Abstract
Background
Graves'-like disease, reflected by thyrotropin receptor (TSHR) antibodies and hyperthyroidism in some mouse strains, can be induced by immunization with adenovirus-expressing DNA for the human TSHR or its A-subunit. The conventional approach involves two or three adenovirus injections at 3-week intervals and euthanasia 10 weeks after the first injection. To investigate TSHR antibody persistence in mice with differing degrees of self-tolerance to the TSHR A-subunit, we studied the effect of delaying euthanasia until 20 weeks after the initial immunization.
Methods
Wild-type (WT) mice and transgenic (tg) mice expressing low intrathyroidal levels of the human TSHR A-subunit were immunized with A-subunit-adenovirus on two occasions; a second group of mice was immunized on three occasions. Sera obtained 4, 10, and 20 weeks (euthanasia) after the initial immunization were tested for thyrotropin (TSH) binding inhibition (TBI), antibody binding to TSHR A-subunit protein-coated enzyme-linked immunosorbent assay (ELISA) plates, and thyroid stimulating antibody activity (TSAb; cyclic adenosine monophosphate [cAMP] generation). Serum thyroxine (T4) and thyroid histology were studied at euthanasia.
Results
The majority of WT mice retained high TSHR antibody levels measured by TBI or ELISA at euthanasia but only about 50% were TSAb positive. Low-expressor tgs exhibited self-tolerance, with fewer mice positive by TBI or ELISA and antibody levels were lower than in WT littermates. In WT mice, antibody persistence was similar after two or three immunizations; for tgs, only mice immunized three times had detectable TSAb at 20 weeks. Unlike our previous observations of hyperthyroidism in WT mice examined 4 or 10 weeks after immunization, all mice were euthyroid at 20 weeks.
Conclusions
Our findings for induced TSHR antibodies in mice, similar to data for human thyroid autoantibodies, indicate that the parameters that contribute to the concentration of the antibody and thereby play a critical role in long-term persistence of TSHR antibodies are the degree of self-tolerance to the TSHR and chronic stimulation.
Introduction
Mouse models of induced Graves' disease require in vivo expression of the thyrotropin receptor (TSHR) or its A-subunit by injecting TSHR-expressing cells or immunization with plasmid or adenoviral vectors encoding TSHR DNA [reviewed in Nagayama (1)]. The Nagayama model involves repeated intramuscular injection of adenovirus expressing the human TSHR (2). Subsequent investigations were performed to optimize induction of Graves'-like disease (reflected by TSHR antibodies and hyperthyroidism in some mouse strains) by testing the efficacy of the A-subunit versus the full-length TSHR, comparing low versus high adenovirus dose, and injecting dendritic cells expressing the A-subunit [reviewed in Nagayama (1)]. However, none of these studies changed the timing of the protocol, namely, three injections of adenovirus or cells at 3-week intervals and euthanasia 4 weeks after the third injection. In addition, until recently, no studies were directed at determining the long-term persistence of adenovirus-induced TSHR antibodies.
It should be emphasized that both the adenovirus and the immune system can contribute to long-term responses against the TSHR. First, the protein encoded by the adenovirus continues to be expressed for some time after a single injection and is, therefore, available for antigen uptake and presentation to the immune system. In developing the adenovirus model, Nagayama and colleagues confirmed TSHR expression in vivo by demonstrating radiolabeled TSH binding to muscle preparations from mice injected 5 days previously (2). Further, expression of a herpes simplex virus type 1 thymidine kinase persisted for 3 months in the pituitary of mice injected once with adenovirus encoding the thymidine kinase (3). Second, IgG class antibodies have relatively long half-lives, up to 8 days depending on the subclass (4,5). Third and even more important, plasma cells persist long term (months rather than weeks) and continue to secrete antibody independently of antigenic stimulation (6,7).
Against this background, we investigated the long-term (up to 20 weeks) persistence of TSHR antibodies in BALB/c mice immunized twice or three times with human A-subunit-adenovirus (A-sub-Ad). While our investigation was in progress, two publications provided information on the same topic (8,9). As will be discussed later, the focus of these two studies differed from each other as well as from the current investigation. In addition to wild-type (WT) mice, our study was performed in transgenic (tg) mice that exhibit self-tolerance to the immunogen because they express the human TSHR A-subunit in the thyroid. Our findings provide insight into the parameters that contribute to the concentration of the antibody and thereby play a critical role in long-term persistence of TSHR antibodies, namely, the degree of self-tolerance to the TSHR and chronic stimulation.
Methods
Mice and TSHR A-sub-Ad immunization
We studied tg mice that express low intrathyroidal levels of the human TSHR A-subunit (Lo-tgs) (10) and WT littermates. Generation and characterization of tg mice with the human TSHR A-subunit targeted to the thyroid has been described (11). Founders were bred to BALB/cJ (Jackson Laboratories, Bar Harbor, ME) and transgene-positive offspring were repeatedly crossed to BALB/cJ. Mice of this line have been cryopreserved by the Mutant Mouse Regional Resource Center (University of California, Davis) and are available under the following designation: C.Cg-Tg(TG-TSHR)51.9Smcl, #014125. In the present studies, Lo-tg mice had been crossed to BALB/c for 15 generations.
