Abstract
RNA binding proteins are characterized as a new family of apoptosis inducers; however, the mechanism by which they induce apoptosis is poorly understood. KHDC1 family members were recently identified as K-homology (KH)-domain containing RNA binding proteins that are unique to eutherian mammals and highly expressed in oocytes. In this study, we report that the expression of KHDC1A induces caspase-3 dependent apoptosis and inhibits mRNA translation, and the translational repression is independent of apoptosis. We demonstrate that both the N-terminus and C-terminus of KHDC1A are required for its pro-apoptotic and translational repression activities. Furthermore, in the C-terminus of KHDC1A, a putative trans-membrane motif (TMM) is critical for these activities. In addition, the ectopically expressed KHDC1A is localized to the endoplasmic reticulum (ER) and changes the morphology of the ER. The inhibition of ER-specific caspase-12 successfully rescues KHDC1A-induced apoptosis, but not Fas-induced apoptosis. Taken together, we conclude that KHDC1A functions as a global translational repressor and induces apoptosis through an ER-dependent signaling pathway.
This article describes a novel apoptosis pathway requiring an RNA binding protein, KHDC1A, which has a transmembrane domain and localizes to the endoplasmic reticulum.
Introduction
Programmed cell death, or apoptosis, is a physiological process required in mammals for normal development and tissue homeostasis. Alterations in the apoptotic program have been implicated in pathogenesis (Thompson, 1995; Vaux and Korsmeyer, 1999; Meier et al., 2000). Three major apoptotic pathways have been identified, which are orchestrated by distinct cellular loci and organelles (Adams, 2003). One apoptotic pathway is mediated by extrinsic ligand and specified cell-surface death receptors, resulting in the caspase-8 activation. The second pathway is signaled by diverse intrinsic metabolic stresses that converge to the mitochondria, consequently triggering the release of the cytochrome c and the activation of caspase-9. The third apoptotic pathway is implicated in endoplasmic reticulum (ER) by activating caspase-12 (Budihardjo et al., 1999). Eventually, all three pathways activate the executive caspase-3 and induce the cleavage of many downstream mediators, including poly (ADP-ribose) polymerase (PARP). While the first two pathways have been extensively investigated, details of the ER-mediated apoptotic pathway and its relationship with the mitochondrial pathway remain to be further elucidated (Szegezdi et al., 2003; Shiraishi et al., 2006; Puthalakath et al., 2007).
K-homology (KH) domain-containing RNA binding proteins are involved in various aspects of RNA metabolism, ranging from transcription to RNA splicing, transportation, translation, and stability (Valverde et al., 2008). Recently, these proteins have been reported as being a family of apoptotic inducers. For example, the mouse single KH domain containing protein, quaking protein isoform 7 (QKI-7), induces cell death independent of any known signals. The expression of QKI-7 causes apoptosis in transfected cells, and the C-terminal 14-amino-acid fragment of the QKI-7 appears necessary and sufficient to induce apoptosis (Pilotte et al., 2001). Kep1 is a single KH domain-containing protein identified in Drosophila. Ectopic expression of Kep1 in the fly cell line or HeLa cells induces apoptosis (Di Fruscio et al., 1998, 2003).The overexpression Sam68 (Src-associated in mitosis, 68 kDa) in fibroblasts results in both cell-cycle arrest and apoptosis (Taylor et al., 2004). In addition, proteins carrying multiple KH domains also play a role in apoptosis. MCG10, a p53-regulated KH domain-containing gene, suppresses cell proliferation by inducing apoptosis and arresting cell cycle at the G2/M transition (Zhu and Chen, 2000). Similarly, overexpression of the Drosophila homolog of the fragile X protein leads to apoptotic cell loss in all the adult tissues examined (Wan et al., 2000). In Jurkat cells, the expression pattern of 21 proteins in 2D gels was significantly changed during Fas-induced apoptosis, of which 15 contain RNA binding motifs, suggesting an important role for RNA binding proteins in the apoptotic process (Thiede et al., 2001). Although emerging evidence has indicated that KH domain-containing RNA binding proteins are closely associated with apoptosis, the mechanism has not yet been fully understood.
