Abstract
Cardiac involvement has been reported in as many as 45–55% of patients with human immunodeficiency virus (HIV) infection and acquired immune deficiency syndrome (AIDS), and significant cardiac morbidity is reported in 6–7% of HIV patients. We investigated the inhibitory effects of isothiocyanates (ITCs) on heart dysfunction and mortality by regulating apoptosis in the left ventricle of the heart in a murine AIDS model. Mice were divided into six groups: an uninfected group, an untreated LP-BM5 retrovirus-infected group, and four LP-BM5 retrovirus-infected groups treated with one of four ITCs (sulforaphane [SUL], indolo[3,2-b]carbazole, benzyl isothiocyanate [BITC], or phenethyl isothiocyanate [PEITC]). After 16 weeks, the median survival time of the LP-BM5 retrovirus-infected mice was 87 days, whereas that of the uninfected control group and all ITC treatment groups was over 112 days. SUL, PEITC, and BITC significantly inhibited apoptosis in the left ventricle by increasing the Bcl-2/Bax ratio compared with LP-BM5-infected mice. In addition, SUL and PEITC suppressed inducible nitric oxide synthase (iNOS) expression at both the mRNA and protein levels in the left ventricle of heart tissue infected with the LP-BM5 retrovirus by inactivating cytoplasmic nuclear factor κB (NF-κB). In conclusion, LP-BM5 retrovirus infection was related to survival of murine AIDS mice, and NF-κB-mediated iNOS expression may be an important mediator of left ventricle dysfunction of the heart. Furthermore, certain ITCs may have the potential to improve AIDS-related heart dysfunction due to their inhibition of apoptosis by decreasing iNOS and Bax expression through suppression of NF-κB.
Key Words: apoptosis, inducible nitric oxide synthase, isothiocyanates, murine AIDS
Introduction
Human immunodeficiency virus (HIV) infection is the fourth leading cause of death worldwide.1 An estimated 2.5 million new HIV infections and 2.1 million acquired immune deficiency syndrome (AIDS) deaths occurred in 2007, according to the United Nations Joint Program on HIV/AIDS, and almost 33.2 million adults and children worldwide are currently living with HIV/AIDS.2,3 With >7000 new infections each day, the HIV pandemic remains an important public health issue.4
HIV infection and AIDS have a well-recognized association with myocarditis and dilated cardiomyopathy.5 In particular, HIV-related heart muscle disease can present as a dilated cardiomyopathy, as isolated left ventricular dysfunction, or as nonspecific right heart changes.6 Cardiac involvement has been reported in as many as 45–55% of patients with HIV infection and AIDS, and significant cardiac morbidity from HIV disease is estimated to be 6–7% and mortality is estimated to be 1–6%.7,8 Dilated cardiomyopathy, myocarditis, endocarditis, pericardial disease, and pulmonary hypertension remain major cardiac manifestations of HIV disease.7,9
Two classes of the most abundant naturally occurring dietary chemopreventive compounds are polyphenolic and isothiocyanate (ITC)-containing compounds, which have completely different chemical structures. Polyphenolic compounds are characterized by phenolic functional groups, whereas ITC-containing compounds are characterized by the sulfur-containing N=C=S functional group.10 ITCs are synthesized and stored in cruciferous vegetables as glucosinolates. Allyl ITC from cabbage, mustard, and horseradish, benzyl ITC (BITC), phenethyl ITC (PEITC) from watercress and garden cress, and sulforaphane (SUL) from broccoli, cauliflower, brassicas, and kale are commonly used ITC-containing compounds.10,11 Their biological activities are strongly related to modulation of cellular redox status, and numerous studies have documented their antioxidant properties. Furthermore, they have also been shown to inhibit cancer growth and metastasis in animal carcinogenesis models as well as cell culture models and to induce the apoptosis of cancer cells.11,12
For the past two decades, most therapeutic research in the field of HIV/AIDS has focused on the development of vaccines and antiretrovirals to block specific steps in the virus life cycle. Antiretroviral therapy has been extremely effective at controlling viral replication in HIV-infected individuals.4 Highly active antiretroviral therapy is currently widely used, which may have resulted in the changes in the prevalence of antiretroviral drug resistance in drug-naive patients.13,14
Various natural products without any experimental evidence of anti-HIV activities, such as garlic, ginseng, and shiitake mushrooms, are being used by AIDS patients in some countries.15,16 Therefore, in this study, we investigated the inhibitory effects of indolo[3,2-b]carbazole (ICZ), BITC, PEITC, and SUL from cruciferous vegetables on apoptosis induced by overexpression of inducible nitric oxide (NO) synthase (iNOS) and induction of Bax in the heart left ventricle of a murine AIDS (MAIDS) model. The present study is the first to demonstrate that ITCs have inhibitory effects on AIDS-related heart dysfunction.
Materials and Methods
Animals and treatment
Female C57BL/6 mice, 4 weeks old, were obtained from Charles River Laboratories (Wilmington, DE, USA). They were housed in transparent plastic cages with stainless wire lids (three or four mice per cage) in the animal facility of Kyung Hee University (Yongin, Korea). Our animal use protocol was approved by the Institutional Animal Care and Use Committee of Kyung Hee University. The housing facility was maintained at 20–22°C and 60–80% relative humidity with a 12-h light/dark cycle. For the study, mice were randomly assigned to the uninfected control group or infected groups that received various ICTs by oral administration. Oral administration of treatments began at 2 days postinfection with the LP-BM5 murine leukemia retrovirus and continued for 16 weeks for a survival test (24 mice per group) and 12 weeks for immunological analysis (10 mice per group). All mice were fed the AIN-93M maintenance diet during the experiments.