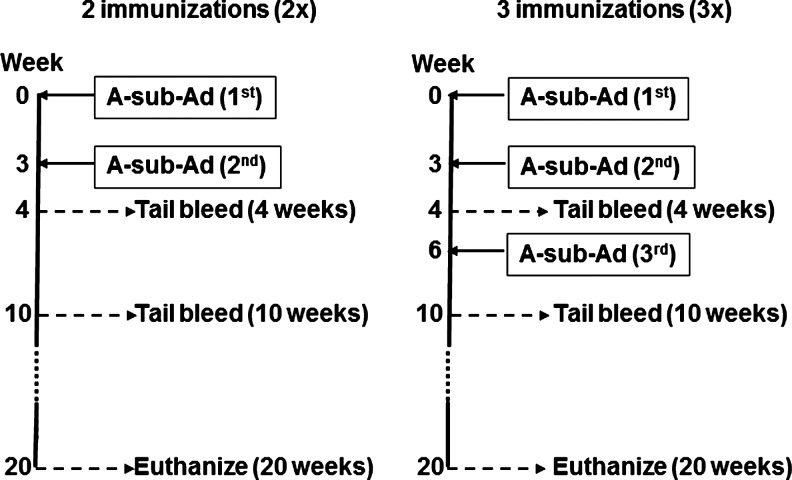
Adenovirus expressing the human TSHR A-subunit (A-subunit-Ad, amino acids 1–289, in the vector pAdHM4) (12) was propagated in HEK293 cells (American Type Culture Collection, Manassas, VA) and purified on CsCl density gradients, and viral particle concentration was determined from the absorbance at 260 nm (13). As illustrated in the schedule (Fig. 1), 6–7-week-old mice received two (2×protocol) or three (3× protocol) injections of A-subunit-Ad (∼1010 particles/injection) at 3-week intervals. Blood was drawn 4, 10, and 20 weeks after the first immunization. Thyroid glands were harvested when mice were euthanized (20 weeks after the first immunization). Animal studies were approved by the Institutional Animal Care and Use Committee at Cedars-Sinai Medical Center and performed with the highest standards of care in a pathogen-free facility.
FIG. 1.
Protocol for immunization with TSHR A-sub-Ad, blood drawing, and euthanasia. WT and A-subunit tg mice (Lo-tg) were immunized twice (2×) or three times (3×) with TSHR A-sub-Ad. Blood was obtained 4, 10, and 20 weeks after the first immunization. TSHR, thyrotropin receptor; A-sub-Ad, A-subunit adenovirus; WT, wild type; tg, transgenic.
TSHR antibody assays
TSHR antibodies were investigated using three assays: thyrotropin (TSH) binding inhibition (TBI), ELISA using TSHR A-subunit protein, and a bioassay for thyroid stimulating antibody (TSAb). TBI and TSAb measure antibody binding to conformationally intact TSHR. In contrast, ELISA detects nonfunctional antibodies. The following protocols were used:
(i) TBI was determined using a commercial kit (Kronus, Boise, ID). Serum aliquots (25 μL) were incubated with detergent-solubilized porcine TSHR; 125I-TSH was added and the TSHR-antibody complexes were precipitated with polyethylene glycol. TBI values were calculated from the formula: [1−(TSH binding in test serum−nonspecific binding)/(TSH binding in normal serum−nonspecific binding)]×100.
(ii) TSHR antibodies (IgG class) were measured by ELISA as previously described (12). Recombinant TSHR A-subunit protein secreted by Chinese Hamster Ovary cells (CHO) with an amplified transgenome (14) was purified from culture supernatants by affinity chromatography (15). ELISA wells were coated with A-subunit protein (5 μg/mL) and incubated with duplicate aliquots of test sera (diluted 1:100). Antibody binding was detected with horseradish peroxidase-conjugated mouse anti-IgG (Sigma Chemical Co., St. Louis, MO) and the signal was developed with o-phenylenediamine and H2O2. Data are reported as the optical density at 490 nm.
(iii) TSAb activity was assayed as reported previously (16), using cells expressing the human TSHR in 96-well plates that were incubated (60 min, 37°C) with test sera diluted 1:20 in Ham's F12 (containing NaCl) supplemented with 10 mM HEPES (pH 7.4) and 1 mM isobutylmethylxanthine. After aspirating the medium, intracellular cyclic adenosine monophosphate (cAMP) was extracted with ethanol, evaporated to dryness, and resuspended in 0.2 mL Dulbecco's phosphate-buffered saline. Aliquots (12 μL) were assayed using the LANCE cAMP kit (PerkinElmer, Boston, MA). TSAb activity was expressed as a percentage of cAMP values attained with sera from control, unimmunized mice.
Serum thyroxine levels and thyroid histology
Total thyroxine (T4) was measured at euthanasia in undiluted mouse serum (25 μL) by radioimmunoassay using a kit (Siemens Health Diagnostics, Tarrytown, NY). T4 values were computed from kit standards and expressed as μg/dL. Thyroids were fixed in buffered formaldehyde (pH 7.4) and paraffin embedded, and serial sections were stained with hematoxylin and eosin (Research Animal Diagnostic Laboratory, University of Missouri, Columbia, MO).
Statistical analyses
Significance of the differences in responses of different groups or at different times was determined by Mann Whitney rank sum test or, when normally distributed, by Student's t test. Multiple comparisons were performed using analysis of variance (ANOVA). Chi-square analysis was used to test differences in the proportions of hyperthyroid versus euthyroid mice. Tests were performed using SigmaStat (Jandel Scientific Software, San Rafael, CA).
Results
TSHR antibody levels over time
TSHR antibody levels were studied 4, 10, and 20 weeks after the first of two immunizations (2×) or three immunizations (3×) with human A-subunit-Ad (Fig. 1). We compared responses in WT mice versus Lo-tg mice that express low intrathyroidal levels of the human TSHR A-subunit (Lo-transgenics) (10) and in the thymus (16). Compared with WT littermates, the Lo-tgs are tolerant to the human A-subunit as shown by their inability to respond to low-dose A-subunit-Ad as well as by lower antibody levels induced by high-dose adenovirus immunization (10,16).
