Abstract
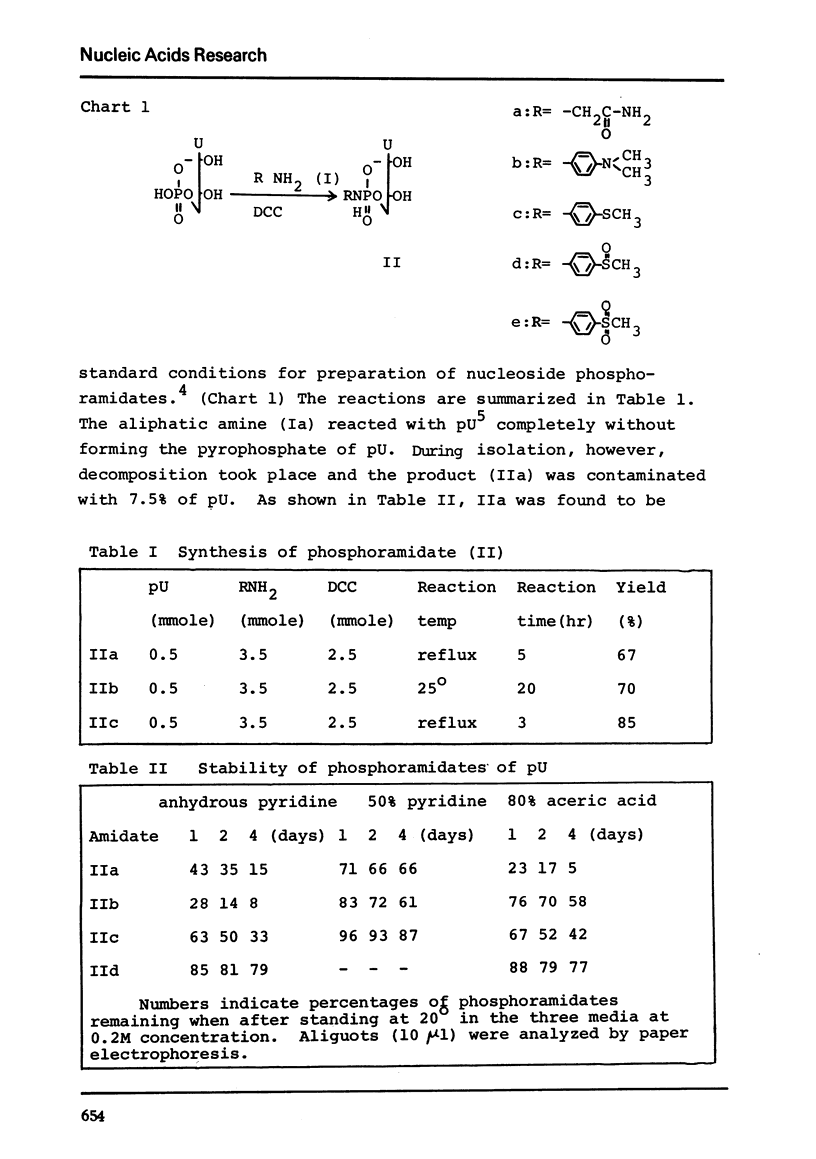
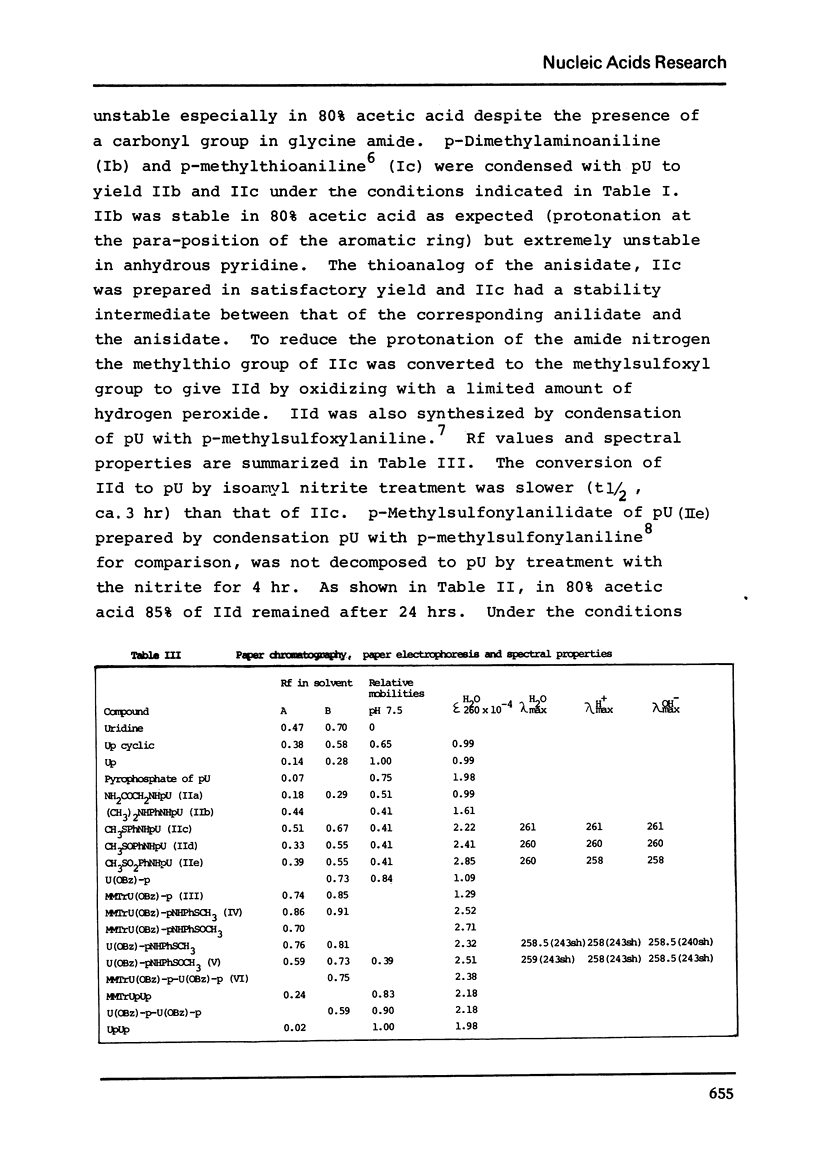
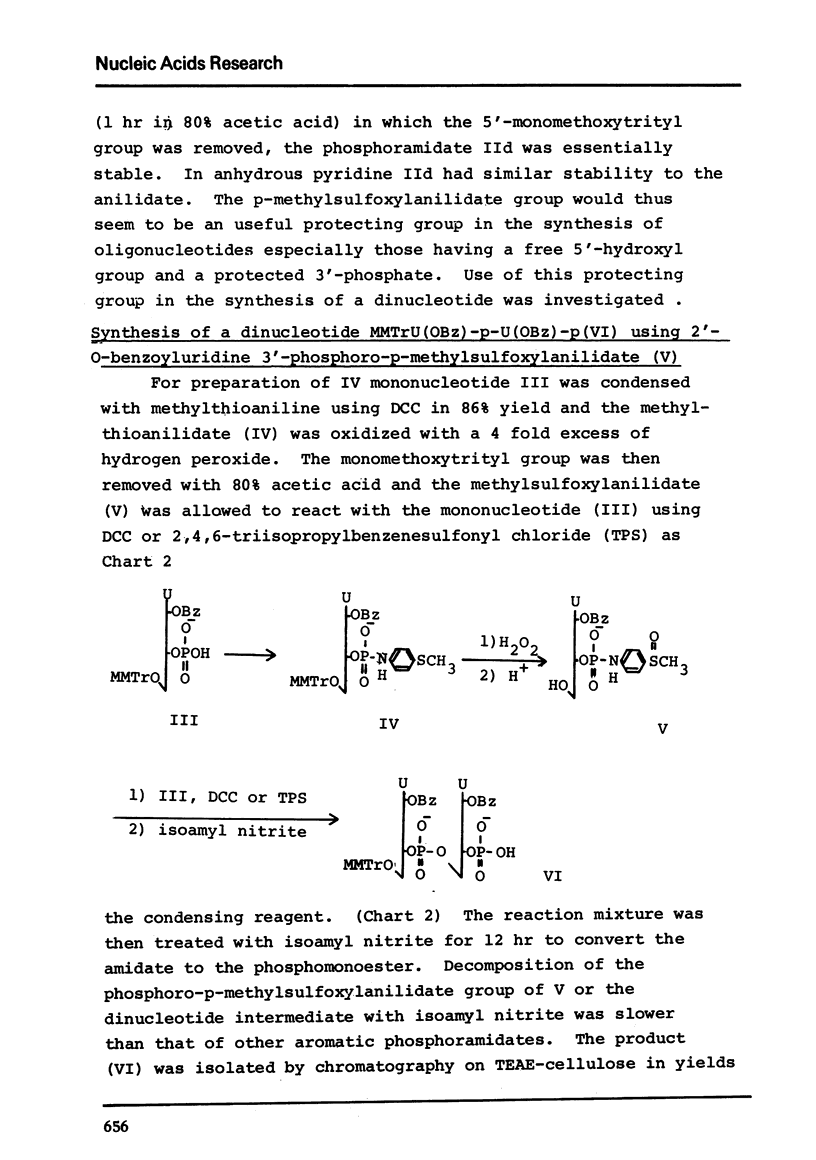
N, N-Dimethyl-p-phenylenediamine, glycine amide and p-methylthioaniline were condensed with uridine 5'-phosphate and the phosphoramidates obtained were tested for their stability in anhydrous pyridine, 50% aqueous pyridine or 80% acetic acid. The p-methylthioanilidate (IIc) was oxidized to give p-methylsulfoxylanilidate of uridine 5'-phosphate (IId) which was found to be 5 times more stable than the p-methylthio compound. The p-methylsulfoxylanilidate of 2'-O-benzoyluridine 3'-phosphate was condensed with the mononucleotide to yield the dinucleotide, MMTrU(OBz)-p-U(OBz)-p in 28% yield.