Abstract
Rock inhabiting fungi are among the most stress tolerant organisms on Earth. They are able to cope with different stressors determined by the typical conditions of bare rocks in hot and cold extreme environments. In this study first results of a system biological approach based on two-dimensional protein profiles are presented. Protein patterns of extremotolerant black fungi – Coniosporium perforans, Exophiala jeanselmei – and of the extremophilic fungus – Friedmanniomyces endolithicus – were compared with the cosmopolitan and mesophilic hyphomycete Penicillium chrysogenum in order to follow and determine changes in the expression pattern under different temperatures. The 2D protein gels indicated a temperature dependent qualitative change in all the tested strains. Whereas the reference strain P. chrysogenum expressed the highest number of proteins at 40 °C, thus exhibiting real signs of temperature induced reaction, black fungi, when exposed to temperatures far above their growth optimum, decreased the number of proteins indicating a down-regulation of their metabolism. Temperature of 1 °C led to an increased number of proteins in all of the analysed strains, with the exception of P. chrysogenum. These first results on temperature dependent reactions in rock inhabiting black fungi indicate a rather different strategy to cope with non-optimal temperature than in the mesophilic hyphomycete P. chrysogenum.
Keywords: Adaptation, Extreme environments, Proteome pattern, Rock inhabiting fungi, Temperature
Highlights
► The paper is the first investigation of protein patterns in rock inhabiting fungi. ► We show the first results of temperature response in black fungi. ► Protein patterns of black fungi from different ecological niches are compared. ► Adaptation to similar conditions led to a convergent response in black fungi. ► Ecological strategies of MCF are different from Penicillium chrysogenum ones.
Introduction
‘Exposure of cells to suboptimal growth conditions or to any environment that reduces cell viability or fitness can be considered stresses’ (de Nadal et al. 2011). Stress has been classified as either biotic or abiotic, these including thermal (hot or cold) and non-thermal stress, such as acid, water, or pressure (Mafart et al. 2001). Both the physiological state and the natural environment in which an organism has been evolutionarily selected, influence its adaptive responses and rapid adaptations are crucial to maximizing cell survival (de Nadal et al. 2011). Eukaryotic cells have evolved sophisticated cellular mechanisms in response to the stresses that regulate several aspects of cell physiology as e.g. gene expression, metabolism, cell cycle progression, cytoskeletal organization, protein expression and homeostasis, and modification of enzymatic activity. These stress tolerance responses can generate both immediate and long-term adaptations, which are especially crucial for the survival of organisms in environments with extreme physicochemical parameters. Within eukaryotes, a specialized group of fungi – the black yeasts and microcolonial fungi (MCF) – have been identified as conquerors of an extremely stressful habitat: the bare rock in hot and cold environments (Staley et al. 1982; de Hoog & Grube 2008; Sterflinger et al. 2012).
Due to their stress tolerance, MCF and black yeasts have a wide distribution that includes some of the most extreme environments of the Earth as well as extraterrestrial conditions (Onofri et al. 2012). Originally black fungi – also named dematiaceous fungi – were described as inhabitants of living and dead plant material. However, in the last 30 y they have been isolated from hypersaline waters (Gunde-Cimerman et al. 2000), acidic environments (Baker et al. 2004), radioactive areas (Dadachova et al. 2007), as human pathogens or opportunists (Matos et al. 2002) and as a dominant part of the epi- and endolithic microbial communities (Friedmann 1982; Sterflinger 2000; Burford et al. 2003; Ruibal et al. 2005; Sert et al. 2007; Selbmann et al. 2008). Together with cyanobacteria and lichens, they contribute to the global biogeochemical cycling by active weathering of natural rocks and stone monuments (Sterflinger & Krumbein 1997).
These habitats share some important characteristics: osmotic stress, UV and oxidative stress and rapid variation of temperature, water supply, and nutrient availability (Sterflinger et al. 1999; Vember & Zhdanova 2001; Sterflinger 2005). To withstand these changes, organisms living in such environments need either permanently existing or exceptionally fast adaptive cellular or metabolic responses. Although MCF and black yeasts are a diverse taxonomic group having polyphyletic origins within the Ascomycota, they have similar morphological and physiological characters. These similarities were interpreted as a ‘principle of uniformity’ by Urzí et al. (2000) being an obligate basis to tolerance of physical and chemical stress on rock and plant surfaces. Slow growth rates, an optimal surface/volume ratio of the cauliflower-like colonies, thick and strongly melanised cell walls, exopolysaccharides production, the high intracellular content of trehalose, and polyoles as well as lack of sexual reproductive structures, are considered as adaptations to the extreme environments (Sterflinger 1998; Selbmann et al. 2005; Onofri et al. 2007; Gostinčar et al. 2010).
Temperature is undoubtedly one of the major factors affecting the growth and survival of any microorganism (Deegenaars & Watson 1998): for this reason it is of great interest to investigate how MCF and black yeasts withstand temperatures that are significantly out of their growth range. Unlike in other Ascomycetes as Neurospora crassa, Candida albicans, Saccharomyces cerevisiae and Schizosaccharomyces pombe (Kraus & Heitman 2003; Bahn et al. 2007; Alonso-Monge et al. 2009), the stress-response mechanisms of MCF have not yet been investigated, either on the genomic or on the proteomic level. A very recent investigation has revealed the complexity of protein composition in cosmopolitan fungi as Penicillium chrysogenum and Aspergillus sp. (Jami et al. 2010a; Rizwan et al. 2010). Further studies that analyse the ecological differences and analogies among these fungi in a systematic approach and on the molecular level are missing. The production of molecular chaperons (MC), so called ‘heat shock proteins’ (HSPs), small HSPs, and also ‘cold shock proteins’ (CSPs) belong to the most important stress reactions of cells in general (Becker & Craig 1994; Albanese et al. 2006; Nakamoto & Vígh 2007; Nevarez et al. 2008) and are known to represent the main effect to temperature stress in mesophilic fungi such as P. chrysogenum (Raggam et al. 2011). Thus, protein expression profiling was chosen as the first tool to shed light on the biological response of MCF and black fungi towards suboptimal temperatures.
The main goal of the present paper was to reveal if black fungi and mesophilic hyphomycetes present a similar reaction to temperature stress, as reflected by the protein patterns. Three strains of black rock inhabiting fungi were chosen for this study: Exophiala jeanselmei MA 2853, Coniosporium perforans MA 1299 and Friedmanniomyces endolithicus CCFEE 5208. Fungi were grown at different temperatures and the protein profiles were analysed in comparison with each other and with Penicillium chrysogenum (strain MA 3995), as reference strain.