ITCs including SUL, BITC, ICZ, and PEITC were purchased from Sigma (St. Louis, MO, USA). Female C57BL/6 mice were administered one of the ITCs or vehicle once daily by oral gavage at a dose of 1 mM/kg in 100 μL of ethanol per day. A dose of 1 mM/kg was chosen as this dose has been shown to be effective against several types of cancer in a previous study.17 LP-BM5 murine leukemia retrovirus (generously donated from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA) was administered intraperitoneally in 0.1 mL of minimum essential medium with an esotropic titer of 4.5 log10 plaque-forming units×10−3/L; this titer induces disease with a time course comparable with that previously published.18 Infection of female C57BL/6 mice with LP-BM5 retrovirus results in the rapid induction of clinical symptoms with virtually no latent phase. The mice were infected 2 days prior to initiation of oral administration of ITCs. The infection and treatment period for immunological analysis was 12 weeks for all groups. When MAIDS developed, all mice were sacrificed by ethyl ether anesthesia. Hearts were rapidly excised, and the left ventricle was separated for TdT-mediated dUTP nick-end labeling (TUNEL) assay and histological analysis. Heart left ventricle tissue for real-time polymerase chain reaction (PCR) and western blot was frozen in liquid nitrogen and stored at −70°C until analysis.
Survival test
Female C57BL/6 mice, 4 weeks old, were randomly assigned to one of six groups (24 mice per group) and fed one of the ITCs or vehicle for 16 weeks to assess the effects of ITCs intake on survival. Mice were monitored daily for survival and conditions, while their drinking water and AIN-93M diet were changed every 3 days. Median survival time is expressed as the number of days until 50% of mice in the group had died.
TUNEL assay
The degree of apoptosis was evaluated by the TUNEL method. Paraffin blocks containing left ventricle tissues were cut in 6-μm-thick sections, and the sections were stained for the detection of apoptosis using the TUNEL apoptosis detection kit (GenScript, Piscataway, NJ, USA). In brief, the sections were deparaffinized and rehydrated, incubated with blocking solution (3% H2O2 in methanol) for 10 min, and permeabilized in a solution containing 0.1% Triton X-100 and 0.1% sodium citrate in water. A TUNEL reaction mixture containing fluorescein ITC-12-dUTP was applied to the fixed cells, and apoptotic cells were determined under a fluorescence microscope with an excitation wavelength of 450 nm and an emission wavelength of 565 nm.
Real-time PCR
Heart left ventricle tissue was disrupted and homogenized with rotor-stator homogenizers in the presence of RLT buffer (lysis buffer) (Qiagen, Valencia, CA, USA) with β-mercaptoethanol until a completely homogeneous lysate was obtained. Total RNA was isolated from left ventricle tissue lysate using an RNeasy® Mini kit (Qiagen). One microgram of total RNA obtained from the left ventricle tissues was reverse-transcribed using reverse-transcript II reverse transcriptase (Invitrogen, Carlsbad, CA, USA) to produce cDNAs. Real-time PCR was performed using the selective primer sets with Universal SYBR® Green PCR Master Mix according to the manufacturer's instruction (Qiagen). The primer sequences used for amplification of the sense and antisense strands were as follows: GAPDH, 5′-CCATGAGAAGTATGACAACAGCC-3′ and 5′-TGGCAGGTTTTTCTAGACGG-3′; iNOS, 5′-TCTTGGGTCTCCGCTTCTCGTC-3′ and 5′-TGGCTGGTACATGGGCACAGAG-3′; Bcl-2, 5′-TTCCAGCCTGAGAGCAACCCAATG-3′ and 5′-TGACCCCACCGAACTCAAAGAAGG-3′; and Bax, 5′-AGGATGATTGCTGACGTGGACACG-3′ and 5′-AAGATGGTAACTGTCTGCCATGTGG-3′. Data analyses were carried out using a 7500 System with SDS software version 1.3.1 (Applied Biosystems Inc., Foster City, CA, USA).
Histological analysis
Detection of iNOS was performed by immunostaining. Heart left ventricles were fixed in 4% formaldehyde. Following dehydration and embedding in paraffin by routine protocol, heart left ventricle tissue sections (6 μm) were deparaffinized, rehydrated, and blocked with 1.5% normal horse serum. Expression of iNOS was detected using anti-iNOS (diluted 1:200) and Vectastain® avidin-biotin peroxidase and biotinylated secondary antibody (Vector Laboratories, Burlingame, CA, USA). The sections were visualized under a microscope.
Western blot analysis
Heart left ventricle tissues were lysed using RIPA buffer containing 50 mM phosphate buffer (pH 7.4), 0.5% Nonidet P-40, 0.1% sodium dodecyl sulfate, and a protease inhibitor cocktail (Sigma). Equal amounts of protein were separated using 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and then electrotransferred onto nitrocellulose membranes (Millipore, Bedford, MA, USA). Membranes were blocked with 5% nonfat dry milk at 4°C for 1 h and then incubated with anti-nuclear factor κB (NF-κB), inhibitory κB (IκB), and iNOS antibodies (Cell Signaling, Beverly, MA, USA) overnight at 4°C. After washing and binding with horseradish peroxidase–conjugated secondary antibodies at room temperature for 1 h, the reaction products were visualized with chemiluminescent reagents (Amersham, Piscataway).
