1.7% and 5.3% for TSH; 5.1 pmol/L, 3.6% and 7.8% for fT4; 1.53 pmol/L, 2.3% and 5.0% for fT3; 1.0 IU/mL, 2.5% and 9.8% for TPO-Ab; and 1.0 IU/mL, 2.7% and 8.2% for TG-Ab. The number of serum samples analyzed was 5805 (61.2% of the whole cohort); only samples of a sufficient volume (≥1 mL) were included in this study. The excluded population did not differ significantly from those included. The mean gestational age at sampling was 11.0 week (standard deviation 3.6) and only samples drawn before or at the 20th gestational week were accepted (98% of the samples). When the sample size was not sufficient for all analyses, thyroid hormone analyses were carried out primarily.
All mothers with known thyroid disease (n=86) before pregnancy were excluded from the analyses. Diagnoses of previous thyroid diseases were obtained via a questionnaire and confirmed in hospital records. All mothers with thyroid dysfunction during pregnancy, diagnosed by early pregnancy serum sampling, were excluded from the analyses (n=909). Thus, only euthyroid mothers (those with serum TSH concentrations of 0.1–3.0 mU/L and serum fT4 concentrations of 11–22 pmol/L) were included in the analyses. These cut-off levels are based on the reference intervals calculated previously for this population (29).
Mothers were deemed to be positive for TPO-Ab or TG-Ab if the antibody concentration was over the 95th percentile, that is, >167.7 IU/mL for TPO-Ab and >47.7 IU/mL for TG-Ab. There were 143 (3.0%) TPO-Ab-positive and 176 (3.6%) TG-Ab-positive mothers in the cohort. All data were analyzed with the antibody-positive mothers included and excluded.
Follow-up data of the mothers
Three register-based data sets were used in this study to evaluate the health of the mothers after their index pregnancy, the follow-up time being 23 years. First, data from the Social Insurance Institute of Finland comprises information on diagnosed diseases, with medication and reimbursement of medical expenses. Second, the national Finnish Hospital Discharge Register includes information on diagnoses at discharge from all hospital wards or outpatient clinics, using the International Classification of Diseases (ICD). The register has an accuracy of 83–95%.
During the follow-up period, three different ICD versions were used: ICD-8 through December 31, 1986; ICD-9 from January 1, 1987, through December 31, 1996; and ICD-10 from January 1, 1997, onward. Third, the Population Register and Register of Causes of Death contain information on causes of death, covering all deaths in Finland. Data from these registers were obtained for the period 1985–2008. Data from the national Hospital Discharge Register were complete from 2000 onward. The register-based data were combined with the NFBC 1986 data by using individual social security numbers, which are given to all Finns. This was carried out by personnel uninvolved in this study, and the researchers had no access to identifiable data concerning the participants.
Receiving reimbursement for medication to treat hypothyroidism or having discharge diagnoses of hypothyroidism (defined as ICD-8 codes 244.0–244.9 and 245, ICD-9 codes 244.9, 245.2, and 245.9, and ICD-10 codes E03.5, E03.80, E03.82, E03.89, E03.9, and E06) was considered to be verification of the disease.
Statistical analyses
Laboratory data were skewed and logarithmically transformed. Comparisons were carried out by using the Students t test for continuous variables and the Fisher's exact test for categorical variables. The linear regression analysis was used when evaluating the effect of smoking on total variation of variables. The one-way analysis of variance (ANOVA) with the Dunnet's post hoc test was used to evaluate the effect of number of pack-years on TSH and thyroid hormones. The number of pack-years was divided into six categories for the analyses: 0, 0.1–1, 1.1–2, 2.1–5, 5.1–9.9, and 10 or more pack-years. The risk of subsequent hypothyroidism among smokers and nonsmokers was established by using Cox's regression analyses. Risks are presented as hazard ratios (HRs) with 95% confidence intervals (95% CIs). The results were further adjusted for maternal age and parity. A p-value<0.05 was deemed significant. All statistical analyses were performed with SPSS 18.0 (IBM SPSS Statistics).