Materials and methods
Fungal strains
Three strains of black fungi, clustering within two different orders of Dothideomyceta (Chaetothyriales and Capnodiales), were used in the present study (Fig 1). The isolates were selected according to their bio-ecological characteristics. They all colonize rock epi- or endolithically but they have a diverse geographical distribution:
-
(1)
Exophiala jeanselmei (MA 2853) is a mesophilic black yeast detected as a frequent colonizer of rock in moderate climates (Warscheid & Braams 2000; Sterflinger & Prillinger 2001). It has a close phylogenetic and physiological relation to human opportunists and pathogens (de Hoog 1993) which makes this strain a highly interesting model to study the evolution of virulence (Gostinčar et al. 2011).
-
(2)
Coniosporium perforans (MA 1299) is a widely distributed microcolonial rock inhabitant fungus in both moderate and Mediterranean climates (Sterflinger et al. 1997; M. Owczarek, in preparation). Although it can be considered as mesophilic with respect to its growth optimum, this strain has a remarkable high temperature and desiccation tolerance (Sterflinger 1998).
-
(3)
Friedmanniomyces endolithicus (CCFEE 5208) is a psychrophilic fungus with an outstanding and unique ecology and phylogeny. The considerable sequence deviation from known taxa, reflected by the phylogenetically isolated position, suggest F. endolithicus as an endemic species for the Antarctic (Selbmann et al. 2005), where it occurs cryptendolithically in rocks, having a strong degree of extremotolerant specialisation (Onofri et al. 1999).
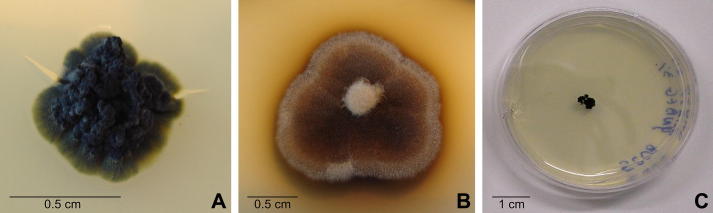
Fig 1.
Colony morphology of the analysed black fungi grown on MEA: (A) Coniosporium perforans MA 1299; (B) Exophiala jeanselmei MA 2853 (photos Tesei D); and (C) Friedmanniomyces endolithicus CCFEE 5208 (photo Selbmann L).
The mesophilic hyphomycete Penicillium chrysogenum (MA 3995) was chosen as a reference strain since it is very well characterized under different growth conditions in nature and at laboratory scale. Further, the ecology and proteome of P. chrysogenum has been studied extensively (Tresner & Hayes 1971; Dantigny et al. 2007; Jami et al. 2010a, 2010b).
Exophiala jeanselmei, C. perforans, and P. chrysogenum, were obtained from the Austrian Center of Biological Resources and Applied Mycology culture collection (ACBR, Vienna, Austria; www.acbr-database.at). Friedmanniomyces endolithicus was provided by the Culture Collection of Fungi from Extreme Environments (CCFEE, Università della Tuscia, Viterbo, Italy; http://www.sma.unitus.it/index.php/museo-nazionale-dellantartide-sezione-micologica.html).
Thermal preferences, cultivation, and exposure conditions
Temperature optima and growth rates were tested for all Exophiala jeanselmei, Coniosporium perforans, and P. chrysogenum. Data for Friedmanniomyces endolithicus were previously published by Selbmann et al. (2005). Strains were inoculated on malt extract agar (2 %, MEA (Malt Extract Agar)) and incubated for a maximum of 21 d at 0, 5, 10, 15, 20, 25, 28, 30, 35, 37 °C. The diameter of the colonies was recorded each day. All tests were performed in triplicate.
For further experiments, 28 °C was chosen as incubation temperature for E. jeanselmei, C. perforans, and P. chrysogenum mainly because this is standard incubation temperature in microbiology and it was still in the growth range of all three fungi. Since F. endolithicus does not grow above 15 °C and its growth optimum is within the range 10–15 °C (Selbmann et al. 2005), the incubation was performed at 15 °C. All the isolates were grown on 2 % MEA for 4 weeks in order to obtain enough biomass for protein extraction.
For stress simulation fungi were exposed to 1 °C and to 40 °C for 1 week; F. endolithicus was exposed to 1 °C and 28 °C. The viability of the colonies was evaluated after temperature treatment for 12, 24, 48, 72 h, and after 1 week. Biomass for protein profiling was harvested by scratching the material from the plates using a scalpel, immediately frozen and stored at −80 °C until protein extraction.
Protein extraction
Protein extracts were obtained as described by Isola et al. (2011). Briefly, cell disruption was performed by a mechanical method adding an EDTA based lysis buffer. Precipitation was based on phenol: 3 ml of Tris–buffered phenol solution pH 8.0 (Sigma–Aldrich, Steinheim, Germany) were added to every sample after the mechanical disruption, in a 15 ml polypropylene centrifuge tube. Centrifugation at 7834× g for 10 min at 4 °C was performed in order to separate the phenolic phase, subsequently transferred to a new pre-weighed tube. Five volumes of ice-cold 0.1 M ammonium acetate in methanol was added and after overnight precipitation (−20 °C), the protein pellet was obtained by centrifugation at 7834× g for 30 min at 4 °C. After washing it with ice-cold methanol (absolute) and then with ice-cold acetone (80 % v/v), the dried protein pellet (−20 °C) was suspended in Modified Sample Buffer (MSB) according to final pellet weight. The protein concentrations were determined by the BioRad protein assay (BioRad Lab., Hartfordshire, USA) by establishing a standard curve using serial dilutions from 0.8–100 μg/ml−1 of bovine serum albumin (Thermo Scientific, Rockford, IL, USA).