Statistics
All experiments were presented as mean±SD values. The significance of treatment effects was assessed by Duncan's multiple range tests after one-way analysis of variance using SAS (Cary, NC, USA) software. Test statistics were considered significant at the P<.05 level.
Results and Discussion
Effect of ITCs on the survival of LP-BM retrovirus-infected mice
In the MAIDS model, the pathogenesis of LP-BM5 viral infection is characterized by immune dysfunction with many changes similar to those reported in human HIV infection. It is well established that LP-BM5 viral infection provokes an enlargement of lymphoid organs accompanied by systemic imbalance of T helper cytokines and dysfunction of immune cells.19 During recent decades, many clinicians have suggested that significant cardiac dysfunction is an important cause of AIDS-related mortality that was estimated to be 6–7%.7 In fact, many people with HIV infection generally have depressed left ventricular function as measured by either fractional shortening or contractility that is associated with cardiomyopathy, resulting in the increased incidence of mortality.20 Although few studies have estimated the life expectancy of HIV-infected people with cardiomyopathy, it has been well accepted that antiviral treatment against HIV improves the symptoms of heart dysfunction, suggesting that proper treatment for those people could increase the life expectancy. In animal studies, two murine leukemia virus models have been advocated to study HIV: the MAIDS model and a temperature-sensitive virus (ts1) model. The MAIDS model, which involves the LP-B5 virus, has been widely used as a model comparable to HIV.21,22 The LP-BM5 retrovirus-induced MAIDS model has some similarities with HIV-induced AIDS in humans.23 To examine the protective effect of ITCs in LP-BM5 retrovirus-infected mice, we determined mortality in the uninfected and infected groups. As shown in Table 1, the median survival time of the infected control mice was 87 days, whereas the median survival time of the uninfected control and all ITC-treated mice was over 112 days. Death from profound immunodeficiency usually occurred within 16–24 weeks after LP-BM5 retrovirus infection.22 In this study, viral activity of LP-BM5 might be more virulent because it was injected directly without frozen storage. Therefore, the median survival time of the infected control mice was shorter than expected based on other studies. The importance of cardiac dysfunction is demonstrated by the reported median survival to AIDS-related death of 101 days in patients with left ventricular dysfunction versus 472 days in patients with normal hearts as analyzed by echocardiography at a similar infection stage.24 The increased survival rate achieved by oral administration of ITCs indicates that ITCs have protective effects against LP-BM5 retrovirus infection-induced immunodeficiency.
Table 1.
Median Survival Time of Mice in the Study of LP-BM5 Retrovirus Infection and Isothiocyanate Administration
| LP-BM5 retrovirus/treatment | Median survival time (days)a | Number of mice (%)b |
|---|---|---|
| −/− | >112 | 16 (100) |
| +/− | 87 | 5 (31.3) |
| +/ICZ | >112 | 12 (75) |
| +/BITC | >112 | 16 (100) |
| +/PEITC | >112 | 16 (100) |
| +/SUL | >112 | 16 (100) |
Median survival time was represented by the interval at which 50% death in the treatment group occurred.
Number of mice surviving after 16 weeks (%).
BITC, benzyl isothiocyanate; ICZ, indolo[3,2-b]carbazole; PEITC, phenethyl isothiocyanate; SUL, sulforaphane.
Effect of ITCs on apoptosis of heart left ventricle tissue cells in LP-BM5 retrovirus-infected mice
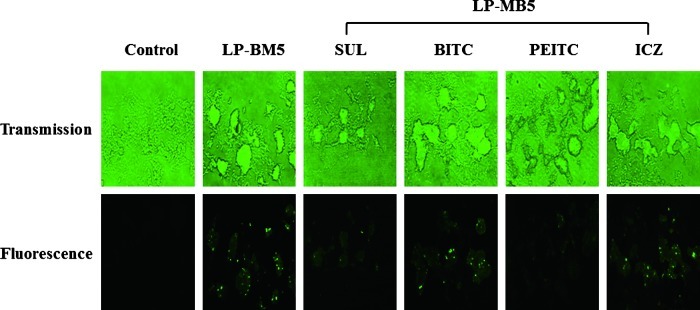
Apoptosis or programmed cell death is a major form of cell death characterized by stereotypic morphological and biochemical features including plasma membrane blebbing and shrinkage, chromosomal DNA fragmentation, and chromatin condensation.25,26 Aberrant regulation of apoptosis has been observed in many serious disorders, including neuronal disease, AIDS, autoimmune diseases, and cancer.27 To examine whether the protective effect of ITCs were mediated by the inhibition of apoptosis in LP-BM5 retrovirus-infected mice, we performed the TUNEL assay. As shown in Figure 1, the number of TUNEL-positive or apoptotic cells increased significantly in LP-BM5 retrovirus-infected mice compared with uninfected control mice. The number of TUNEL-positive cells was significantly lower in the SUL- and PEITC-treated groups compared with the LP-BM5 retrovirus-infected control group. These results indicate that oral administration of ITCs inhibited apoptosis in heart left ventricle tissues of LP-BM5 retrovirus-infected mice. However, there might be an argument against the function of ITCs because those have been long used as apoptotic reagents in cancer studies. It may be suggested that, because cancer cells are known to be more susceptible to certain chemical reagents, ITCs show different effects on apoptosis depending upon the types of tissues or organs. In addition, unlike in cancer cells, ITCs could regulate the HIV replication that ameliorates the symptoms of HIV-related diseases.28 Cardiomyocyte apoptosis underlies many forms of heart disease, including viral cardiomyopathy. Inducers of cardiomyocyte apoptosis include myocardial cell stretch, pressure overload, oxygen radicals, NO, cytokines such as tumor necrosis factor-α, and viruses.29 Pozzan et al.24 detected cardiomyocyte apoptosis in the isolated cells of 10 of 23 patients (43.5%) with AIDS. Cardiomyocyte apoptosis may also contribute to HIV-related heart muscle failure.24
FIG. 1.