Results
A total of 1335 (27.6%) euthyroid mothers (with/without TPO-Ab or TG-Ab positivity) and 1270 (27.8%) euthyroid antibody-negative mothers reported smoking before pregnancy. These mothers had a smoking history of a median of 3.5 pack-years (range 0.1–37.5). Of the smokers, 298 (8.3%) quit before the serum sampling and total of 607 mothers (12.5%) quit or reduced smoking during pregnancy.
Among the fathers, 1671 (34.5%) smoked. In 805 families, both parents smoked, in 822 families, only the father smoked, and in 428 families, only the mother smoked. Smoking mothers were significantly younger, more often nulliparous and had nonsignificantly lower BMI (Table 1).
Table 1.
Demographic Characteristics of the Study Population
| Prepregnancy smokers | Nonsmokers | |
|---|---|---|
| n | 1335 | 3305 |
| Maternal age (years) | 25.7±4.9 | 28.3±5.3a |
| Body–mass index (kg/m2) | 22.0±3.5 | 22.2±3.4 |
| Nulliparous | 572 (42.9%) | 1008 (30.5%)a |
| Gestational age of the child at birth | 39.4±1.9 | 39.4±1.7 |
All figures are mean±standard deviation or n (%).
p<0.05 when comparing smokers with nonsmokers.
Mothers who smoked before pregnancy had a lower prevalence of TG-Ab positivity, as only 2.3% (n=30/1332) vs. 4.2% (n=139/3303) of smokers versus nonsmokers were positive for TG-Abs (p=0.001). Smokers and nonsmokers had a similar rate of TPO-Ab positivity (39/1331 vs. 95/3300, 2.9% vs. 2.9%, p=0.923).
Euthyroid mothers who smoked before pregnancy had higher serum fT3 and lower fT4 concentrations during early pregnancy than nonsmokers. However, smoking before pregnancy explained only 1.28% and 0.12% of total variation in linear regression analyses regarding fT3 and fT4 concentrations, respectively.
We observed similar findings among those who smoked before pregnancy, but managed to completely quit smoking in early pregnancy (before serum sampling) when comparing these women with nonsmokers. Interestingly, these mothers also had higher TSH concentrations than those who had never smoked. No differences were seen between the concentrations of TSH or thyroid hormones when these mothers were compared with those who continued smoking during pregnancy.
Of the mothers, 806 (18.7%) continued smoking during the first trimester. Smoking in the first trimester was also associated with higher serum fT3 and lower serum fT4 concentrations. Only 108 (2.5%) mothers continued to smoke in the second trimester, and it was associated only with higher serum fT3 levels, and no difference was observed in the concentrations of fT4 between smokers versus nonsmokers. Paternal smoking had no individual effect on maternal early pregnancy thyroid hormone levels (Table 2).
Table 2.
Comparison of Serum Thyrotropin and Thyroid Hormone Concentrations Among Euthyroid Mothers (With or Without Thyroid Autoantibodies) of Different Smoking Status
| Smoking status | n | TSH (mU/L) | fT4 (pmol/L) | fT3 (pmol/L) |
|---|---|---|---|---|
| Smoking before pregnancy | ||||
| Smokers | 1335 | 1.06 | 15.02 | 5.26 |
| Nonsmokers | 3305 | 1.03 | 15.17 | 5.05 |
| p-Value | NS | 0.019 | <0.001 | |
| Smoking in the first trimester | ||||
| Smokers | 806 | 1.02 | 15.02 | 5.27 |
| Nonsmokers | 3511 | 1.02 | 15.24 | 5.07 |
| p-Value | NS | 0.006 | <0.001 | |
| Smoking in the second trimester | ||||
| Smokers | 108 | 1.20 | 14.53 | 5.48 |
| Nonsmokers | 316 | 1.27 | 14.38 | 5.10 |
| p-Value | NS | NS | <0.001 | |
| Quit smoking before serum sampling | ||||
| Quit smoking | 298 | 1.14 | 14.92 | 5.19 |
| Nonsmokers | 3288 | 1.03 | 15.18 | 5.05 |
| p-Value | 0.008 | 0.037 | 0.005 | |
| Quit smoking or continued smoking in early pregnancy | ||||
| Quit smoking | 298 | 1.14 | 14.93 | 5.19 |
| Continued smoking | 459 | 1.07 | 14.96 | 5.22 |
| p-Value | NS | NS | NS | |
| Only husband smokes | ||||
| Smokers | 822 | 1.05 | 15.16 | 5.07 |
| Nonsmokers | 2131 | 1.02 | 15.17 | 5.04 |
| p-Value | NS | NS | NS | |
| Both smoke | ||||
| Smokers | 805 | 1.06 | 15.00 | 5.21 |
| Nonsmokers | 3305 | 1.04 | 15.17 | 5.05 |
| p-Value | NS | 0.012 | <0.001 | |
Figures are geometric means. A p-value <0.05 was deemed significant.