2D gel electrophoresis
For each temperature tested (1, 15, 28, 40 °C) two technical replicates were performed. The 13 cm strips (IPG™ DryStrip 3-10 NL, GE Healthcare Bio-Sciences AB, Uppsala, Sweden) were rehydrated in a total volume of 255 μl rehydration buffer [8 M urea, 2 % (w/v) CHAPS, 10 mM dithiotreitol (DTT), 0.1 % bromophenol blue, and 0.5 % (v/v) Servalyte] including 20 μg of protein, at room temperature and for 16 h. Isoelectric focussing was carried out according to manufacturer instructions at 20 °C and a total of 14 kV h, using a Protean IEF cell system (Bio-Rad Hartfordshire, USA). The strips were re-equilibrated for 15 min under gentle shaking in 2 ml equilibration solution [50 mM Tris–HCl pH = 8.4, 6 M urea, 30 % (v/v) glycerol, 2 % (w/v) sodium dodecyl sulphate (SDS)], with 2 % (w/v) DTT and subsequently, for 15 min in 2 ml equilibration solution [50 mM Tris–HCl pH = 6.8, 6 M urea, 30 % (v/v) glycerol, 2 % (w/v) SDS], with 2.5 % (w/v) iodoacetamide (IAA) and trace of bromophenol blue (Bjellqvist et al. 1993). Second dimension was performed in 10 % (w/v) SDS polyacrylamide gel electrophoresis (SDS-PAGE, 14 cm × 14 cm) with running buffer [24 mM Tris pH = 8.3, 192 mM glycine, 0.1 % (w/v) SDS]. For the electrophoretic run 160 V and variable mA were applied using the Perfect Blue Twin Gel System (PeqLab GmbH, Erlangen, Germany). The chamber was cooled at 4 °C (type CBN 8-30, Heto, Birkerød, Denmark).
Staining and analysis of 2D gels
Protein spots were visualized by a high sensitive mass spectrometric compatible silver staining (Shevchenko et al. 1996). The gels were fixed in 50 % (v/v) methanol and 5 % (v/v) acetic acid for 20 min, then washed in 50 % (v/v) methanol for 10 min and rinsed with MilliQ water (Millipore, MA, USA) overnight at 4 °C. Subsequently, the 2D gels were sensitized using a 0.02 % (w/v) sodium thiosulphate solution for 1 min and then incubated in 0.1 % (w/v) silver nitrate solution for 20 min at 4 °C, rinsing twice with MilliQ water for 1 min each, after incubation. The gel development was carried out by the incubation in 0.04 % (v/v) formalin and 2 % (w/v) sodium carbonate solution until the desired intensity of staining was achieved. Gels were washed with a 5 % (v/v) acetic acid. All the washing solutions used were prepared in MilliQ water (Millipore, MA, USA).
Stained gels were scanned in TIFF 16 bit format. Image Master 2D Platinum version 5.0 (Amersham Biosciences, Swiss Institute of Bioinformatics, Geneva, Switzerland) was used for spot-matching and image analysis. The spots intensity, densitometrically determined and expressed as spot volume, was evaluated (see Supplementary material for examples). Comparison reports of the qualitative differences of the samples were generated and served for the evaluation of the presence/absence of protein spots under the tested temperatures.
Results
Thermal preferences
As shown in Table 1, for Exophiala jeanselmei, Coniosporium perforans, and Penicillium chrysogenum the upper temperature limit was 30 °C, thus 40 °C can be considered as serious stress for these organisms. Since the Friedmanniomyces endolithicus growth limit is 15 °C (Selbmann et al. 2005), 28 °C, that were applied as uppermost temperature for the treatment of this fungus, can be also considered as serious stress.
Table 1.
Thermal preferences of model fungi. Thermal preferences have been reported as diameter of colonies (in cm) as the average of three different tests. MA Nr: strain number in the ACBR/BOKU Vienna culture collection.
| Strain | Thermal preferences (°C) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | 25 | 28 | 30 | 35 | 37 | |
| P. chrysogenum MA 3995 | 0.50 | 0.93 | 1.50 | 1.90 | 2.80 | 2.95 | 1.30 | 0.75 | _ | _ |
| E. jeanselmei MA 2853 | _ | 0.40 | 0.75 | 1.20 | 1.40 | 1.70 | 1.60 | 1.25 | _ | _ |
| C. perforans MA 1299 | _ | _ | _ | 0.65 | 1.10 | 0.70 | 0.70 | 0.65 | _ | _ |
Thermal preferences have been reported as diameter of colonies (in cm) as the average of three different tests. MA Nr: strain number in the ACBR/BOKU Vienna culture collection.
Analysis of protein patterns
Each 2D protein gel – for each fungus and each of the temperatures tested – was carried out in duplicate resulting in a total of 24 gels. From the two technical replicates the gel exhibiting the highest number of spots was used for the following analysis: (1) to evaluate if the black fungi respond towards different temperatures by a change in the protein pattern; (2) to compare the changes of the protein patterns of the black fungi with Penicillium chrysogenum as reference; (3) to compare the changes of the protein patterns of the mesophilic fungi Exophiala jeanselmei and Coniosporium perforans with the extremophilic fungus Friedmanniomyces endolithicus as reference. For each comparison, after spot detection, the gels were aligned and matched to the reference gel. The analysis of groups of matching spots allowed the evaluation of changes and similarities in protein expression patterns.
2D protein patterns at different temperatures
At all conditions tested the protein pattern of the four fungal strains differed concerning the total number of spots (Table 2), their molecular weight as well as their isoelectric point [pI]-related distribution. At 28 °C the number of spots was 381 in Penicillium chrysogenum, 382 in Exophiala jeanselmei, and 325 in Coniosporium perforans; at 15 °C the number was 425 in Friedmanniomyces endolithicus. In E. jeanselmei the major protein spots had molecular weights within 70 and 170 kDa and pIs from 6 to 10 while C. perforans and P. chrysogenum expression profiles showed a high number of spots having molecular weight within 50 and 10 kDa. In F. endolithicus mainly protein spots with pI between 3 and 8 and molecular weights from 25 to 170 kDa, were detected.
Table 2.
Number of protein spots detected in the 2-DE gels of the analysed strains at each exposure condition.