Inhibitory effects of isothiocyanates on apoptosis in heart left ventricle tissues of LP-BM5 retrovirus-infected C57BL/6 mice. Apoptosis of left ventricle tissues was measured by TdT-mediated dUTP nick-end labeling assay, as described in Materials and Methods. Color images available online at www.liebertpub.com/jmf
Effect of ITCs on mRNA expression of Bcl-2 and Bax in the heart left ventricles of LP-BM5 retrovirus-infected mice
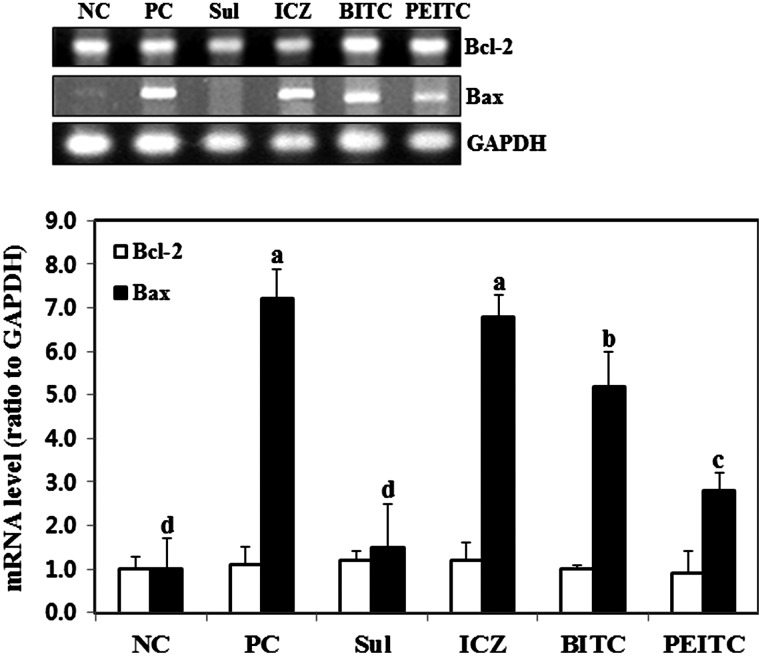
An imbalance in the expression of Bcl-2 family proteins in mitochondrial membranes, specifically anti-apoptotic Bcl-2 and pro-apoptotic Bax, leads to cytochrome c-mediated apoptosis.30 Therefore, to further explore the effects of the ITCs on apoptosis, we measured the expression of Bcl-2 and Bax by western blotting. There were no significant differences in Bcl-2 expression between groups, whereas Bax expression was significantly higher (sevenfold) in the LP-BM5 retrovirus-infected control group than in the uninfected control group (Fig. 2). This increased Bax/Bcl-2 ratio probably triggered apoptosis in the LP-BM5 retrovirus-infected control mice. There were no significant changes in the mRNA expression of Bcl-2 after oral administration of ITCs. However, ITCs decreased the mRNA levels of Bax significantly, thereby reducing the Bax/Bcl-2 ratio. In HIV infection, oxidative stress leads to apoptosis by increased expression of pro-apoptotic genes, such as those coding for caspase-2, caspase-3, caspase-9, and Bax.31–33 According to several studies, ITCs possess potent antioxidative capacity against oxidative stress, including menadione, tert-butyl hydroperoxide, 4-hydroxynonenal, and peroxynitrite.34,35 Taken together, these results suggested that inhibition of apoptosis by oral administration of ITCs, at least in part, is caused by decreasing Bax expression via antioxidative effects of ITCs.
FIG. 2.
Effect of isothiocyanates on mRNA expression of Bcl-2 and Bax in the left ventricles of C57BL/6 mice infected with the LP-BM5 retrovirus: uninfected control (negative control [NC]), infected control (positive control [PC]), SUL, ICZ, BITC, and PEITC. Left ventricle tissues were prepared and used for real-time polymerase chain reaction, as described in Materials and Methods. Data are mean±SD values of at least three independent experiments, each performed in triplicate. abcdDifferent letters indicate a significant difference (P<.05) as determined by Duncan's multiple range test. GAPDH, glyceraldehyde 3-phospahte dehydrogenase.