Khdc1a/Ndg1 was originally identified in T-cells as being a transcript induced by Nur77 orphan nuclear receptor. Rajpal et al. (2003) have shown that the overpression of Khdc1 induces apoptosis in 293T cells in vitro as well as T cells in vivo. Subsequently, studies indicated that the Khdc1 genes are unique to eutherian mammals and abundantly expressed in growing oocytes (Pierre et al., 2007). Our previous work revealed that KHDC1A and KHDC1B proteins interact with cytoplasmic polyadenylation element-binding protein (CPEB), a key translational regulator controlling the polyA length of mRNAs. We also found that the expression of KHDC1A induced cell death in Xenopus embryos (Cai et al., 2010). In the present study, we investigate how KHDC1A induces apoptosis and the domain that is critical for this activity. We demonstrate that KHDC1A induces apoptosis through the ER pathway and that the C-terminal putative trans-membrane motif (TMM) is critical for its activities.
Materials and Methods
Plasmid construction
pCS2 vector and its derivative of pCS2-Flag were used in this study. Information on pCS2 is available at http://sitemaker.umich.edu/dlturner.vectors/home. The coding sequence of Khdc1a, Khdc1b, and embryonic stem cell-specific gene 1 (Esg1) were cloned into pCS2-Flag, and the coding sequences of Firefly and Renilla luciferases were cloned into pCS2. Characteristics of the constructs were listed in Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/dna).
Cell culture and transfection
Cell culture and transfection were previously described (Cai et al., 2010). Briefly, HeLa cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) at 37°C and 5% CO2. Transfection was conducted in a six-well plate or a 60 mm culture plate. About 200 or 500 ng plasmids were used for each transfection in a six-well plate or a 60 mm plate, respectively. For the luciferase assay, 25 ng Firefly and 10 ng Renilla luciferase plasmids were used for each transfection in the 60 mm plate. For the apoptosis inhibition assay, caspase-3 or caspase-12 inhibitors Z-DEVD FMK or Z-ATAD FMK (BioVision) were added to the media 6 h after transfection with a final concentration of 20 μM. For the control experiment, the nontransfected Hela cells were pretreated with mock or 1 μg/mL of anti-Fas/Apo1 antibodies (Biovision) for 6 h and then exposed to 20 μM of caspase-12 inhibitors Z-ATAD FMK for another 48 h.
Western blot analysis and antibodies
The cells were first harvested and lysis buffer containing Tris-HCl, NaCl, and NP-40 (EBC) (50 mM Tris-HCl, [pH 7.5], 120 mM NaCl, 0.5% Nonidet P-40 supplemented with protease inhibitor cocktail [Roche] and PhosSTOP Phosphatase Inhibitor Cocktail [Roche]). Lysates were then clarified by centrifugation with 16,100 g for 15 min at 4°C, and the supernatant was collected as protein lysate. Proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to Immobilon-P membrane (Millipore). The membrane was blocked in buffer containing Tris, NaCl, and Tween (TBST) (10 mM Tris [pH 8.0], 150 mM NaCl, and 0.1% Tween 20) supplemented with 5% (wt/vol) powdered milk and then incubated with primary antibodies at 4°C overnight. Anti-Flag, anti-β-tubulin antibodies were used with a dilution of 1:5000, and antibodies against caspase-12, cleaved caspase-3, and cleaved PARP were used at a 1:2000 dilution. The membrane were then washed thrice with TBST at room temperature and incubated with a horseradish peroxidase-conjugated secondary antibody (GE healthcare) for 1 h. After the membrane had been washed thrice with TBST, the signal was detected by ECL plus (GE Healthcare) according to the manufacturer's protocol. Primary antibodies specific to Flag and β-tubulin were purchased from Sigma with catalog numbers of F1804 and T5201, respectively. Antibodies against cleaved PARP and cleaved caspase-3 were from Cell Signaling technology with catalog numbers of #9541 and #9664.
Immunofluorescence staining
For immunofluorescence staining, cultured cells were fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100 in phosphate-buffered saline, and blocked in 3% bovine serum albumin in phosphate-buffered saline (PBS) at room temperature for 1 h. After incubation with primary antibody at room temperature for 1.5 h, the samples were washed thrice with PBS and incubated in diluted secondary antibody at room temperature for 1 h. The samples were then washed thrice with PBS and mounted in VECTASHIELD Mounting Medium (Vector labs). Images were collected with the TCS SP2 confocal microscope (Leica) and analyzed with Volocity software (Perkin Elmer). Calnexin-specific antibody was from Sigma (Catalog number: C4731).