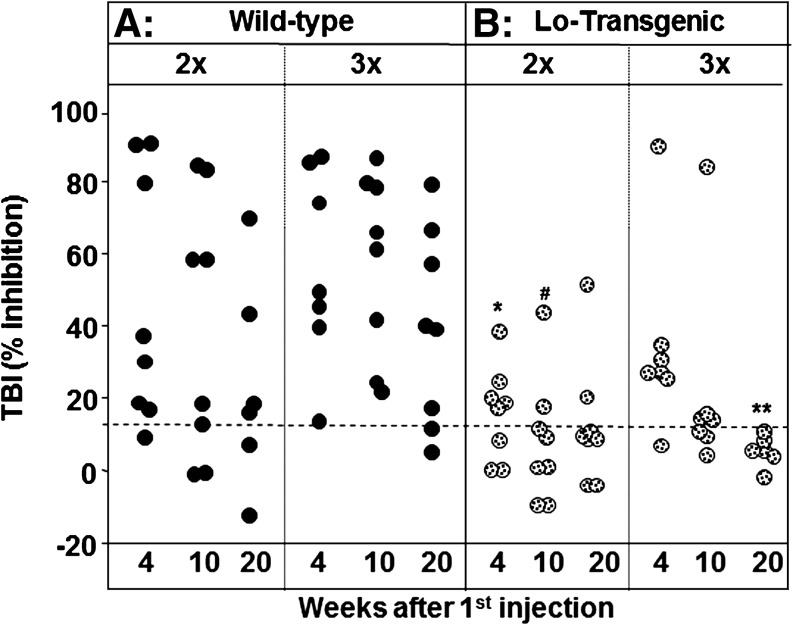
TSHR antibodies were measured using three different assays. First, the antibodies were detected by their ability to inhibit TSH binding to the TSHR (TBI). The great majority of WT mice immunized 3× had high TBI levels even 20 weeks after the first immunization (Fig. 2A). Although TSHR antibody levels were more variable in WT animals that received only two immunizations, some mice in the 2× group had TBI values comparable to those in the 3× group, even at 20 weeks. In contrast, due to self-tolerance, far fewer Lo-tgs were TBI positive compared with WT mice immunized in parallel, particularly in the 2× tg group (Fig. 2B). Among the tgs, the highest TBI values were observed in a mouse injected three times with A-subunit-Ad. Unexpectedly, two Lo-tgs immunized twice, but none immunized three times, remained TBI positive at 20 weeks.
FIG. 2.
TSH binding inhibition (TBI) activity remains detectable 20 weeks after the first immunization in WT (A) and a few tg (B) mice. Mice were immunized twice (2×) or three times (3×) with A-subunit-Ad and sera tested after 4, 10, and 20 weeks. Data for individual mice are shown as black circles for WT mice and as stippled circles for tg mice. Dashed horizontal line: mean+2SD for mice immunized with control adenovirus (16,17). Statistically significant differences: *Tg (2×) 4 weeks versus WT (2×) 4 weeks and WT (3×) 4 weeks, ANOVA, p<0.05; #Tg (2×) 10 weeks versus WT (3×) 10 weeks and WT (3×) 4 weeks, ANOVA, p<0.05; **Tg (3×) 20 weeks versus WT (3×) 20 weeks (Rank sum test, p=0.006). SD, standard deviation; ANOVA, analysis of variance; thyrotropin, TSH.
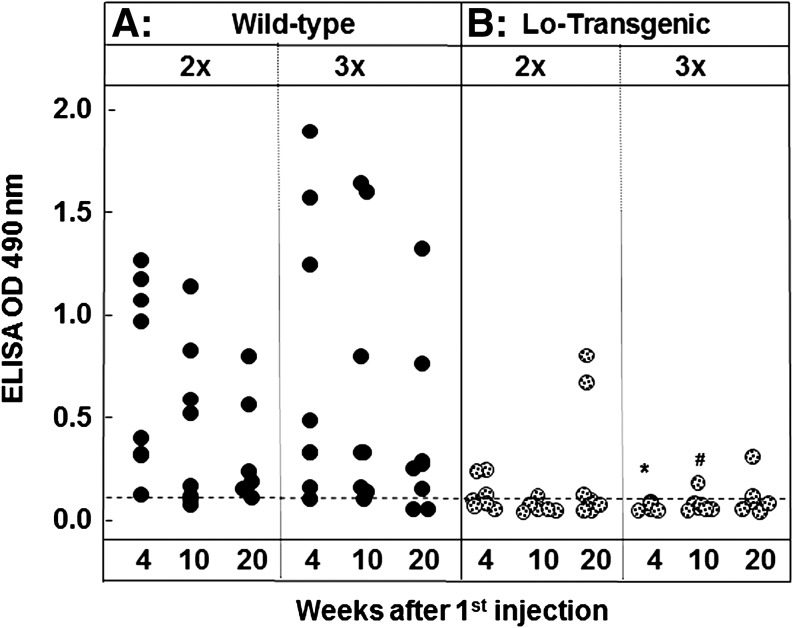
In the second type of TSHR antibody assay, sera from immunized mice were examined for their ability to bind to A-subunit protein-coated ELISA plates. Antibodies detected in this assay are not biologically active (18) but TSHR ELISA antibody levels provide a measure of the strength of the induced immune response. Most WT mice that received two or three adenovirus injections were TSHR ELISA antibody positive at 4, 10, and 20 weeks postimmunization (Fig. 3A). However, the highest TSHR ELISA antibody levels were observed in mice immunized three times. The Lo-tgs developed strikingly lower TSHR ELISA antibody levels than WT littermates but low antibody levels persisted in a few mice even at the 20-week time point (Fig. 3B).
FIG. 3.
TSHR antibody measured by ELISA remains detectable 20 weeks after the first immunization in most WT (A) but in only a few tg (B) mice. Mice were immunized twice (2×) or three times (3×) with A-subunit-Ad and sera tested after 4, 10, and 20 weeks. Data are shown as black circles for individual WT mice and as stippled circles for tg mice. Dashed horizontal line: mean+2SD for mice immunized with control adenovirus (16,17). Statistically significant differences: #lower in tg (3×) than in WT (3×) 10 weeks (ANOVA p<0.05); *lower in tg (3×) 4 weeks than in WT (3×) 10 weeks (ANOVA p<0.05). ELISA, enzyme-linked immunosorbent assay.