iNOS expression in the heart left ventricles of LP-BM5 retrovirus-infected mice
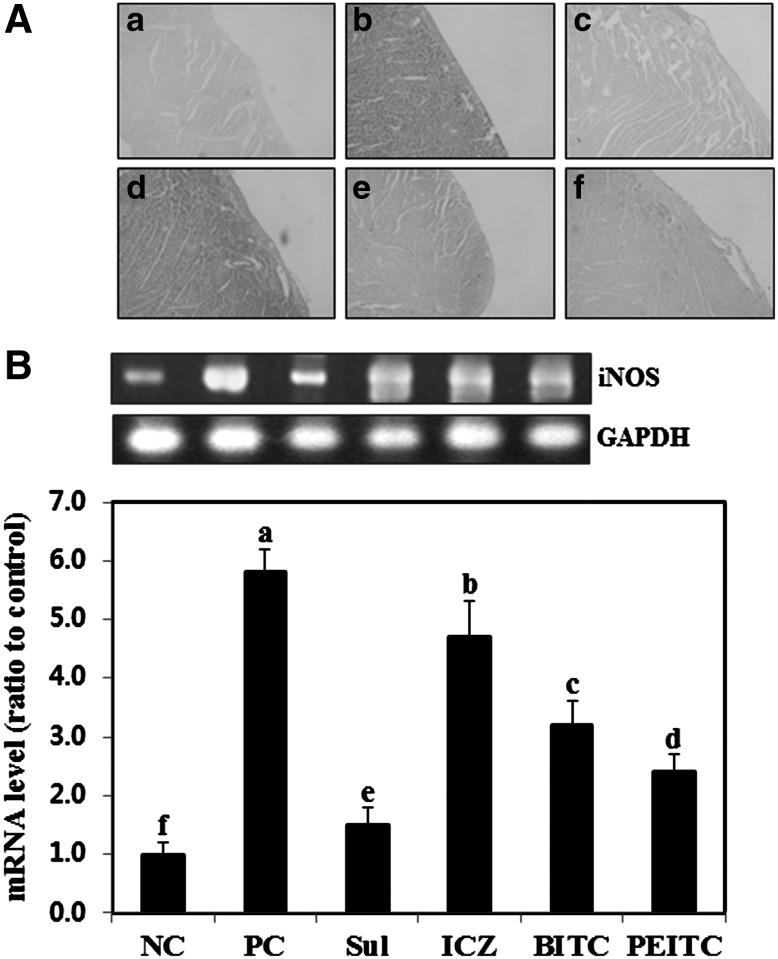
NO is one of the most important regulatory factors of the cardiovascular system. NO is synthesized from l-arginine by NO synthases, including neuronal type I synthase, endothelial type III synthase, and inducible type II synthase (iNOS), a family of isoenzymes with distinct functional, biological, and regulatory properties.36,37 The overexpression of specific cardiac iNOS results in cardiac fibrobrosis, dilation, and premature death.38 Barbaro et al.39 reported that iNOS staining intensity was higher in patients with HIV-associated cardiomyopathy than in patients with idiopathic dilated cardiomyopathy. In addition, Sam et al.40 reported that heart left ventricular dysfunction and myocardial apoptosis were diminished in a mouse iNOS knockout model. Therefore, to examine the effects of ITCs on LP-BM5 retrovirus-induced apoptosis, we measured iNOS expression in the left ventricle of the heart of LP-BM5 retrovirus-infected control mice. The optical density of iNOS staining in LP-BM5 retrovirus-infected mice was significantly higher than in uninfected control mice (Fig. 3A), indicating that LP-BM5 induced iNOS expression in the left ventricle. It is interesting that SUL, PEITC, and BITC showed a diminished amount of brown color in left ventricle specimens, suggesting that long-term administration of those ITCs for LP-BM5 retrovirus-infected mice is, in part, able to inhibit the induction of iNOS in the left ventricle due to viral infection. However, there was no current evidence for ICZ administration on decreasing iNOS expression in LP-BM5 retrovirus-infected left ventricle. For more detailed measurements, as shown in Figure 3B, the level of iNOS mRNA was significantly increased (5.7-fold) in LP-BM5 retrovirus-infected mice compared with uninfected control mice. Expression of iNOS was significantly lower in mice that received oral administration of ITCs, compared with LP-BM5 retrovirus-infected control mice. In particular, SUL and PEITC decreased iNOS mRNA levels significantly by 430% and 370%, respectively, compared with LP-BM5 retrovirus-infected control mice. These results are in agreement with a previous study that showed that iNOS immunoreactivity was stronger in the myocardial cells of HIV-infected patients with dilated cardiomyopathy compared with the myocardial cells of controls.39
FIG. 3.
Inhibitory effect of isothiocyanates on inducible nitric oxide synthase (iNOS) and iNOS mRNA expression in the left ventricle tissues of C57BL/6 mice infected with the LP-BM5 retrovirus. Left ventricle tissues were prepared and used for (A) immunohistochemistry for tissues from mice treated with (a) NC, (b) PC, (c) SUL, (d) ICZ, (e) BITC, and (f) PEITC and (B) real-time polymerase chain reaction, as described in Materials and Methods. Data are mean±SD values of at least three independent experiments, each performed in triplicate. abcdefDifferent letters indicate a significant difference (P<.05) as determined by Duncan's multiple range test.