TSH, thyrotropin; fT3, free triiodothyronine; fT4, free thyroxine; NS, nonsignificant.
All results remained similar when excluding those with TPO-Ab and/or TG-Ab positivity from the analyses or when excluding those who quit smoking before serum sampling (data not shown).
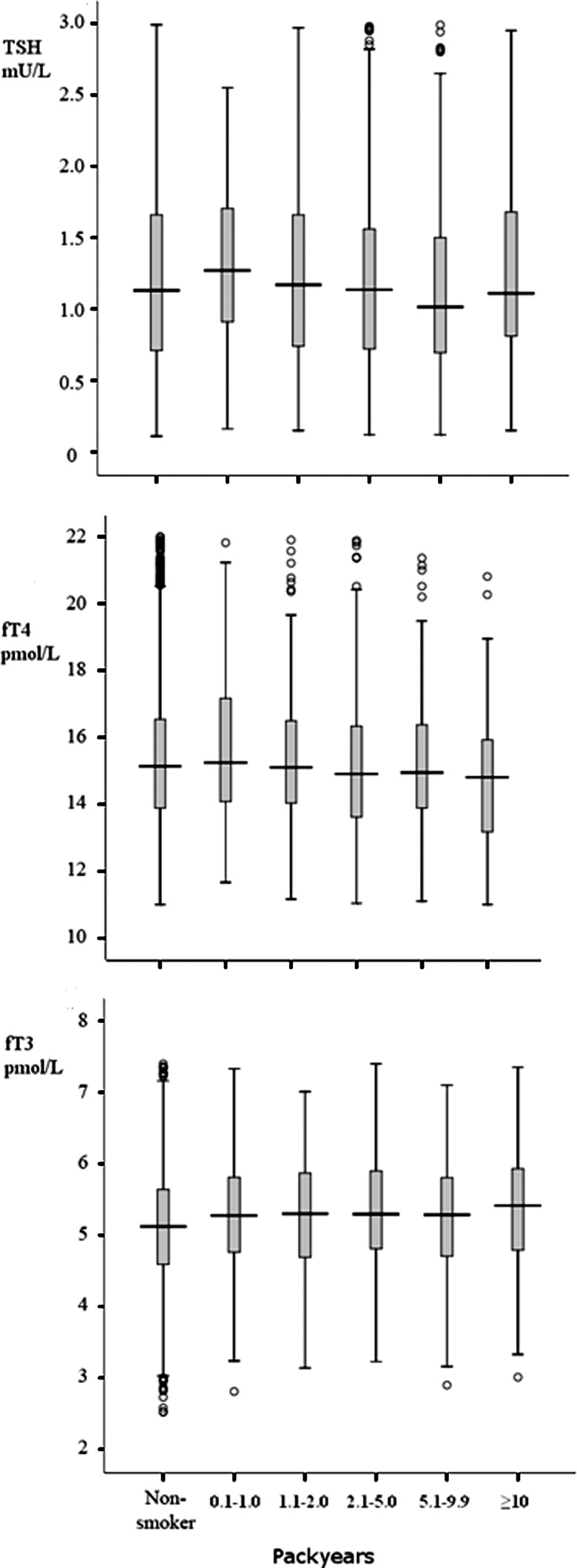
We observed no differences in TSH concentrations between the nonsmoking and smoking groups, even with a long-term smoking history defined by number of pack-years. There seemed to be a declining trend in fT4 concentrations with an extended smoking history (one-way ANOVA, test for linear trend, p=0.007) and an upward trend in fT3 concentrations (ANOVA, test for linear trend, p<0.001). These results are summarized in Figure 1.