Apoptosis analysis by flow cytometry
Cells were harvested at 24 h after transfection, and fixed in 70% ethanol. Then, the cells were washed thrice with PBS and stained in 50 μg/mL propidium iodide and 0.1 mg/mL RNaseA in PBS. The DNA content was analyzed by flow cytometry (Coulter Epics XL, Beckman), and apoptosis was determined by measuring the sub-G1 peak. The data were analyzed with the WinMDI software.
Luciferase assays
Cells were transfected and harvested 24 h after transfection. Luciferase assays were performed using Dual-glo luciferase reagents (Promega) following the manufacturer's protocol. Data were collected with GloMax™ 20/20 Luminometer (Promega).
Real-time reverse transcription–polymerase chain reaction
Cells were transfected and harvested at different time points as indicated. RNA isolation, reverse transcription, and real-time polymerase chain reaction (PCR) were performed as previously described (Cai et al., 2010). Raw values were normalized against β-actin (forward primer: 5-AGCGGGAA ATCGTGCGTGAC, reverse primer: 5-CAATGGTGATGAC CTGGCCGT). PCR primers for Renilla luciferase were 5-GCTTATCTACGTGCAAGTGA (forward) and 5-AGAA CTCGCTCAACGAACGA (reverse), and PCR primers for Firefly luciferase were 5-GGAATCCATCTTGCTCCAAC (forward) and 5-GTTACTTGACTGGCGACGTA (reverse).
35S incorporation
HeLa cells grown in 6-well plates were transfected with specified plasmids. Twenty hours after transfection, cells were labeled for 1 h in free DMEM (Invitrogen) with 5% dialyzed FBS (Invitrogen) and 35S methionine-cysteine protein labeling mix (Perkin Elmer, NEG072014MC). Following the labeling reaction, the media were aspirated, and the cells were washed with PBS and lysed in EBC buffer. Proteins were precipitated with cold 20% trichloroacetic acid. 10 μL of each cell lysate was dotted onto glass fiber filters and washed with 5% trichloroacetic acid and 90% ethanol. 35S incorporation was measured by using a beta scintillation counter. The results were normalized to the amount of total proteins analyzed by bicinchoninic acid assay.
Results
Overexpression of KHDC1A induces apoptosis
The Khdc1a and Khdc1b genes encode for a pair of closely related KH-domain containing proteins. Both genes are highly expressed in growing oocytes and early-stage embryos. In a previous study, we demonstrated that KHDC1B specifically interacts with CPEB and exclusively inhibits the activity of CPEB (Cai et al., 2010). In contrast, although KHDC1A interacts with CPEB, the function of KHDC1A is largely elusive. Protein sequence analyses indicate that both KHDC1A and KHDC1B contain an atypical KH domain at their N-termini and share 85% identity with each other. Compared with KHDC1B, however, KHDC1A contains an extra stretch of amino acids at the C-terminus (Supplementary Fig. S1A). This extra C-terminus contains 40 amino acids, among which 21 are hydrophobic and 6 are polar amino acids (Supplementary Fig. S1B). To test the function of KHDC1A and KHDC1B, we overexpressed them in HeLa cells. Despite their high degree of sequence homology, the transfection of KHDC1A, but not KHDC1B, resulted in a phenotype resembling apoptosis. A significant portion of KHDC1A-transfected cells had a shrunk morphology and were found floating in the growth media. Given that KHDC1A has been suggested to induce apoptosis (Rajpal et al., 2003), we decided to verify our observation in HeLa cells by immunostaining, flow cytometry, and Western blot analysis.