| Strains | Number of spots at each exposure condition |
||
|---|---|---|---|
| 28 °C | 1 °C | 40 °C | |
| P. chrysogenum MA 3995 | 381 | 358 | 601 |
| E. jeanselmei MA 2853 | 382 | 387 | 174 |
| C. perforans MA 1299 | 325 | 494 | 255 |
| 15 °C | 1 °C | 28 °C | |
| F. endolithicus CCFEE 5208 | 425 | 466 | 284 |
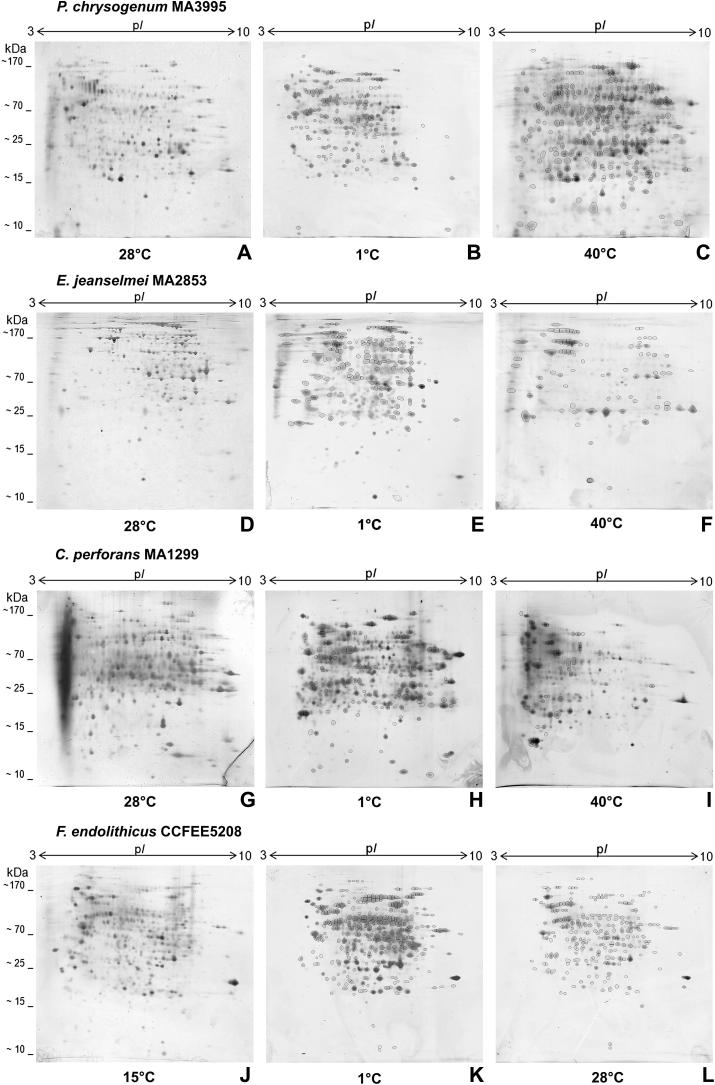
The exposure of P. chrysogenum to different temperatures influenced both the expression pattern and the spots abundance (Fig 2, A–C). At 1 °C spots decreased from 381 to 358 and mainly proteins with basic pIs (8–10) were missing. At 40 °C, the number of detected protein spots increased to 601. Overlapping the gels obtained from three temperatures showed that 153 spots were matching at 28 °C and 1 °C while 211 spots were matching at 28 °C and 40 °C, thus indicating a greater similarity of proteins at the higher temperature range. A number of 100 protein spots was found to match at all temperature (Table 3).
Fig 2.
2D gel protein patterns obtained after exposure to different temperatures. P. chrysogenum MA 3995 (A–C), E. jeanselmei MA 2853 (D–F), Coniosporium perforans MA 1299 (G–I), and F. endolithicus CCFEE 5208 (J–L). Pairs of protein spots detected by overlapping are highlighted in black.
Table 3.
Number of matching protein spots detected. Gel matching was carried out selecting the temperatures 28 °C and 15 °C as reference.
| Strain | Reference gel | Number of pairs at each exposure condition |
||
|---|---|---|---|---|
| 1 °C | 40 °C | All temperatures | ||
| P. chrysogenum MA 3995 | 28 °C | 153 | 211 | 100 |
| E. jeanselmei MA 2853 | 28 °C | 147 | 81 | 46 |
| C. perforans MA 1299 | 28 °C | 134 | 47 | 21 |
| 1 °C | 28 °C | All temperatures | ||
| F. endolithicus CFEE 5208 | 15 °C | 278 | 224 | 187 |
Exophiala jeanselmei exposed to low temperature (Fig 2, D–F) showed no significant variations in the absolute number of spots; 387 spots were found at 1 °C whereas 382 spots were detected at 28 °C. However, a substantial change in the expression pattern, caused by the increase of proteins with acidic pIs (3–5) and molecular weight in the range of 25 and 100 kDa, was observed. The number of protein spots with a higher mol. wt – between 100 and 170 kDa – was instead reduced. A decrease to 174 spots occurred at 40 °C, mostly concerning spots within the pI range 5–7 and the mol. wt between 30 and 170 kDa. The number of spots commonly expressed at both 28 °C and 1 °C was 147 and thus it was significantly higher than the number of overlapping spots found both in the 28 °C and 40 °C gels (81). A total of 46 spots matched at all temperatures (Table 3).
In C. perforans (Fig 2, G–I), growth at 1 °C resulted in the expression of the highest number of protein spots (494 vs 325) which was especially related to an increase in the number of high molecular weight spots. Only 255 spots were observed as a consequence of the strain exposure to 40 °C; the reduction mostly concerned spots in the pIs range 5–9 and with molecular weight between 30 and 90 kDa. Common protein spots at 28 °C and 1 °C were 134 and only 47 at 28 °C and 40 °C. Altogether 21 protein spots were found to be expressed at all three temperatures (Table 3).
For F. endolithicus the analysis of the 2D gels (Fig 2, J–L) at 1 °C revealed an increase from 425 to 466 spots with an obvious change in the protein pattern. Mostly high molecular weight spots – within the range 70–170 kDa – and with pI values between 6 and 7, were observed. At 28 °C many proteins, respectively in the pI and mol. wt range 5–7 and 25–90 kDa, disappeared resulting in a total of 284 protein spots. When overlapping the gels, 278 spots were found at both 15 °C and 1 °C, while comparing the growth at 15 °C and 28 °C, 224 spots were matching. At all temperatures tested 187 common spots were detected (Table 3).
Protein pattern of black fungi as compared to Penicillium chrysogenum
The protein patterns of all the strains were analysed using P. chrysogenum as reference strain (Table 4). At standard incubation temperature (28 °C) P. chrysogenum shared 46 protein spots with Exophiala jeanselmei and 43 with Coniosporium perforans. An equal number of protein spots (43) was also shared by P. chrysogenum and Friedmanniomyces endolithicus – this incubated at 15 °C – while only 3 spots were detected as common among all samples. A similar result was obtained at 1 °C: 37 spots matched in P. chrysogenum and E. jeanselmei, while 59 spots and 46 in P. chrysogenum and, respectively, C. perforans and F. endolithicus. Only 6 protein spots were commonly found in all strains at 1 °C. The analysis of the samples exposed to 40 °C revealed the presence of 50 common protein spots in P. chrysogenum and E. jeanselmei and of 62 and 46 in P. chrysogenum and C. perforans and F. endolithicus respectively; merely 2 spots matched in all the strains. At 1 °C, but not at 28 °C and 40 °C, the matching spots were mainly represented by proteins with high molecular weight.
Table 4.