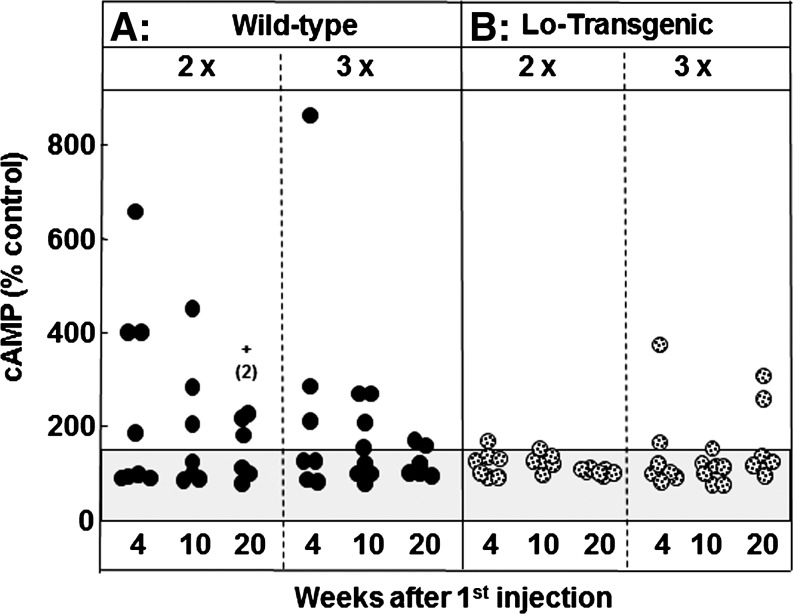
The third assay, TSAb activity, measures the ability of sera to induce cAMP generation by human-TSHR-expressing CHO cells. TSAb activity was positive in ∼50% of WT 2× or WT 3× mice (Fig. 4A), much lower than the proportion of WT mice positive for TBI or ELISA (Figs. 2, 3). It should be noted that two WT mice, with strong positive TSAb after the second immunization, died before the end of the study, likely because of hyperthyroidism as observed by others and ourselves (19,20). In contrast, the mouse with the highest TSAb survived, possibly because the initially high TSAb level fell over the subsequent weeks. It should be emphasized that TSAb levels in all WT mice were low or border-line positive at euthanasia (20 weeks).
FIG. 4.
Low levels of TSAb activity are detectable 20 weeks after the initial immunization in the majority of WT mice (A) but in only a few tg mice (B). Mice were immunized twice (2×) or three times (3×) with A-subunit-Ad and sera tested after 4, 10, and 20 weeks. TSAb activity was measured in terms of cAMP generation and expressed as a percentage of the activity induced by sera from control mice (% control; see the Methods section). Data are shown as black circles for individual WT mice and as stippled circles for tg mice. Dashed horizontal line: mean+2SD for mice immunized with control adenovirus (16,17). Statistically significant differences: TSAb, thyroid stimulating antibody; cAMP, cyclic adenosine monophosphate.
TSAb activity in Lo-tg mice was barely detectable after two A-subunit-Ad immunizations and was present at very low levels in only a few mice immunized three times (Fig. 4B). In previous studies, we observed that despite TSAb activity measured using human-TSHR-expressing cells, these antibodies were unable to stimulate the thyroid gland in Lo-tg mice (16).
Thyroid function and thyroid histology
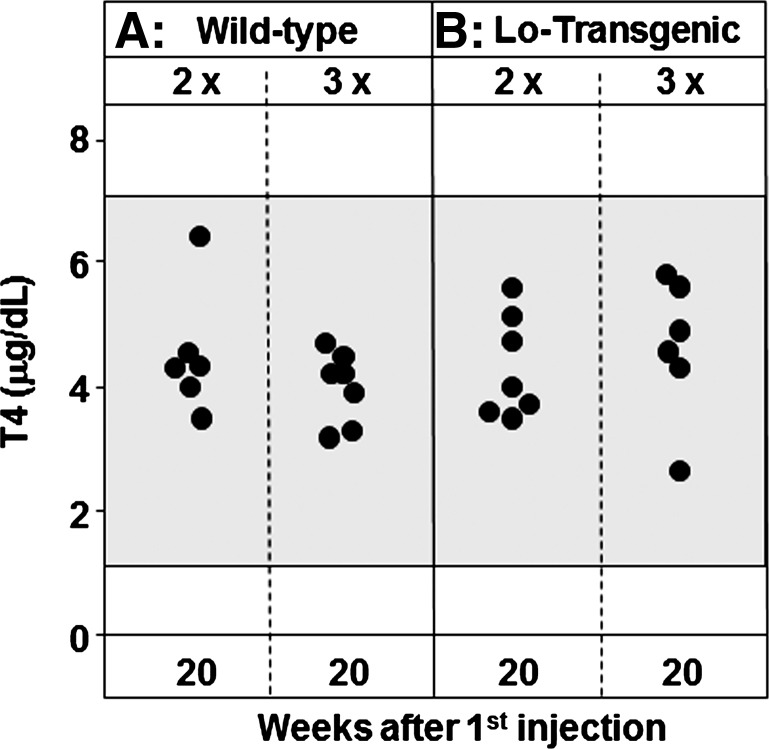
Sera were tested for total T4 and thyroid histology at euthanasia, 20 weeks after the first immunization. All T4 levels were within the range previously observed for WT and tg mice immunized with control adenovirus (Fig. 5A). The lack of elevated T4 levels was consistent with thyroid histology that showed no evidence of thyrocyte hyperplasia. Thyroid lymphocytic infiltrates were absent from both WT and tg mice.
FIG. 5.
Thyroxine (T4) levels at euthanasia, 20 weeks after the initial immunization. The shaded area represents the normal range for WT (A) and tg (B) mice immunized with control adenovirus measured 4 weeks after the third immunization (17); this time interval corresponds to 10 weeks in the present study.
Insufficient sera were available for testing 4 and 10 weeks after immunization, the time intervals when we (and others) normally observe hyperthyroidism in WT BALB/c. However, compiling the findings in six separate studies (11,12,16,21–23), the proportion of hyperthyroid mice was significantly greater at the 4-week time point than after 10 weeks, namely, 70% hyperthyroid decreasing to 48% hyperthyroid (55/79 vs. 41/85 hyperthyroid; p=0.009, χ2 test). The lack of hyperthyroidism at the 20-week time point (Fig. 5) is therefore consistent with the downward trend associated with the immunization and blood sampling protocol previously used in the adenovirus model of Graves'-like disease.