FIG. 1.
Serum thyrotropin and thyroid hormone concentrations among women with different smoking histories presented as pack-years.
Smokers and nonsmokers had a similar prevalence of subsequent hypothyroidism, 1.5% (n=20) and 1.3% (n=44), respectively (HR 1.1, 95% CI 0.7–1.9).
Discussion
In the current study, we found that cigarette smoking in early pregnancy influenced serum thyroid hormone concentrations and prevalence of thyroid autoantibodies. Smokers had significantly lower fT4 and higher fT3 concentrations and were less often TG-Ab-positive than nonsmokers in the first trimester. Cessation of smoking during early pregnancy had no influence on maternal TSH or thyroid hormone levels compared with smokers.
Smoking has been thought to affect thyroid function via several mechanisms. Thiocyanate, a toxin in cigarette smoke, might interfere with iodide transport to the thyroid and cause intrathyroidal iodine depletion, at least in areas with iodine deficiency (30). This mechanism is also thought to have a protective effect against the development of thyroid autoantibodies. Another contributing factor might be the overall influence of smoking on the immune system as smoking has been found to increase the risk of autoimmune diseases such as rheumatoid arthritis and Graves' disease (31). It has been speculated that smoking, through the effects of nicotine or other constituents, might have a mild effect on thyroid hormone secretion and influence the activity of deiodinase enzymes, especially that of type 2 deiodinase (which converts T4 to T3 in the tissues) (32).
We found that pregnant women who had smoked before or during pregnancy had higher serum fT3 and lower fT4 concentrations than nonsmokers. Studies in nonpregnant (3–6,14) and pregnant populations (17) have shown previously that smokers tend to have higher fT3 concentrations, which are in accordance with our results. Previous studies in nonpregnant populations have mostly shown higher fT4 levels among smokers (5,6). The previous results of pregnant women have been inconsistent, with some studies showing no association with fT4 concentrations and smoking (17,20) and some showing decreased fT4 concentrations among smokers (18,19). These disparities may reflect variation in populations, especially with iodine intake and exclusion criteria used in different studies. Our results show that in a strictly euthyroid pregnant population, smokers have higher fT3 and lower fT4 levels, suggesting that smoking may have an effect on the type 2 deiodinase enzyme activity. These changes in thyroid hormone concentrations are, however, very subtle and explain only a small proportion of total variation in the analyses. The implications of such changes on an individual level regarding pregnant women cannot be resolved with this study, but it has been established that normal maternal thyroid function is essential during pregnancy (22). On a population level, even these subtle changes inflicted by smoking may be important, especially among populations with iodine deficiency, which may be more prone to the effects of smoking (33,34). Our population was based on Finnish euthyroid women, who are known to have long-term iodine sufficiency (35), which might explain why the association was so subtle.
A new finding in this study was that an extended smoking history had an effect on thyroid hormone levels in early pregnancy, as those with more pack-years in their smoking history had a declining trend in fT4 and an upward trend in fT3 concentrations. One study has shown opposite results, with the length of smoking having no effect on fT4 concentrations (33), but that study was not based on a pregnant or euthyroid population, which might have skewed the results. Based on our results, the length of smoking in pack-years affects thyroid hormone metabolism in euthyroid pregnant women adversely.
Smokers in nonpregnant populations have been found to less often have elevated TSH levels (8,12,24) or have lower TSH concentrations than nonsmokers (3–13). Similarly, some studies based on pregnant populations have shown lower TSH levels among smokers (17,20), while others have not (18,19). We could not confirm an association between smoking and TSH in our euthyroid pregnant population. The discrepancy with other studies might be explained by including only those who were strictly euthyroid into the analyses.