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ohtsuka E., Murao K., Ubasawa M., Ikehara M. A new method for the synthesis of protected ribooligonucleotides with 3'-phosphate end groups. J Am Chem Soc. 1969 Mar 12;91(6):1537–1538. doi: 10.1021/ja01034a047. [DOI] [PubMed] [Google Scholar]
- Ohtsuka E., Murao K., Ubasawa M., Ikehara M. Studies on transfer ribonucleic acids and related compounds. I. Synthesis of ribooligonucleotides using aromatic phosphoramidates as a protecting group. J Am Chem Soc. 1970 Jun 3;92(11):3441–3445. doi: 10.1021/ja00714a036. [DOI] [PubMed] [Google Scholar]
- Otsuka E., Honda A., Shigyo H., Morioka S., Sugiyama T. Studies on transfer ribonucleic acids and related compounds. 8(1). Further studies on aromatic phosphoramidates as a protecting group for phosphomonoesters. Nucleic Acids Res. 1974 Feb;1(2):223–234. doi: 10.1093/nar/1.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka E., Ubasawa M., Morioka S., Ikehara M. Studies on transfer ribonucleic acids and related compounds. VI. Synthesis of yeast alanine transfer ribonucleic acid 3'-terminal nonanucleotides and 5'-terminal hexanucleotides. J Am Chem Soc. 1973 Jul 11;95(14):4725–4733. doi: 10.1021/ja00795a042. [DOI] [PubMed] [Google Scholar]