Discussion
We investigated the long-term persistence of TSHR antibodies in WT and human TSHR A-subunit tg BALB/c mice immunized twice or three times with human TSHR A-sub-Ad. The Nagayama approach for induced Graves' disease has been used extensively to study the role of cytokines and genetic susceptibility [reviewed in Nagayama (1)] as well as to test novel therapies to prevent or treat induced disease [e.g., studies by Misharin et al. (18), Nagayama et al. (24), Gilbert et al. (25), and Ueki et al. (26)].
At euthanasia, 5 months (20 weeks) after the first immunization, virtually all WT mice retained high TSHR antibody levels measured by TBI or binding to TSHR protein-coated ELISA plates. However, at all time points, and particularly at euthanasia, only about 50% of mice were positive for TSAb activity. Lo-tg mice that express the human TSHR A-subunit in the thyroid and thymus exhibit self-tolerance. In accordance with previous studies (10,16), fewer mice were positive for TBI or ELISA and the levels were lower than in WT littermates. Nevertheless, at the final time point studied, some Lo-tg mice were positive for TSHR antibodies measured by TBI or ELISA. In WT mice, antibody persistence was similar in animals that received two or three A-subunit-Ad injections. However, only tgs immunized three times had detectable TSAb (although low) at the final time point.
No mice examined at euthanasia (20 weeks after immunization) were hyperthyroid and all had normal thyroid histology. In previous studies, we observed hyperthyroidism in BALB/c mice tested 4 and 10 weeks after the first adenovirus injection (11,12,21–23). However, the proportion of hyperthyroid mice was significantly lower at 10 weeks than at 4 weeks (48% vs. 70%). The absence of hyperthyroidism at 20 weeks (present study) continues the downward trend, consistent with very low or undetectable TSAb levels at this time point.
The long-term presence of TSHR antibodies measured by TBI in our study resembles the observations of Zhao et al. (8). TBI levels were higher in the latter study, possibly because their assay used human TSHR whereas the kit we used employed porcine TSHR. On the other hand, in both studies, TSAb activity was measured using cells expressing the human TSHR. The higher TSAb levels, together with the persistent hyperthyroidism observed by Zhao et al. (8), suggest that DNA vaccination plus electroporation is more effective than adenovirus immunization for inducing TSHR antibodies with the long-term potential to stimulate the thyroid gland. The reason for this difference is not known. However, it is possible that the greater frequency of DNA vaccination plus electroporation (on six occasions) is more effective than three injections of adenovirus.
A second long-term study differed from that of Zhao et al. (8) and our investigation in that TSHR knockout mice were immunized with adenovirus expressing the mouse TSHR (9). The reason for this protocol was that, unlike Lo-tgs that respond to high-dose human A-sub-Ad, tolerance to the mouse TSHR A-subunit cannot be broken using adenovirus in WT mice (27). Nakahara et al. (9) measured persistence of TSHR antibodies in the knockout mice, as well as in athymic nude mice following adoptive transfer of splenocytes from immunized knockout mice. In these elegant studies, TSHR antibodies (measured by flow cytometry) remained detectable for 24 weeks after the second immunization in the knockouts and in the recipients of transferred splenocytes. Unexpectedly, TSAb activity detected 4–8 weeks after the second immunization in the knockouts was replaced by TSH blocking activity (TBAb) at the 24-week time interval. Moreover, in the recipients of transferred splenocytes, initial TSAb activity followed by TBAb activity was associated with a change from hyperthyroidism to hypothyroidism (9).
It should be emphasized that, unlike mice immunized with the mouse TSHR (9), long-term studies in mice immunized with the human TSHR revealed hyperthyroidism in some mice and euthyroidism in the others (8). In previous short-term investigations, we detected both TSAb and TBAb activity in immunized BALB/c (or C57BL/6) mice (21). Moreover, as already noted, significantly fewer mice were hyperthyroid at the 10-week time interval compared with the 4-week time interval (23). We found no shift to hypothyroidism previously or in our current long-term study. However, shifts from TSAb to TBAb (or vice versa) in association with thyroid function changes have been observed in some humans [e.g., studies by Zakarija et al. (28), Tamai et al. (29), Kraiem et al. (30), Evans et al. (31)].
A question we addressed was whether the presence of TSHR antibodies over a long time period would lead to thyroiditis, as reported for administration of a monoclonal mouse TSAb (32). The thyroid glands in our WT and tg mice lacked lymphocytic infiltrates, in agreement with short-term studies by others and ourselves [reviewed in Nagayama (1)]. In contrast, thyroid lymphocytic infiltrates were observed in some mice in the other two long-term studies (8,9).
TSHR antibody persistence for many weeks, together with variability between individual mice, complicates interpreting the outcome of novel therapies to treat mice with induced Graves' disease. Several approaches reduced disease development, including immune deviation away from T helper 1 toward T helper 2 type responses using cytokines (33,34) or Schistosoma infection (24), injecting A-subunit protein before immunization (18), and injecting anti-CD20 to eliminate B cells (26). None of these approaches could treat animals with on-going hyperthyroidism. In retrospect, the failure to reduce TSHR antibody levels in mice with Graves' disease could have been anticipated because the animals were tested at times when long-term persistence of TSHR antibodies has been demonstrated (8,9; present study). In contrast, hyperthyroidism was reduced in mice with Graves' disease by targeting B lymphocyte proliferation or survival factors using decoy molecules of tumor-necrosis-family ligand inhibitors (25).
It is of interest to compare our observations with those involving thyroglobulin (Tg) as immunogen. Mice immunized conventionally on two or three occasions with Tg protein and adjuvant, or with Tg-boosted splenocytes from Tg-primed donors, are euthanized after short time intervals, 6–8 weeks for granulomatous thyroiditis in DBA/1 mice [e.g., report by Braley-Mullen and Sharp (35)] and 2–3 weeks for classical experimental autoimmune thyroiditis in C3H/HeN mice (36). In contrast, immunization with Tg cDNA plus electroporation requires multiple weekly immunizations (three or four) and mice are euthanized 2–3 weeks after the final boost for antibody analyses and after 2–3 months for splenocyte-based assays (36). These time intervals for Tg cDNA vaccination correspond to a maximum of 16 weeks for splenocyte recall assays (in vitro restimulation with Tg) but only 7 weeks for antibody assays. The length of time for which antibodies persist after 20 weeks (as in studies of TSHR antibodies) was not reported.