Number of matching protein spots detected. Gel matching was carried out selecting P. chrysogenum as reference strain.
| Strain | Reference strain | Number of pairs at each exposure condition |
||
|---|---|---|---|---|
| 28 °C | 1 °C | 40 °C | ||
| E. jeanselmei | P. chrysogenum | 46 | 37 | 50 |
| C. perforans | P. chrysogenum | 43 | 59 | 62 |
| 15 °C/28 °C | 1 °C | 28 °C/40 °C | ||
| F. endolithicus | P. chrysogenum | 43 | 46 | 46 |
| All strains | P. chrysogenum | 3 | 6 | 2 |
Common protein spots within black fungi as compared to Friedmanniomyces endolithicus
The comparison among the black fungal strains was carried out choosing the extremophilic fungus Friedmanniomyces endolithicus as reference strain (Table 5). At 15 °C and 28 °C respectively, 65 matching protein spots were detected in F. endolithicus and Exophiala jeanselmei, 68 in F. endolithicus and Coniosporium perforans and 17 spots were matching in all the samples. At 1 °C, 41 matching spots were observed as common between F. endolithicus and E. jeanselmei and 62 were common with C. perforans. Among the three fungi only 9 spots matched at 1 °C. The number of common spots decreased after exposure to 40 °C and 28 °C (the latter for F. endolithicus): While only 7 protein spots matched in all strains, 29 were detected as common in F. endolithicus and E. jeanselmei and 44 in F. endolithicus and C. perforans.
Table 5.
Number of matching protein spots detected. Gel matching was carried out selecting F. endolithicus as reference strain.
| Strain | Reference strain | Number of pairs at each exposure condition |
||
|---|---|---|---|---|
| 28 °C/15 °C | 1 °C | 40 °C/28 °C | ||
| E. jeanselmei | F. endolithicus | 65 | 41 | 29 |
| C. perforans | F. endolithicus | 68 | 62 | 44 |
| All strains | F. endolithicus | 17 | 9 | 7 |
Discussion
This study is the first contribution on the response of extremotolerant and extremophilic black fungi towards suboptimal temperatures, through the investigation of protein patterns. After exposure to different temperatures, qualitative changes – concerning the total number of spots, their molecular weight as well as their pI – suggested that the temperature response of black fungi differs considerably from mesophilic fungi and involves proteins that are hitherto unidentified and unexploited.
Generally, growth at low and high temperature requires diverse adaptations (Maheshwari et al. 2000; Margesin et al. 2007; Casanueva et al. 2010) and ‘proteins are the main targets of these adaptations as they control the equilibrium between substrate and products, influx of nutrients, outflow of waste products, macromolecular assemblies, nucleic-acid dynamics and appropriate folding’ (D'Amico et al. 2006). Also in mesophilic fungi stabilizing proteins as MC, HSPs, and CSPs are the most important effects to temperature stress (Haslbeck et al. 2005; Piette et al. 2010, 2011) but another consequence of non-optimal growth conditions can also be the down-regulation of the metabolism and the proteins involved. Because the proteome of Penicillium chrysogenum has been widely characterized (Jami et al. 2010a, 2010b) it was used as a reference to the black fungi. In this study and in accordance with literature data (Raggam et al. 2011) P. chrysogenum, when exposed to 40 °C, exhibited a remarkable over-expression of proteins, which can clearly be interpreted as the synthesis of HSPs (Fig 2, A–C). The slight decrease in the number of spots exhibited at 1 °C indicates a downregulation of the metabolic activity.
The black fungi, when exposed to a temperature that is significantly above their growth regime, showed a reaction different from P. chrysogenum: All three stains responded to 40 °C and 28 °C – the latter for Friedmanniomyces endolithicus – with a reduction of the total number of protein spots (Fig 2, F, I, L) thus indicating a lack of a heat-shock response on the protein level. Interestingly, spots from the same pI and molecular weight range (respectively 5–7 and 30–90 kDa) were extinct after temperature increase thus suggesting that the strains probably downregulated similar sets of proteins. From this it can be concluded that the basic set of proteins necessary to survive high temperature is stable without the help of HSPs or that other, non-protein protective metabolites and molecules are involved. In E. jeanselmei and Coniosporium perforans the lack of a heat shock response might on the one hand reflect the necessity to survive temperatures up to 60 °C that are easily reached on the sun exposed rock surfaces inhabited by these fungi and moreover it helps to save energy – otherwise needed for the production of protective proteins – in an extremely oligotrophic habitat. Also in F. endolithicus an explanation for the lack of a heat shock response can be found in its ecology: The fungus is endemic in a permanently cold habitat where a heat-shock response was not developed during evolution (Hofmann et al. 2000). However, for non-endemic psychrophilic Antarctic yeasts a heat shock response was demonstrated (Deegenaars & Watson 1998).
In contrast to what was observed at high temperatures, the black fungi considerably increased the number of proteins at 1 °C (Fig 2, E, H, K). Friedmanniomyces endolithicus and C. perforans especially exhibited high molecular weight spots in the mol. wt range from 70 to 170 kDa. In E. jeanselmei the total number of spots did not change significantly but a remarkable modification of the expression pattern – mostly spots with a molecular weight between 25 and 100 kDa – was detected in response to the temperature decrease. The change of the protein patterns that occurred in the mesophilic fungi C. perforans and E. jeanselmei at 1 °C can be interpreted as a cold-shock response. Especially the significant increase of protein spots in C. perforans suggests the transient up-regulation of CSPs and HSPs, key proteins directly involved in the cell protection against the stress induced by temperature (Jones et al. 1987; Berry & Foegeding 1997; Phadtare & Inouye 2004). Also for psychrophilic organisms the production of CSPs is well known, however with the addition of special adaptations which are absent in mesophiles and also include the lack of repression of house-keeping protein synthesis (D'Amico et al. 2006). Antifreeze proteins (AFPs) and other cold-acclimation proteins (CAPs) have been demonstrated in some organisms (De Cross & Bidochka 2001; Jia & Davies 2002; Feller & Gerday 2003; Gocheva et al. 2006; Collins et al. 2007; Timperio et al. 2008). According to the results of this study, the production of CAPs can be hypothesized also for F. endolithicus.