Suppressive effect of ITCs on NF-κB activation in the heart left ventricles of LP-BM5 retrovirus-infected mice
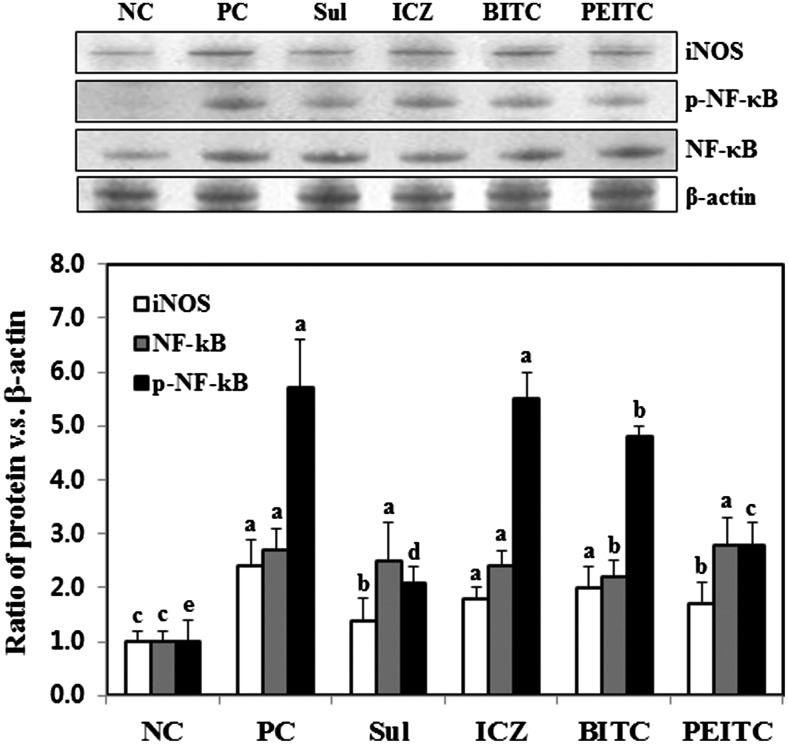
NF-κB is present in all cells in a resting state in the cytoplasm. Only when it is activated and translocated to the nucleus is the usual sequence of events generated. Under resting conditions, NF-κB consists of a heterotrimer of p50, p65, and I-κBα in the cytoplasm. The phosphorylation, ubiquitination, and degradation of I-κBα lead to the release of the p50–p65 heterodimer, which then translocates to the nucleus, binds its specific 10-basepair consensus site, and regulates the genetic expression of key pro-inflammatory cytokines during progression of HIV infection.41 It has been well accepted that iNOS expression is induced by cytokine-induced transcription factors, such as interferon and NF-κB, which recognize response elements within the iNOS promoter.38,42 NF-κB, a pro-inflammatory transcription factor, is involved in iNOS, cyclooxygenase-2, and tumor necrosis factor-α expression as well. As shown in Figure 4, the expression of iNOS was clearly increased by LP-BM5 retrovirus infection. Oral administration of SUL clearly decreased the expression of iNOS close to the level seen in uninfected controls. However, ICZ and BITC treatment did not result in a significant difference in iNOS expression compared with the LP-BM5 retrovirus-infected control. To determine the mechanism underlying the suppression of iNOS by ITCs, we analyzed the expression and translocation of NF-κB, which is known to regulate the expression of iNOS. Oral administration of each of the four ITCs after infection resulted in a dramatic reduction in the translocation of NF-κB. In particular, SUL and PEITC inhibited phosphorylated NF-κB to a greater extent than equal concentrations of ICZ and BITC. These results suggested that SUL and PEITC suppress iNOS induction significantly by inhibiting NF-κB translocation. Furthermore, NF-κB transcribes the mRNAs of several anti-apoptotic members of the Bcl-2 family. Because the expression of Bcl-2 and Bax is directly activated by NF-κB,43,44 we speculated that LP-BM5 retrovirus infection would increase the expression of NF-κB and that ITCs would suppress this response. When these findings are taken together, SUL and PEITC dramatically suppressed apoptosis by reducing Bax mRNA levels through the inhibition of NF-κB.
FIG. 4.
Inhibitory effect of isothiocyanates on overexpression of iNOS and activation of nuclear factor κB (NF-κB) in the left ventricle tissues of C57BL/6 mice infected with the LP-BM5 retrovirus. Left ventricle tissues were prepared and used for western blot, as described in Materials and Methods. Data are mean±SD values of at least three independent experiments, each performed in triplicate. abcdeDifferent letters indicate a significant difference (P<.05) as determined by Duncan's multiple range test. p-NF-κB, phosphorylated NF-κB.
In conclusion, oral administration of SUL and PEITC significantly inhibited apoptosis in heart left ventricle tissues of LP-BM5 retrovirus-infected mice by reducing iNOS expression, the Bax/Bcl-2 ratio, and the NF-κB signaling pathway. These results suggest that ITCs are potential therapeutic candidates for treatment of AIDS-related heart dysfunction.