Finally, it is useful to consider changes in thyroid autoantibody levels before and after treatment in humans. Serum concentrations of TSHR antibodies are low (15,37), much lower than those of autoantibodies to thyroid peroxidase or Tg (38,39). These differences reflect the natural history of the two diseases: Hashimoto's thyroiditis leads to overt hypothyroidism after many years of smoldering disease (reflecting chronic immune responses) whereas the potency of TSAb ensures that Graves' disease is clinically evident early in the immune process [reviewed by McLachlan and Rapoport (40) and Affraimidis and colleagues (41)]. Thyroidectomy removes autoantigen as well as the cells responsible for thyroid autoantibody secretion, although other lymphoid sites may be involved [e.g., studies by McLachlan and colleagues (42,43)]. Clearly, it is relatively simple to eliminate the precursors responsible for a small amount of antibody. Not surprisingly, TSHR antibody levels returned to normal 1.5 years after thyroidectomy to treat Graves' disease (44). In contrast, autoantibodies to Tg or thyroid peroxidase only disappeared 18 years after treating thyroid cancer patients with surgery and 131I remnant ablation (45).
The above findings in humans, demonstrating differences in concentration and antibody persistence to three thyroid autoantigens, provide parallels for the long-term persistence of induced murine TSHR antibodies. Thus, murine TSHR antibodies present in high concentrations (as measured by TBI or ELISA) are detectable longer than those detected as TSAb activity, particularly the rare antibodies cross-reactive with the mouse TSHR.
In conclusion, our observations for induced antibodies in mice, together with findings for thyroid autoantibodies in humans, provide insight into the parameters that play a critical role in determining the concentration of the long-term antibody responses to the TSHR. Thus, TSHR antibody persistence involves the degree of self-tolerance to the autoantigen and chronic immune stimulation.
Acknowledgments
This work was supported by National Institutes of Health Grants DK54684 (S.M.M.) and DK19289 (B.R). The authors are also grateful for contributions by Dr. Boris Catz, Los Angeles, CA.
Disclosure Statement
No competing financial interests exist.
References
- 1.Nagayama Y. Graves' animal models of Graves' hyperthyroidism. Thyroid. 2007;17:981–988. doi: 10.1089/thy.2007.0161. [DOI] [PubMed] [Google Scholar]
- 2.Nagayama Y. Kita-Furuyama M. Ando T. Nakao K. Mizuguchi H. Hayakawa T. Eguchi K. Niwa M. A novel murine model of Graves' hyperthyroidism with intramuscular injection of adenovirus expressing the thyrotropin receptor. J Immunol. 2002;168:2789–2794. doi: 10.4049/jimmunol.168.6.2789. [DOI] [PubMed] [Google Scholar]
- 3.Southgate TD. Stone D. Williams JC. Lowenstein PR. Castro MG. Long-term transgene expression within the anterior pituitary gland in situ: impact on circulating hormone levels, cellular and antibody-mediated immune responses. Endocrinology. 2001;142:464–476. doi: 10.1210/endo.142.1.7898. [DOI] [PubMed] [Google Scholar]
- 4.Fahey JL. Sells S. The immunoglobulins of mice.V. The metabolic (catabolic) properties of five immunoglobulin classes. J Exp Med. 1965;122:418–458. doi: 10.1084/jem.122.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vieira P. Rajewsky K. The bulk of endogenously produced IgG2a is eliminated from the serum of adult C57BL/6 mice with a half-life of 6–8 days. Eur J Immunol. 1986;16:871–874. doi: 10.1002/eji.1830160727. [DOI] [PubMed] [Google Scholar]
- 6.Slifka MK. Antia R. Whitmire JK. Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998;8:363–372. doi: 10.1016/s1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- 7.Manz RA. Lohning M. Cassese G. Thiel A. Radbruch A. Survival of long-lived plasma cells is independent of antigen. Int Immunol. 1998;10:1703–1711. doi: 10.1093/intimm/10.11.1703. [DOI] [PubMed] [Google Scholar]
- 8.Zhao SX. Tsui S. Cheung A. Douglas RS. Smith TJ. Banga JP. Orbital fibrosis in a mouse model of Graves' disease induced by genetic immunization of thyrotropin receptor cDNA. J Endocrinol. 2011;210:369–377. doi: 10.1530/JOE-11-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakahara M. Johnson K. Eckstein A. Taguchi R. Yamada M. Abiru N. Nagayama Y. Adoptive transfer of anti-thyrotropin receptor (TSHR) autoimmunity from TSHR knockout mice to athymic nude mice. Endocrinology. 2012;153:2034–2042. doi: 10.1210/en.2011-1846. [DOI] [PubMed] [Google Scholar]
- 10.McLachlan SM. Nagayama Y. Pichurin PN. Mizutori Y. Chen CR. Misharin A. Aliesky HA. Rapoport B. The link between Graves' disease and Hashimoto's thyroiditis: a role for regulatory T cells. Endocrinology. 2007;148:5724–5733. doi: 10.1210/en.2007-1024. [DOI] [PubMed] [Google Scholar]
- 11.Pichurin PN. Chen C-R. Chazenbalk GD. Aliesky H. Pham N. Rapoport B. McLachlan SM. Targeted expression of the human thyrotropin receptor A-subunit to the mouse thyroid: insight into overcoming the lack of response to A-subunit adenovirus immunization. J Immunol. 2006;176:668–676. doi: 10.4049/jimmunol.176.1.668. [DOI] [PubMed] [Google Scholar]