The results of this study gave significant evidence that the temperature response – and possibly the general stress response in this special group of fungi – differs considerably from the response of mesophilic hyphomyctes as P. chrysogenum. Further it can be concluded that survival of cells without expression of protective proteins is either based on the thermostability of the basic sets of proteins present, on other protective molecules in the cell or on cellular mechanisms that are still unknown. The hypothesis that a special set of proteins is present in black fungi is supported by the fact that the maximum overlap between protein patters found in black fungi and in P. chrysogenum was 13 %. Thus, the results of this study give promising indications that the black fungi might be sources for a number of new proteins that do not commonly occur in mesophilic fungi and that could be of great biotechnological interest. This hypothesis, however, will have to be evaluated based on protein identification and de-novo sequencing. Also the influence of nutrient availability, water activity (aw), osmotic stress, the solutes, and chemical composition of the rock as well as other biochemical and physical parameters on the stress tolerance and on the protein expression of these fungi, will be the focus of future investigations (Grant 2004; Chin et al. 2010). Currently, a unique climate chamber to simulate multiple stress factors on terrestrial organisms is constructed at BOKU University and will allow reproducing different environmental conditions separately or in combination with each other.
Acknowledgements
The work was financed by an FWF grant No. P24206-B16. We thank the VIBT-EQ GmbH and the City of Vienna (Zentrum für Innovation und Technologie) for supporting the VIBT-Extremophile Center and the research on black fungi. We thank also the University of Tuscia, Faculty of MM FF SS, for students grant. Martina Marchetti (TU Vienna) is acknowledged for valuable discussions on the topic of protein analysis.
Corresponding Editor: Anna Rosling
Footnotes
Supplementary data related to this article can be found online at http://dx.doi.org/10.1016/j.funbio.2012.06.004.
Contributor Information
Donatella Tesei, Email: donatella.tesei@boku.ac.at.
Gorji Marzban, Email: gorji.marzban@boku.ac.at.
Kristina Zakharova, Email: k.zakharova@boku.ac.at.
Daniela Isola, Email: isola@unitus.it.
Laura Selbmann, Email: selbmann@unitus.it.
Katja Sterflinger, Email: katja.sterflinger@boku.ac.at.
Appendix A. Supplementary data
References
- Albanese V., Yen-Wen Yam A., Baughman J., Parnot C., Frydman J. Systems analyses reveal two chaperone networks with distinct functions in eukaryotic cells. Cell. 2006;124:75–88. doi: 10.1016/j.cell.2005.11.039. [DOI] [PubMed] [Google Scholar]
- Alonso-Monge R., Román E., Arana D.M., Pla J., Nombela C. Fungi sensing environmental stress. Clinical Microbiology and Infection. 2009;15:17–19. doi: 10.1111/j.1469-0691.2008.02690.x. [DOI] [PubMed] [Google Scholar]
- Bahn Y., Xue C., Idnurm A., Rutherford J.C., Heitman J., Cardenas M.E. Sensing the environment: lessons from fungi. Nature Reviews. 2007;5:57–69. doi: 10.1038/nrmicro1578. [DOI] [PubMed] [Google Scholar]
- Baker B.J., Lutz M.A., Dawson S.C., Bond P.L., Banfield J.F. Metabolically active eukaryotic communities in extremely acidic mine drainage. Applied and Environmental Microbiology. 2004;70:6264–6271. doi: 10.1128/AEM.70.10.6264-6271.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J., Craig E.A. Heat-shock proteins as molecular chaperons. European Journal of Biochemistry. 1994;219:11–23. doi: 10.1007/978-3-642-79502-2_2. [DOI] [PubMed] [Google Scholar]
- Bjellqvist B., Pasquali C., Ravier F., Sanchez J.C., Hochstrasser D. A nonlinear wide-range immobilized pH gradient for two-dimensional electrophoresis and its definition in a relevant pH scale. Electrophoresis. 1993;14:1357–1365. doi: 10.1002/elps.11501401209. [DOI] [PubMed] [Google Scholar]
- Berry E.D., Foegeding P.M. Cold temperature adaptation and growth of microorganisms. Journal of Food Protection. 1997;60:1583–1594. doi: 10.4315/0362-028X-60.12.1583. [DOI] [PubMed] [Google Scholar]
- Burford E.P., Kierans M., Gadd G.M. Geomycology: fungi in mineral substrata. Mycologist. 2003;17:98–107. [Google Scholar]
- Casanueva A., Tuffin M., Cary C., Cowan D.A. Molecular adaptations to psychrophily: the impact of ‘omic’ technologies. Trends in Microbiology. 2010;18:374–381. doi: 10.1016/j.tim.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Chin J.P., Megaw J., Magill C.L., Nowotarski K., Williams J.P., Bhaganna p, Linton M., Patterson M.F., Underwood J.C., Mswaka A.Y., Hallsworth E. Solutes determine the temperature windows for microbial survival and growth. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:7835–7840. doi: 10.1073/pnas.1000557107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins T., Roulling F., Piette F., Marx J.C., Feller G., Gerday C., D'Amico S. Fundamentals of cold-adapted enzymes. In: Margesin R., Schinner F., Gerday C., Marx J.C., editors. Psychrophiles: from biodiversity to biotechnology. Springer; Berlin: 2007. pp. 211–227. [Google Scholar]
- D'Amico S., Collins T., Marx J.C., Feller G., Gerday C. Psychrophilic microorganisms: challenges for life. EMBO Reports. 2006;7:385–389. doi: 10.1038/sj.embor.7400662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadachova E., Bryan R.A., Huang X., Moadel T., Schweitzer A.D., Aisen P., Nosanchuk J.D., Casadevall A. Ionizing radiation changes the electronic properties of melanin and enhances the growth of melanized fungi. PLoS One. 2007;2:e457. doi: 10.1371/journal.pone.0000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantigny P., Marín S., Beyer M., Magan N. Mould germination: data treatment and modeling. International Journal of Food Microbiology. 2007;114:17–24. doi: 10.1016/j.ijfoodmicro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- De Cross J.N.A., Bidochka M.J. Cold-induced proteins in cold-active isolates of the insect-pathogenic fungus Metharhizium anisopliae. Mycological Research. 2001;105:868–873. [Google Scholar]