Acknowledgments
This work was supported by grant KHU-20100157 from Kyung Hee University in 2009.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Sudano I. Spieker LE. Noll G. Corti R. Weber R. Lüscher TF. Cardiovascular disease in HIV infection. Am Heart J. 2006;151:1147–1155. doi: 10.1016/j.ahj.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 2.Rodrigo C. Rajapakse S. Current status of HIV/AIDS in South Asia. J Glob Infect Dis. 2009;1:93–101. doi: 10.4103/0974-777X.56249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lau KA. Wang B. Saksena NK. Emerging trends of HIV epidemiology in Asia. AIDS Rev. 2007;9:218–229. [PubMed] [Google Scholar]
- 4.Keedy KS. Margolis DM. Therapy for persistent HIV. Trends Pharmacol Sci. 2010;31:206–211. doi: 10.1016/j.tips.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogers JS. Zakaria S. Thom KA. Flammer KM. Kanno M. Mehra MR. Immune reconstitution inflammatory syndrome and human immunodeficiency virus-associated myocarditis. Mayo Clin Proc. 2008;83:1275–1279. doi: 10.4065/83.11.1275. [DOI] [PubMed] [Google Scholar]
- 6.Currie PF. Boon NA. Immunopathogenesis of HIV-related heart muscle disease: current perspectives. AIDS. 2003;1:S21–S28. doi: 10.1097/00002030-200304001-00004. [DOI] [PubMed] [Google Scholar]
- 7.Mehta NJ. Khan IA. HIV-associated coronary artery disease. Angiology. 2003;54:269–275. doi: 10.1177/000331970305400302. [DOI] [PubMed] [Google Scholar]
- 8.De Castro S. Migliau G. Silvestri A, et al. Heart involvement in AIDS: a prospective study during various stages of the disease. Eur Heart J. 1992;13:1452–1459. doi: 10.1093/oxfordjournals.eurheartj.a060085. [DOI] [PubMed] [Google Scholar]
- 9.Barbaro G. Di Lorenzo GD. Grisorio B. Barbarini G. Cardiac involvement in the acquired immunodeficiency syndrome: a multicenter clinical-pathological study. AIDS Res Hum Retroviruses. 1998;14:1071–1077. doi: 10.1089/aid.1998.14.1071. [DOI] [PubMed] [Google Scholar]
- 10.Cheung KL. Kong AN. Molecular targets of dietary phenethyl isothiocyanate and sulforaphane for cancer chemoprevention. AAPS J. 2010;12:87–97. doi: 10.1208/s12248-009-9162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thornalley PJ. Isothiocyanates: mechanism of cancer chemopreventive action. Anticancer Drugs. 2002;13:331–338. doi: 10.1097/00001813-200204000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Valgimigli L. Iori R. Antioxidant and pro-oxidant capacities of ITCs. Environ Mol Mutagen. 2009;50:222–237. doi: 10.1002/em.20468. [DOI] [PubMed] [Google Scholar]
- 13.Bang JI. Song KH. Kim SH, et al. Prevalence of primary antiretroviral resistance: trends in Korea. AIDS Res Hum Retroviruses. 2008;24:83–85. doi: 10.1089/aid.2007.0116. [DOI] [PubMed] [Google Scholar]
- 14.Montagnier L. 25 years after HIV discovery: prospects for cure and vaccine. Virology. 2010;397:248–254. doi: 10.1016/j.virol.2009.10.045. [DOI] [PubMed] [Google Scholar]
- 15.Mills E. Montori V. Perri D. Phillips E. Koren G. Natural health product–HIV drug interactions: a systematic review. Int J STD AIDS. 2005;16:181–186. doi: 10.1258/0956462053420103. [DOI] [PubMed] [Google Scholar]
- 16.Gerencer M. Turecek PL. Kistner O. Mitterer A. Savidis-Dacho H. Barrett NP. In vitro and in vivo anti-retroviral activity of the substance purified from the aqueous extract of Chelidonium majus L. Antiviral Res. 2006;72:153–156. doi: 10.1016/j.antiviral.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Morse MA. Wang C. Stoner GD. Mandal S. Conran PB. Amin SG. Inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced DNA adduct formation and tumorigenicity in the lung of F344 rats by dietary phenethyl isothiocyanate. Cancer Res. 1989;49:549–553. [PubMed] [Google Scholar]
- 18.Liang B. Jiang S. Zhang Z, et al. Anti-inflammatory effects of theophylline: modulation of immune functions during murine leukemia virus infection. Immunopharmacol Immunotoxicol. 2001;23:307–319. doi: 10.1081/iph-100107332. [DOI] [PubMed] [Google Scholar]
- 19.Dias AS. Bester MJ. Britz RF. Apostolides Z. Animal models used for the evaluation of antiretroviral therapies. Curr HIV Res. 2006;4:431–446. doi: 10.2174/157016206778560045. [DOI] [PubMed] [Google Scholar]
- 20.Fisher SD. Easley KA. Orav EJ, et al. Mild dilated cardiomyopathy and increased left ventricular mass predict mortality: the prospective P2C2 HIV multicenter study. Am Heart J. 2005;150:439–447. doi: 10.1016/j.ahj.2005.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark S. Duggan J. Chakraborty J. ts1 and LP-BM5: a comparison of two murine retrovirus models for HIV. Viral Immunol. 2001;14:95–109. doi: 10.1089/088282401750234475. [DOI] [PubMed] [Google Scholar]
- 22.Gallicchio VS. Hughes NK. Tse KF. Ling J. Birch NJ. Effect of lithium in immunodeficiency: improved blood cell formation in mice with decreased hematopoiesis as the result of LP-BM5 MuLV infection. Antiviral Res. 1995;26:189–202. doi: 10.1016/0166-3542(94)00075-j. [DOI] [PubMed] [Google Scholar]