- 12.Chen C-R. Pichurin P. Nagayama Y. Latrofa F. Rapoport B. McLachlan SM. The thyrotropin receptor autoantigen in Graves' disease is the culprit as well as the victim. J Clin Invest. 2003;111:1897–1904. doi: 10.1172/JCI17069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mittereder N. March KL. Trapnell BC. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J Virol. 1996;70:7498–7509. doi: 10.1128/jvi.70.11.7498-7509.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chazenbalk GD. Jaume JC. McLachlan SM. Rapoport B. Engineering the human thyrotropin receptor ectodomain from a non-secreted form to a secreted, highly immunoreactive glycoprotein that neutralizes autoantibodies in Graves' patients' sera. J Biol Chem. 1997;272:18959–18965. doi: 10.1074/jbc.272.30.18959. [DOI] [PubMed] [Google Scholar]
- 15.Chazenbalk GD. Wang Y. Guo J. Hutchison JS. Segal D. Jaume JC. McLachlan SM. Rapoport B. A mouse monoclonal antibody to a thyrotropin receptor ectodomain variant provides insight into the exquisite antigenic conformational requirement, epitopes and in vivo concentration of human autoantibodies. J Clin Endocrinol Metab. 1999;84:702–710. doi: 10.1210/jcem.84.2.5481. [DOI] [PubMed] [Google Scholar]
- 16.Misharin A. Aliesky H. Nagayama Y. Rapoport B. McLachlan SM. Studies in mice deficient for the autoimmune regulator and transgenic for the thyrotropin receptor reveal a role for the Autoimmune Regulator in tolerance for thyroid autoantigens. Endocrinology. 2009;150:2948–2956. doi: 10.1210/en.2008-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mizutori Y. Nagayama Y. Flower D. Misharin A. Aliesky HA. Rapoport B. McLachlan SM. Role of the transgenic human thyrotropin receptor A-subunit in thyroiditis induced by A-subunit immunization and regulatory T cell depletion. Clin Exp Immunol. 2008;154:305–315. doi: 10.1111/j.1365-2249.2008.03769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Misharin A. Nagayama Y. Aliesky H. Mizutori Y. Rapoport B. McLachlan SM. Attenuation of induced hyperthyroidism in mice by pre-treatment with thyrotropin receptor protein: diversion of thyroid stimulating antibody to non-functional antibody induction. Endocrinology. 2009;150:3944–3952. doi: 10.1210/en.2009-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kita M. Ahmad R. Marians R. Graves P. Davies TF. Thyrotoxic death in mice with Graves' disease. Thyroid. 1997;7 SC#2 (Abstract). [Google Scholar]
- 20.Chen C-R. Pichurin P. Chazenbalk GD. Aliesky H. Nagayama Y. McLachlan SM. Rapoport B. Low-dose immunization with adenovirus expressing the thyroid-stimulating hormone receptor A-subunit deviates the antibody response toward that of autoantibodies in human Graves' disease. Endocrinology. 2004;145:228–233. doi: 10.1210/en.2003-1134. [DOI] [PubMed] [Google Scholar]
- 21.Chen CR. Aliesky H. Pichurin PN. Nagayama Y. McLachlan SM. Rapoport B. Susceptibility rather than resistance to hyperthyroidism is dominant in a thyrotropin receptor adenovirus-induced animal model of Graves' disease as revealed by BALB/c-C57BL/6 hybrid mice. Endocrinology. 2004;145:4927–4933. doi: 10.1210/en.2004-0716. [DOI] [PubMed] [Google Scholar]
- 22.Aliesky HA. Pichurin PN. Chen CR. Williams RW. Rapoport B. McLachlan SM. Probing the genetic basis for thyrotropin receptor antibodies and hyperthyroidism in immunized CXB recombinant inbred mice. Endocrinology. 2006;147:2789–2800. doi: 10.1210/en.2006-0160. [DOI] [PubMed] [Google Scholar]
- 23.Misharin A. Hewison M. Chen C-R. Lagishetty V. Aliesky HA. Mizutori Y. Rapoport B. McLachlan SM. Vitamin D deficiency modulates Graves' hyperthyroidism induced in BALB/c mice by thyrotropin receptor immunization. Endocrinology. 2009;150:1051–1060. doi: 10.1210/en.2008-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagayama Y. Watanabe K. Niwa M. McLachlan SM. Rapoport B. Schistosoma mansoni and alpha-galactosylceramide: prophylactic effect of Th1 Immune suppression in a mouse model of Graves' hyperthyroidism. J Immunol. 2004;173:2167–2173. doi: 10.4049/jimmunol.173.3.2167. [DOI] [PubMed] [Google Scholar]
- 25.Gilbert JA. Kalled SL. Moorhead J. Hess DM. Rennert P. Li Z. Khan MZ. Banga JP. Treatment of autoimmune hyperthyroidism in a murine model of Graves' disease with TNF-family ligand inhibitors suggests a key role for BAFF in disease pathology. Endocrinology. 2006;147:4561–4568. doi: 10.1210/en.2006-0507. [DOI] [PubMed] [Google Scholar]
- 26.Ueki I. Abiru N. Kobayashi M. Nakahara M. Ichikawa T. Eguchi K. Nagayama Y. B cell-targeted therapy with anti-CD20 monoclonal antibody in a mouse model of Graves' hyperthyroidism. Clin Exp Immunol. 2011;163:309–317. doi: 10.1111/j.1365-2249.2010.04301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakahara M. Mitsutake N. Sakamoto H. Chen CR. Rapoport B. McLachlan SM. Nagayama Y. Enhanced response to mouse thyroid-stimulating hormone (TSH) receptor immunization in TSH receptor-knockout mice. Endocrinology. 2010;151:4047–4054. doi: 10.1210/en.2010-0315. [DOI] [PubMed] [Google Scholar]