We also found that those who smoked before pregnancy had a lower prevalence of TG-Abs, but no difference in levels of TPO-Abs. Previously, it has been shown in population-based studies that the prevalence of, both TPO-Ab and TG-Ab positivity are lower in smokers than nonsmokers (8,12,15,36). It has been suggested that smoking is associated with decreased iodide transport and organification, which protect individuals against the development of thyroid autoantibodies (30). Cessation of smoking has also been found to increase the risk of de novo occurrence of serum TPO-Abs and/or TG-Abs (16). We cannot explain why smoking has a different effect on the prevalence of TG-Abs and TPO-Abs, but a similar association has been observed previously in general population studies (8,12,15).
The rates of subsequent hypothyroidism during the follow-up time of 25 years did not differ among smokers and nonsmokers despite alterations in thyroid hormone concentrations and the prevalence of TG-Ab positivity found during early pregnancy. A meta-analysis suggested that smokers may have an increased risk of Hashimoto's thyroiditis and postpartum thyroid dysfunction, but not a statistically significant risk of hypothyroidism (23), a finding also found in our study with a long follow-up.
In our study, smoking was evaluated through a questionnaire without serum cotinine confirmation. Studies have shown a self-reported smoking habit being quite reliable among pregnant women, but it underestimates the real prevalence of smoking by 19–38%, depending on the definitions used (37,38). However, self-reported information correlates well with serum cotinine levels (39). Also, we had no data on the prevalence of smoking habits during the follow-up after delivery and could thus only evaluate the effect of cross-sectional data on the risk of hypothyroidism. The strength of this investigation is the large iodine-sufficient study population, which allowed us to exclude from the analyses all mothers with thyroid dysfunction [diagnosis established using gestational age-specific reference intervals (29)] during pregnancy. In addition, large, reliable Finnish registries have enabled follow-up of the cohort mothers and revealed diagnoses of thyroid diseases over a period of 20 years.
In conclusion, maternal smoking during pregnancy was associated with higher fT3 levels and lower fT4 levels in euthyroid pregnant women. No differences were found in TSH concentrations between smokers and nonsmokers. Previously, in general population studies, it has been suggested that smoking might slightly stimulate thyroid function as previous studies have mostly shown lower TSH with higher fT4 and fT3 among smokers (3–13). Our results would rather indicate changes in the thyroid hormone metabolism induced by smoking. We also cannot resolve the possible implications of these changes in thyroid hormones for the well-being of the fetus. Lower fT4 concentrations among smokers might, however, predispose to hypothyroxinemia during early pregnancy.
Smoking had no effect on the risk of developing subsequent clinical hypothyroidism during a follow-up of 20 years.
Acknowledgments
We thank Ms. Sarianna Vaara, Ms. Tuula Ylitalo, and all other personnel from the National Institute for Health and Welfare for their valuable work regarding the Northern Finland Birth Cohort 1986. We also thank Mr. Jouni Sallinen and Mr. Frank Quinn (Abbott Laboratories) for providing laboratory reagents. This work was supported in part by grants from the Alma and K.A. Snellman Foundation (Oulu, Finland), the Jalmari and Rauha Ahokas Foundation (Finland), the Oulu University Scholarship Foundation (Oulu, Finland), the Finnish Medical Association of Clinical Chemistry, the Foundation of the Northern Ostrobothnia Hospital District (Finland), the Finnish Medical Foundation (Finland), and the Academy of Finland.
Disclosure Statement
The authors have nothing to declare.