- de Hoog G.S. Evolution of black yeasts: possible adaptation to the human host. Antonie Van Leeuwenhoek. 1993;63:105–109. doi: 10.1007/BF00872386. [DOI] [PubMed] [Google Scholar]
- de Hoog G.S., Grube M. vol. 61. 2008. Black Fungal Extremes. (Studies in Mycology). The Netherlands. [Google Scholar]
- de Nadal E., Ammerer G., Posas F. Controlling gene expression in response to stress. Nature Reviews Genetics. 2011;12:833–845. doi: 10.1038/nrg3055. [DOI] [PubMed] [Google Scholar]
- Deegenaars M.L., Watson K. Heat shock response in psychrophilic and psychrotrophic yeast from Antarctica. Extremophiles. 1998;2:41–49. doi: 10.1007/s007920050041. [DOI] [PubMed] [Google Scholar]
- Feller G., Gerday C. Psychrophilic enzymes: hot topics in cold adaptation. Nature Reviews Microbiology. 2003;1:200–208. doi: 10.1038/nrmicro773. [DOI] [PubMed] [Google Scholar]
- Friedmann E.I. Endolithic microorganism in the antartic Cold Desert. Science. 1982;215:1045–1053. doi: 10.1126/science.215.4536.1045. [DOI] [PubMed] [Google Scholar]
- Gocheva Y.G., Krumova ETz, Slokoska L.S., Miteva J.G., Vassilev S.V., Angelova M.B. Cell response of Antarctic and temperate strains of Penicillium spp. to different growth temperature. Mycological Research. 2006;110:1347–1354. doi: 10.1016/j.mycres.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Gostinčar C., Grube M., de Hoog S., Zalar P., Gunde-Cimerman N. Extremotolerance in fungi: evolution on the edge. FEMS Microbiology Ecology. 2010;71:2–11. doi: 10.1111/j.1574-6941.2009.00794.x. [DOI] [PubMed] [Google Scholar]
- Gostinčar C., Grube M., Gunde-Cimerman N. Evolution of fungal pathogens in domestic environments? Fungal Biology. 2011;115:41008–41018. doi: 10.1016/j.funbio.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Grant W.D. Life at low water activity. Philosophical Transactions of the Royal Society. 2004;359:1249–1267. doi: 10.1098/rstb.2004.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunde-Cimerman N., Zalar P., de Hoog G.S., Plemenitaš A. Hypersaline waters in salterns – natural ecological niches for halophilic black yeasts. FEMS Microbiology Ecology. 2000;32:235–240. doi: 10.1111/j.1574-6941.2000.tb00716.x. [DOI] [PubMed] [Google Scholar]
- Haslbeck M., Franzmann T., Weinfurtner D., Buchner J. Some like it hot: the structure and function of small heat-shock proteins. Nature Structural & Molecular Biology. 2005;12:842–846. doi: 10.1038/nsmb993. [DOI] [PubMed] [Google Scholar]
- Hofmann G.E., Buckley B.A., Airaksinen S., Keen J.E., Somero G.N. Heat-shock protein expression is absent in the Antarctic Fish Trematomus bernacchii (Family Nototheniidae) The Journal of Experimental Biology. 2000;203:2331–2339. doi: 10.1242/jeb.203.15.2331. [DOI] [PubMed] [Google Scholar]
- Isola D., Marzban G., Selbmann L., Onofri S., Laimer M., Sterflinger K. Sample preparation and 2-DE procedure for protein expression profiling of black microcolonial fungi. Fungal Biology. 2011;115:971–977. doi: 10.1016/j.funbio.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Jami M.S., Barreiro C., García-Estrada C., Martín J.F. Proteome analysis of the Penicillin producer Penicillium chrysogenum: Characterization of protein changes during the industrial strain improvement. Molecular and Cellular Proteomics. 2010;9:1182–1198. doi: 10.1074/mcp.M900327-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jami M.S., García-Estrada C., Barreiro C., Cuadrado A.A., Salehi-Najafabadi Z., Martín J.F. The Penicillium chrysogenum extracellular proteome. Conversion from a food-rotting strain to a versatile cell factory for white biotechnology. Molecular and Cellular Proteomics. 2010;9:2729–2743. doi: 10.1074/mcp.M110.001412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Z., Davies P.L. Antifreeze proteins: an unusual receptor-ligand interaction. Trends in Biochemical Science. 2002;27:101–106. doi: 10.1016/s0968-0004(01)02028-x. [DOI] [PubMed] [Google Scholar]
- Jones P.G., Van Bogelen R.A., Neidhardt F.C. Induction of proteins in response to low temperature in Escherichia coli. Journal of Bacteriology. 1987;169:2092–2095. doi: 10.1128/jb.169.5.2092-2095.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus P.R., Heitman J. Coping with stress: calmodulin and calcineurin in model and pathogenic fungi. Biochemical and Biophisical Research Communications. 2003;311:1151–1157. doi: 10.1016/s0006-291x(03)01528-6. [DOI] [PubMed] [Google Scholar]
- Mafart P., Couvert O., Leguérinel I. Effect of pH on the heat resistance of spores: comparison of two models. International Journal of Food Microbiology. 2001;63:51–56. doi: 10.1016/s0168-1605(00)00397-4. [DOI] [PubMed] [Google Scholar]
- Maheshwari R., Bharadwaj G., Bhat M.K. Thermophilic fungi: their physiology and enzymes. Microbiology and Molecular Biology Reviews. 2000;64:461–488. doi: 10.1128/mmbr.64.3.461-488.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margesin R., Neuner G., Storey K.B. Cold-loving microbes, plants, and animals-fundamental and applied aspects. Naturwissenschaften. 2007;94:77–99. doi: 10.1007/s00114-006-0162-6. [DOI] [PubMed] [Google Scholar]
- Matos T., de Hoog G.S., de Boer A.G., Haase G. High prevalence of the neurotrope Exophiala dermatidis and related oligotrophic black yeasts in sauna facilities. Mycoses. 2002;45:373–377. doi: 10.1046/j.1439-0507.2002.00779.x. [DOI] [PubMed] [Google Scholar]
- Nakamoto H., Vígh L. The small heat shock proteins and their clients. Cellular and Molecular Life Sciences. 2007;64:294–306. doi: 10.1007/s00018-006-6321-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevarez L., Vasseur V., Le Dréan G., Tanguy A., Guisle-Marsollier I., Houlgatte R., Barbier G. Isolation and analysis of differentially expressed genes in Penicillium glabrum subjected to thermal stress. Microbiology. 2008;154:3752–3765. doi: 10.1099/mic.0.2008/021386-0. [DOI] [PubMed] [Google Scholar]