- 28.Zakarija M. McKenzie JM. Munro DS. Immunoglobulin G inhibitor of thyroid-stimulating antibody is a cause of delay in the onset of neonatal Graves' disease. J Clin Invest. 1983;72:1352–1356. doi: 10.1172/JCI111091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tamai H. Kasagi K. Takaichi Y. Takamatsu J. Komaki G. Matsubayashi S. Konishi J. Kuma K. Kumagai LF. Nagataki S. Development of spontaneous hypothyroidism in patients with Graves' disease treated with antithyroidal drugs: clinical, immunological, and histological findings in 26 patients. J Clin Endocrinol Metab. 1989;69:49–53. doi: 10.1210/jcem-69-1-49. [DOI] [PubMed] [Google Scholar]
- 30.Kraiem Z. Baron E. Kahana L. Sadeh O. Sheinfeld M. Changes in stimulating and blocking TSH receptor antibodies in a patient undergoing three cycles of transition from hypo to hyper-thyroidism and back to hypothyroidism. Clin Endocrinol (Oxf ) 1992;36:211–214. doi: 10.1111/j.1365-2265.1992.tb00960.x. [DOI] [PubMed] [Google Scholar]
- 31.Evans M. Sanders J. Tagami T. Sanders P. Young S. Roberts E. Wilmot J. Hu X. Kabelis K. Clark J. Holl S. Richards T. Collyer A. Furmaniak J. Smith BR. Monoclonal autoantibodies to the TSH receptor, one with stimulating activity and one with blocking activity, obtained from the same blood sample. Clin Endocrinol (Oxf ) 2010;73:404–412. doi: 10.1111/j.1365-2265.2010.03831.x. [DOI] [PubMed] [Google Scholar]
- 32.Costagliola S. Bonomi M. Morgenthaler NG. van Durme J. Panneels V. Refetoff S. Vassart G. Delineation of the discontinuous-conformational epitope of a monoclonal antibody displaying full in vitro and in vivo thyrotropin activity. Mol Endocrinol. 2004;18:3020–3024. doi: 10.1210/me.2004-0231. [DOI] [PubMed] [Google Scholar]
- 33.Nagayama Y. Mizuguchi H. Hayakawa T. Niwa M. McLachlan SM. Rapoport B. Prevention of autoantibody-mediated Graves'-like hyperthyroidism in mice with IL-4, a Th2 cytokine. J Immunol. 2003;170:3522–3527. doi: 10.4049/jimmunol.170.7.3522. [DOI] [PubMed] [Google Scholar]
- 34.Saitoh O. Mizutori Y. Takamura N. Yamasaki H. Kita A. Kuwahara H. Nagayama Y. Adenovirus-mediated gene delivery of interleukin-10, but not transforming growth factor beta, ameliorates the induction of Graves' hyperthyroidism in BALB/c mice. Clin Exp Immunol. 2005;141:405–411. doi: 10.1111/j.1365-2249.2005.02874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braley-Mullen H. Sharp GC. Adoptive transfer murine model of granulomatous experimental autoimmune thyroiditis. Int Rev Immunol. 2000;19:535–555. doi: 10.3109/08830180009088511. [DOI] [PubMed] [Google Scholar]
- 36.Jacobson EM. Concepcion E. Ho K. Kopp P. Vono TJ. Tomer Y. cDNA immunization of mice with human thyroglobulin generates both humoral and T cell responses: a novel model of thyroid autoimmunity. PLoS One. 2011;6:e19200. doi: 10.1371/journal.pone.0019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Forteza R. Smith CU. Amin J. McKenzie JM. Zakarija M. Visualization of the thyrotropin receptor on the cell surface by potent autoantibodies. J Clin Endocrinol Metab. 1994;78:1271–1273. doi: 10.1210/jcem.78.5.7909819. [DOI] [PubMed] [Google Scholar]
- 38.Beever K. Bradbury J. Phillips D. McLachlan SM. Pegg C. Goral A. Overbeck W. Feifel G. Rees Smith B. Highly sensitive assays of autoantibodies to thyroglobulin and to thyroid peroxidase. Clin Chem. 1989;35:1949–1954. [PubMed] [Google Scholar]
- 39.Jaume JC. Kakinuma A. Chazenbalk GD. Rapoport B. McLachlan SM. TSH receptor autoantibodies in serum are present at much lower concentrations than thyroid peroxidase autoantibodies: analysis by flow cytometry. J Clin Endocrinol Metab. 1997;82:500–507. doi: 10.1210/jcem.82.2.3740. [DOI] [PubMed] [Google Scholar]
- 40.McLachlan SM. Rapoport B. Recombinant thyroid autoantigens: the keys to the pathogenesis of autoimmune thyroid disease. J Int Med. 1993;234:347–359. doi: 10.1111/j.1365-2796.1993.tb00756.x. [DOI] [PubMed] [Google Scholar]
- 41.Effraimidis G. Strieder TG. Tijssen JG. Wiersinga WM. Natural history of the transition from euthyroidism to overt autoimmune hypo- or hyperthyroidism: a prospective study. Eur J Endocrinol. 2011;164:107–113. doi: 10.1530/EJE-10-0785. [DOI] [PubMed] [Google Scholar]
- 42.McLachlan SM. Pegg CAS. Atherton MC. Middleton SM. Clark F. Rees Smith B. TSH receptor antibody synthesis by thyroid lymphocytes. Clin Endocrinol. 1986;24:223–230. doi: 10.1111/j.1365-2265.1986.tb00766.x. [DOI] [PubMed] [Google Scholar]
- 43.McLachlan SM. McGregor A. Rees Smith B. Hall R. Thyroid-autoantibody synthesis by Hashimoto thyroid lymphocytes. Lancet. 1979;1:162–163. doi: 10.1016/s0140-6736(79)90559-2. [DOI] [PubMed] [Google Scholar]
- 44.Laurberg P. Wallin G. Tallstedt L. Abraham-Nordling M. Lundell G. Torring O. TSH-receptor autoimmunity in Graves' disease after therapy with anti-thyroid drugs, surgery, or radioiodine: a 5-year prospective randomized study. Eur J Endocrinol. 2008;158:69–75. doi: 10.1530/EJE-07-0450. [DOI] [PubMed] [Google Scholar]
- 45.Chiovato L. Latrofa F. Braverman LE. Pacini F. Capezzone M. Masserini L. Grasso L. Pinchera A. Disappearance of humoral thyroid autoimmunity after complete removal of thyroid antigens. Ann Intern Med. 2003;139:346–351. doi: 10.7326/0003-4819-139-5_part_1-200309020-00010. [DOI] [PubMed] [Google Scholar]