References
- 1.Helakorpi S. Pajunen J. Janninoja P. Virtanen S. Uutela A. Health Behaviour and Health Among the Finnish Adult Population, Spring 2010. National Institute for Health and Welfare (THL); Helsinki: 2011. [Google Scholar]
- 2.Higgins S. Smoking in pregnancy. Curr Opin Obstet Gynecol. 2002;14:145–151. doi: 10.1097/00001703-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Christensen SB. Ericsson UB. Janzon L. Tibblin S. Melander A. Influence of cigarette smoking on goiter formation, thyroglobulin, and thyroid hormone levels in women. J Clin Endocrinol Metab. 1984;58:615–618. doi: 10.1210/jcem-58-4-615. [DOI] [PubMed] [Google Scholar]
- 4.De Pergola G. Ciampolillo A. Alo D. Sciaraffia M. Guida P. Free triiodothyronine is associated with smoking habit, independently of obesity, body fat distribution, insulin, and metabolic parameters. J Endocrinol Invest. 2010;33:815–818. doi: 10.1007/BF03350348. [DOI] [PubMed] [Google Scholar]
- 5.Jorde R. Sundsfjord J. Serum TSH levels in smokers and non-smokers. The 5th Tromso study. Exp Clin Endocrinol Diabetes. 2006;114:343–347. doi: 10.1055/s-2006-924264. [DOI] [PubMed] [Google Scholar]
- 6.Vejbjerg P. Knudsen N. Perrild H. Carle A. Laurberg P. Pedersen IB. Rasmussen LB. Ovesen L. Jorgensen T. The impact of smoking on thyroid volume and function in relation to a shift towards iodine sufficiency. Eur J Epidemiol. 2008;23:423–429. doi: 10.1007/s10654-008-9255-1. [DOI] [PubMed] [Google Scholar]
- 7.Åsvold BO. Bjøro T. Nilsen TI. Vatten LJ. Tobacco smoking and thyroid function: a population-based study. Arch Intern Med. 2007;167:1428–1432. doi: 10.1001/archinte.167.13.1428. [DOI] [PubMed] [Google Scholar]
- 8.Belin RM. Astor BC. Powe NR. Ladenson PW. Smoke exposure is associated with a lower prevalence of serum thyroid autoantibodies and thyrotropin concentration elevation and a higher prevalence of mild thyrotropin concentration suppression in the third National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2004;89:6077–6086. doi: 10.1210/jc.2004-0431. [DOI] [PubMed] [Google Scholar]
- 9.Ericsson UB. Lindgarde F. Effects of cigarette smoking on thyroid function and the prevalence of goitre, thyrotoxicosis and autoimmune thyroiditis. J Intern Med. 1991;229:67–71. doi: 10.1111/j.1365-2796.1991.tb00308.x. [DOI] [PubMed] [Google Scholar]
- 10.Fisher CL. Mannino DM. Herman WH. Frumkin H. Cigarette smoking and thyroid hormone levels in males. Int J Epidemiol. 1997;26:972–977. doi: 10.1093/ije/26.5.972. [DOI] [PubMed] [Google Scholar]
- 11.Karakaya A. Tuncel N. Alptuna G. Kocer Z. Erbay G. Influence of cigarette smoking on thyroid hormone levels. Hum Toxicol. 1987;6:507–509. doi: 10.1177/096032718700600610. [DOI] [PubMed] [Google Scholar]
- 12.Mehran L. Amouzgar A. Delshad H. Azizi F. The association of cigarette smoking with serum TSH concentration and thyroperoxidase antibody. Exp Clin Endocrinol Diabetes. 2012;120:80–83. doi: 10.1055/s-0031-1285910. [DOI] [PubMed] [Google Scholar]
- 13.Soldin OP. Goughenour BE. Gilbert SZ. Landy HJ. Soldin SJ. Thyroid hormone levels associated with active and passive cigarette smoking. Thyroid. 2009;19:817–823. doi: 10.1089/thy.2009.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muller B. Zulewski H. Huber P. Ratcliffe JG. Staub JJ. Impaired action of thyroid hormone associated with smoking in women with hypothyroidism. N Engl J Med. 1995;333:964–969. doi: 10.1056/NEJM199510123331503. [DOI] [PubMed] [Google Scholar]