- Onofri S., Pagano S., Zucconi L., Tosi S. Friedmanniomyces endolithicus (Fungi, hyphomycetes), anam. -gen, and sp. nov., from continental Antarctica. Nova Hedwigia. 1999;68:175–181. [Google Scholar]
- Onofri S., Selbman L., de Hoog G.S., Grube M., Barreca D., Ruisi S., Zucconi L. Evolution and adaptation of fungi at boundaries of life. Advances in Space Research. 2007;40:1657–1664. [Google Scholar]
- Onofri S., de la Torre R., de Vera J.-P., Ott S., Zucconi L., Selbmann L., Scalzi G., Venkateswaran K.J., Rabbow E., Sanchez Iñigo F.J., Horneck G. Survival of rock-colonizing organisms after 1.5 year in outer space. Astrobiology. 2012;12:508–516. doi: 10.1089/ast.2011.0736. [DOI] [PubMed] [Google Scholar]
- Phadtare S., Inouye M. Genome-Wide transcriptional analysis of the cold shock response in wild-type and cold-sensitive, quadruple-csp-deletion strains of Escherichia coli. Journal of Bacteriology. 2004;186:7007–70014. doi: 10.1128/JB.186.20.7007-7014.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piette F., D'Amico S., Struvay C., mazzucchelli G., renaut J., Tutino M.L., Danchin A., Leprince P., Feller G. Proteomics at life at low temperatures: trigger factor is the primary chaperone in the Antarctic bacterium Pseudoalteromonas haloplanktis TAC125. Molecular Microbiology. 2010;76:120–132. doi: 10.1111/j.1365-2958.2010.07084.x. [DOI] [PubMed] [Google Scholar]
- Piette F., Struvay C., Feller G. The protein folding challenge in psychrophiles: facts and current issues. Environmental Microbiology. 2011;13:1924–1933. doi: 10.1111/j.1462-2920.2011.02436.x. [DOI] [PubMed] [Google Scholar]
- Raggam R.B., Salzer H.J.F., Marth E., Heiling B., Paulitsch A., Buzina W. Molecular detection and characterization of fungal heat shock protein 60. Mycoses. 2011;54:394–399. doi: 10.1111/j.1439-0507.2010.01933.x. [DOI] [PubMed] [Google Scholar]
- Rizwan M., Miller I., Tasneem F., Böhm J., Gemeiner M., Razzazi-Fazeli E. Mycotoxin Research. 2010;26:171–180. doi: 10.1007/s12550-010-0051-x. [DOI] [PubMed] [Google Scholar]
- Ruibal C., Platas G., Bills G.F. Isolation and characterization of melanized fungi from limestone formations in Mallorca. Mycological Progress. 2005;4:23–38. [Google Scholar]
- Selbmann L., de Hoog G.S., Mazzaglia A., Friedmann E.I., Onofri S. Fungi at the edge of life: cryptoendolithic fungi from Antarctic desert. Studies in Mycology. 2005;51:1–32. [Google Scholar]
- Selbmann L., de Hoog G.S., Gerrits van den Ende A.H.G., Ruibal C., De Leo F., Zucconi L., Isola D., Ruisi S., Onofri S. Drought meets acid: three new genera in a Dothidealean clade of extremotolerant fungi. Studies in Mycology. 2008;61:1–20. doi: 10.3114/sim.2008.61.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sert H.B., Sümbül H., Sterflinger K. Microcolonial fungi from antique marbles in Perge/Side/Termessos (Antalya/Turkey) Antonie Van Leeuwenhoek. 2007;91:217–227. doi: 10.1007/s10482-006-9111-9. [DOI] [PubMed] [Google Scholar]
- Shevchenko A., Wilm M., Vorm O., Mann M. Mass spectrometric sequencing of proteins from silver-stained polyacrylamide gels. Analytical Chemistry. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- Staley J.T., Palmer F., Adams B. Microcolonial fungi: common inhabitants on desert rocks? Science. 1982;215:1093–1095. doi: 10.1126/science.215.4536.1093. [DOI] [PubMed] [Google Scholar]
- Sterflinger K., De Baere R., de Hoog G.S., De Wachter R., Krumbein W.E., Haase G. Coniosporium perforans and C. apollinis, two new rock-inhabiting fungi isolated from marble in the Sanctuary of Delos (Cyclades, Greece) Antonie Van Leeuwenhoek. 1997;72:349–363. doi: 10.1023/a:1000570429688. [DOI] [PubMed] [Google Scholar]
- Sterflinger K., Krumbein W.E. Dematiacous fungi as a major agent of biopitting for Mediterranean marbles and limestones. Geomicrobiology Journal. 1997;14:219–230. [Google Scholar]
- Sterflinger K. Temperature and NaCl-tolerance of rock-inhabiting meristematic fungi. Antonie Van Leeuwenhoek. 1998;74:271–281. doi: 10.1023/a:1001753131034. [DOI] [PubMed] [Google Scholar]
- Sterflinger K., de Hoog G.S., Haase G. Phylogeny and ecology of meristematic ascomycetes. Studies in Mycology. 1999;43:5–22. [Google Scholar]
- Sterflinger K. Fungi as geologic agents. Geomicrobiology Journal. 2000;17:97–124. [Google Scholar]
- Sterflinger K., Prillinger H. Molecular taxonomy and biodiversity of rock fungal communities in an urban environment (Vienna, Austria) Antonie Van Leeuwenhoek. 2001;80:275–286. doi: 10.1023/a:1013060308809. [DOI] [PubMed] [Google Scholar]
- Sterflinger K. Black yeasts and meristematic fungi: ecology, diversity and identification. In: Seckbach J., editor. The Yeast Handbook. Biodiversity and Ecophysiology of Yeasts. Springer-Verlag, Berlin and Heidelberg GmbH & Co; 2005. pp. 501–514. [Google Scholar]
- Sterflinger K., Tesei D., Zakharova K. Fungi in hot and cold deserts with particular reference to microcolonial fungi. Fungal Ecology. 2012;5:453–462. [Google Scholar]
- Timperio A.M., Egidi M.G., Zolla L. Proteomics applied on plant abiotic stresses: role of heat shock proteins (HSP) Journal of Proteomics. 2008;71:391–411. doi: 10.1016/j.jprot.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Tresner H.D., Hayes J.A. Sodium chloride tolerance of terrestrial fungi. Applied Microbiology. 1971;22:210–213. doi: 10.1128/am.22.2.210-213.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urzí C., De Leo F., de Hoog G.S., Sterflinger K. Recent advances in the molecular biology and ecophysiology of meristematic stone-inhabiting fungi. In: Ciferri O., Tiano P., Mastromei G., editors. Proceedings of the International Congress of Microbes and Art. Plenum Publishing Co. Ltd.; New York, NY: 2000. pp. 3–19. [Google Scholar]
- Vember V.V., Zhdanova N.N. Peculiarities of linear growth of the melanin containing fungi Cladosporium sphaerospermum Perz. And Alternaria alternata (Fr.) Keissler. Mikrobiolohichnyĭ Zhurnal. 2001;63:3–12. [PubMed] [Google Scholar]
- Warscheid T., Braams J. Biodeterioration of stone: a review. International Biodeterioration & Biodegradation. 2000;46:343–368. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.