- 23.Akarid K. Chenais B. Chau F. Sint M. Desforges B. Gougerot-Pocidalo MA. Absence of involvement of nitric oxide in LP-BM5-induced immunodeficiency syndrome. FEMS Immunol Med Microbiol. 1996;15:169–176. doi: 10.1111/j.1574-695X.1996.tb00068.x. [DOI] [PubMed] [Google Scholar]
- 24.Pozzan G. Pagliari C. Tuon FF. Takakura CF. Kauffman MR. Duarte MIS. Diffuse-regressive alterations and apoptosis of myocytes: possible causes of myocardial dysfunction in HIV-related cardiomyopathy. Int J Cardiol. 2009;132:90–95. doi: 10.1016/j.ijcard.2007.10.057. [DOI] [PubMed] [Google Scholar]
- 25.Yu R. Mandlekar S. Harvey KJ. Ucker DS. Kong AN. Chemopreventive isothiocyanates induce apoptosis and caspase-3-like protease activity. Cancer Res. 1998;58:402–408. [PubMed] [Google Scholar]
- 26.Weigert A. Brune B. Nitric oxide, apoptosis and marcrophage polarization during tumor progression. Nitric Oxide. 2008;19:95–102. doi: 10.1016/j.niox.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 27.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 28.Tajima H. Nakamoto Y. Taketo A. Effect of synthetic hydroxyl isothiocyanates on a bacterial virus and DNA. Biosci Biotechnol Biochem. 2007;71:1094–1097. doi: 10.1271/bbb.70001. [DOI] [PubMed] [Google Scholar]
- 29.Twu C. Liu NQ. Popik W, et al. Cardiomyocytes undergo apoptosis in human immunodeficiency virus cardiomyopathy through mitochondrion- and death receptor-controlled pathways. Proc Natl Acad Sci USA. 2002;99:14386–14391. doi: 10.1073/pnas.212327899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reed JC. Double identity for proteins of the Bcl-2 family. Nature. 1997;387:773–776. doi: 10.1038/42867. [DOI] [PubMed] [Google Scholar]
- 31.Agostini M. Di Marco B. Nocentini G. Delfino DV. Oxidative stress and apoptosis in immune diseases. Int J Immunopathol Pharmacol. 2002;15:157–164. doi: 10.1177/039463200201500301. [DOI] [PubMed] [Google Scholar]
- 32.Kannan K. Jain SK. Oxidative stress and apoptosis. Pathophysiology. 2000;7:153–163. doi: 10.1016/s0928-4680(00)00053-5. [DOI] [PubMed] [Google Scholar]
- 33.Balestrieri E. Grelli S. Matteucci C, et al. Apoptosis-associated gene expression in HIV-infected patients in response to successful antiretroviral therapy. J Med Virol. 2007;79:111–117. doi: 10.1002/jmv.20768. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y. Li Jun. Tang L. Cancer-preventive isothiocyanates: dichotomous modulators of oxidative stress. Free Radic Biol Med. 2005;38:70–77. doi: 10.1016/j.freeradbiomed.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 35.Gao X. Dinkova-Kostova AT. Talalay P. Powerful and prolonged protection of human retinal pigment epithelial cells, keratinocytes, and mouse leukemia cells against oxidative damage: the indirect antioxidant effects of sulporaphane. Proc Natl Acad Sci USA. 2001;98:15221–15226. doi: 10.1073/pnas.261572998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Forstermann U. Closs EI. Pollock JS, et al. Nitric oxide synthase isozymes. Characterization, purification, molecular cloning, and functions. Hypertension. 1994;23:1121–1131. doi: 10.1161/01.hyp.23.6.1121. [DOI] [PubMed] [Google Scholar]
- 37.Ferreiro CR. Chagas AC. Carvalho MH, et al. Influence of hypoxia on nitric oxide synthase activity and gene expression in children with congenital heart disease: a novel pathophysiological adaptive mechanism. Circulation. 2001;103:2272–2276. doi: 10.1161/01.cir.103.18.2272. [DOI] [PubMed] [Google Scholar]
- 38.Mungrue IN. Gros R. You X, et al. Cardiomyocyte overexpression of iNOS in mice results in peroxynitrite generation, heart block, and sudden death. J Clin Invest. 2002;109:735–743. doi: 10.1172/JCI13265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barbaro G. Lorenzo GD. Soldini M, et al. Intensity of myocardial expression of inducible nitric oxide synthase influences the clinical course of human immunodeficiency virus-associated cardiomyopathy. Circulation. 1999;100:933–939. doi: 10.1161/01.cir.100.9.933. [DOI] [PubMed] [Google Scholar]
- 40.Sam F. Sawyer DB. Xie Z, et al. Mice lacking inducible nitric oxide synthase have improved left ventricular contractile function and reduced apoptotic cell death late after myocardial infarction. Circ Res. 2001;89:351–356. doi: 10.1161/hh1601.094993. [DOI] [PubMed] [Google Scholar]
- 41.Gilmore TD. The Rel/NF-kB signal transduction pathway: introduction. Oncogene. 1999;18:6842–6844. doi: 10.1038/sj.onc.1203237. [DOI] [PubMed] [Google Scholar]
- 42.Xie QW. Kashiwabara Y. Nathan C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J Biol Chem. 1994;269:4705–4708. [PubMed] [Google Scholar]
- 43.Chen C. Edelstein LC. Gelinas C. The Rel/NF-kappaB family directly activates expression of the apoptosis inhibitor Bcl-xL. Mol Cell Biol. 2000;20:2687–2695. doi: 10.1128/mcb.20.8.2687-2695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shou Y. Li N. Li L. Borowitz JL. Isom GE. NF-kappaB-mediated up-regulation of Bcl-XS and Bax contributes to cytochrome c release in cyanide-induced apoptosis. J Neurochem. 2002;81:842–852. doi: 10.1046/j.1471-4159.2002.00880.x. [DOI] [PubMed] [Google Scholar]