- 15.Pedersen IB. Laurberg P. Knudsen N. Jorgensen T. Perrild H. Ovesen L. Rasmussen LB. Smoking is negatively associated with the presence of thyroglobulin autoantibody and to a lesser degree with thyroid peroxidase autoantibody in serum: a population study. Eur J Endocrinol. 2008;158:367–373. doi: 10.1530/EJE-07-0595. [DOI] [PubMed] [Google Scholar]
- 16.Effraimidis G. Tijssen JG. Wiersinga WM. Discontinuation of smoking increases the risk for developing thyroid peroxidase antibodies and/or thyroglobulin antibodies: a prospective study. J Clin Endocrinol Metab. 2009;94:1324–1328. doi: 10.1210/jc.2008-1548. [DOI] [PubMed] [Google Scholar]
- 17.Shields B. Hill A. Bilous M. Knight B. Hattersley AT. Bilous RW. Vaidya B. Cigarette smoking during pregnancy is associated with alterations in maternal and fetal thyroid function. J Clin Endocrinol Metab. 2009;94:570–574. doi: 10.1210/jc.2008-0380. [DOI] [PubMed] [Google Scholar]
- 18.Pearce EN. Oken E. Gillman MW. Lee SL. Magnani B. Platek D. Braverman LE. Association of first-trimester thyroid function test values with thyroperoxidase antibody status, smoking, and multivitamin use. Endocr Pract. 2008;14:33–39. doi: 10.4158/EP.14.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pearce EN. Lazarus JH. Smyth PP. He X. Dall'amico D. Parkes AB. Burns R. Smith DF. Maina A. Bestwick JP. Jooman M. Leung AM. Braverman LE. Perchlorate and thiocyanate exposure and thyroid function in first-trimester pregnant women. J Clin Endocrinol Metab. 2010;95:3207–3215. doi: 10.1210/jc.2010-0014. [DOI] [PubMed] [Google Scholar]
- 20.McDonald SD. Walker MC. Ohlsson A. Murphy KE. Beyene J. Perkins SL. The effect of tobacco exposure on maternal and fetal thyroid function. Eur J Obstet Gynecol Reprod Biol. 2008;140:38–42. doi: 10.1016/j.ejogrb.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Männistö T. Vääräsmäki M. Pouta A. Hartikainen AL. Ruokonen A. Surcel HM. Bloigu A. Järvelin MR. Suvanto-Luukkonen E. Perinatal outcome of children born to mothers with thyroid dysfunction or antibodies: a prospective population-based cohort study. J Clin Endocrinol Metab. 2009;94:772–779. doi: 10.1210/jc.2008-1520. [DOI] [PubMed] [Google Scholar]
- 22.Stagnaro-Green A. Abalovich M. Alexander E. Azizi F. Mestman J. Negro R. Nixon A. Pearce EN. Soldin OP. Sullivan S. Wiersinga W. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2011;21:1081–1125. doi: 10.1089/thy.2011.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vestergaard P. Smoking and thyroid disorders—a meta-analysis. Eur J Endocrinol. 2002;146:153–161. doi: 10.1530/eje.0.1460153. [DOI] [PubMed] [Google Scholar]
- 24.Knudsen N. Bulow I. Laurberg P. Perrild H. Ovesen L. Jorgensen T. High occurrence of thyroid multinodularity and low occurrence of subclinical hypothyroidism among tobacco smokers in a large population study. J Endocrinol. 2002;175:571–576. doi: 10.1677/joe.0.1750571. [DOI] [PubMed] [Google Scholar]
- 25.Galanti MR. Cnattingius S. Granath F. Ekbom-Schnell A. Ekbom A. Smoking and environmental iodine as risk factors for thyroiditis among parous women. Eur J Epidemiol. 2007;22:467–472. doi: 10.1007/s10654-007-9142-1. [DOI] [PubMed] [Google Scholar]
- 26.Järvelin MR. Hartikainen-Sorri AL. Rantakallio P. Labour induction policy in hospitals of different levels of specialisation. Br J Obstet Gynaecol. 1993;100:310–315. doi: 10.1111/j.1471-0528.1993.tb12971.x. [DOI] [PubMed] [Google Scholar]
- 27.Järvelin MR. Elliott P. Kleinschmidt I. Martuzzi M. Grundy C. Hartikainen AL. Rantakallio P. Ecological and individual predictors of birthweight in a northern Finland birth cohort 1986. Paediatr Perinat Epidemiol. 1997;11:298–312. doi: 10.1111/j.1365-3016.1997.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 28.Männistö T. Surcel HM. Bloigu A. Ruokonen A. Hartikainen AL. Järvelin MR. Pouta A. Vääräsmäki M. Suvanto-Luukkonen E. The effect of freezing, thawing, and short- and long-term storage on serum thyrotropin, thyroid hormones, and thyroid autoantibodies: implications for analyzing samples stored in serum banks. Clin Chem. 2007;53:1986–1987. doi: 10.1373/clinchem.2007.091371. [DOI] [PubMed] [Google Scholar]
- 29.Männistö T. Surcel HM. Ruokonen A. Vääräsmäki M. Pouta A. Bloigu A. Järvelin MR. Hartikainen AL. Suvanto E. Early pregnancy reference intervals of thyroid hormone concentrations in a thyroid antibody-negative pregnant population. Thyroid. 2011;21:291–298. doi: 10.1089/thy.2010.0337. [DOI] [PubMed] [Google Scholar]
- 30.Fukayama H. Nasu M. Murakami S. Sugawara M. Examination of antithyroid effects of smoking products in cultured thyroid follicles: only thiocyanate is a potent antithyroid agent. Acta Endocrinol (Copenh) 1992;127:520–525. doi: 10.1530/acta.0.1270520. [DOI] [PubMed] [Google Scholar]
- 31.Costenbader KH. Karlson EW. Cigarette smoking and autoimmune disease: what can we learn from epidemiology? Lupus. 2006;15:737–745. doi: 10.1177/0961203306069344. [DOI] [PubMed] [Google Scholar]
- 32.Gondou A. Toyoda N. Nishikawa M. Yonemoto T. Sakaguchi N. Tokoro T. Inada M. Effect of nicotine on type 2 deiodinase activity in cultured rat glial cells. Endocr J. 1999;46:107–112. doi: 10.1507/endocrj.46.107. [DOI] [PubMed] [Google Scholar]
- 33.Cho NH. Choi HS. Kim KW. Kim HL. Lee SY. Choi SH. Lim S. Park YJ. Park DJ. Jang HC. Cho BY. Interaction between cigarette smoking and iodine intake and their impact on thyroid function. Clin Endocrinol (Oxf) 2010;73:264–270. doi: 10.1111/j.1365-2265.2010.03790.x. [DOI] [PubMed] [Google Scholar]
- 34.Steinmaus C. Miller MD. Howd R. Impact of smoking and thiocyanate on perchlorate and thyroid hormone associations in the 2001–2002 national health and nutrition examination survey. Environ Health Perspect. 2007;115:1333–1338. doi: 10.1289/ehp.10300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lamberg BA. Haikonen M. Makela M. Jukkara A. Axelson E. Welin MG. Further decrease in thyroidal uptake and disappearance of endemic goitre in children after 30 years of iodine prophylaxis in the east of Finland. Acta Endocrinol (Copenh) 1981;98:205–209. doi: 10.1530/acta.0.0980205. [DOI] [PubMed] [Google Scholar]
- 36.Strieder TG. Prummel MF. Tijssen JG. Endert E. Wiersinga WM. Risk factors for and prevalence of thyroid disorders in a cross-sectional study among healthy female relatives of patients with autoimmune thyroid disease. Clin Endocrinol (Oxf) 2003;59:396–401. doi: 10.1046/j.1365-2265.2003.01862.x. [DOI] [PubMed] [Google Scholar]
- 37.Bardy AH. Seppala T. Lillsunde P. Kataja JM. Koskela P. Pikkarainen J. Hiilesmaa VK. Objectively measured tobacco exposure during pregnancy: neonatal effects and relation to maternal smoking. Br J Obstet Gynaecol. 1993;100:721–726. doi: 10.1111/j.1471-0528.1993.tb14262.x. [DOI] [PubMed] [Google Scholar]
- 38.George L. Granath F. Johansson AL. Cnattingius S. Self-reported nicotine exposure and plasma levels of cotinine in early and late pregnancy. Acta Obstet Gynecol Scand. 2006;85:1331–1337. doi: 10.1080/00016340600935433. [DOI] [PubMed] [Google Scholar]
- 39.Tikkanen M. Surcel HM. Bloigu A. Nuutila M. Ylikorkala O. Hiilesmaa V. Paavonen J. Self-reported smoking habits and serum cotinine levels in women with placental abruption. Acta Obstet Gynecol Scand. 2010;89:1538–1544. doi: 10.3109/00016349.2010.526187. [DOI] [PubMed] [Google Scholar]