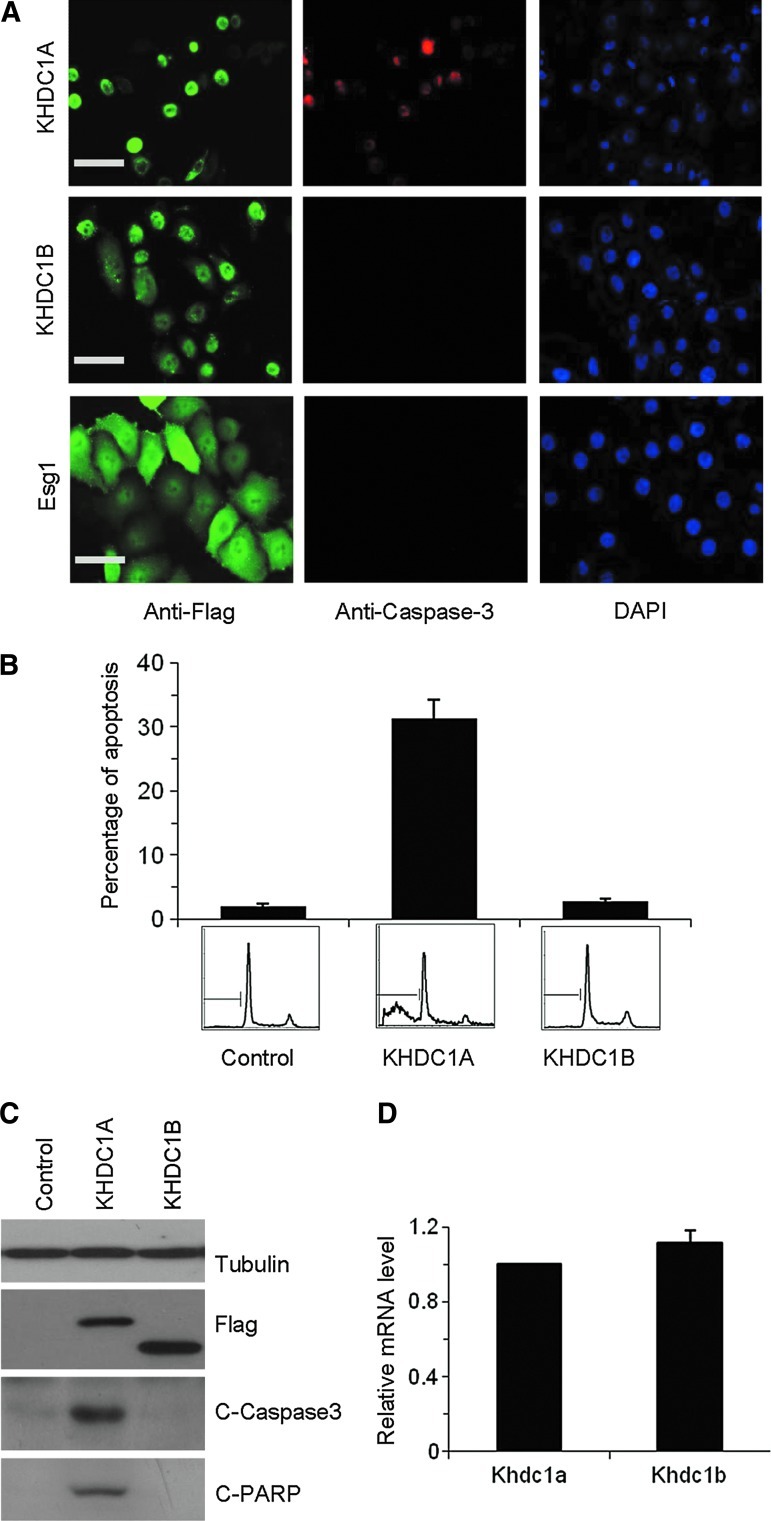
To carry out immunostaining experiments, we expressed Flag-tagged KHDC1A, KHDC1B and the distantly related KH domain containing protein Esg1 in HeLa cells and examined the expression of cleaved caspase-3, a typical apoptosis marker. We included Esg1 as a control, because it is also a KH domain-containing protein that is highly expressed in oocytes, early embryos, and embryonic stem cells. Based on their origination, gene sequence, protein structure, and expression pattern, these three proteins are part of a larger super-family of the KH domain protein (Amano et al., 2006; Tanaka et al., 2006; Pierre et al., 2007). As shown in Figure 1A, while all the three proteins were robustly expressed on transfection, the cleaved caspase-3 was exclusively detected in the KHDC1A expressing cells. Consistently, 4′,6-diamidino-2-phenylindole staining revealed shrunk nuclei and condensed chromatin in the KHDC1A-transfected cells but not the control cells (Fig. 1A). These results suggested that KHDC1A, but not KHDC1B or Esg1, is able to specifically induce apoptosis. We performed flow cytometry analysis to quantify the percentage of apoptosis (sub-G1 peak) in the KHDC1A, KHDC1B, and Esg1-transfected samples. As shown in Figure 1B, the sub-G1 peak was obviously present in the cells transfected with KHDC1A but absent in the cells expressing the control proteins. On average, 30% of cells were apoptotic on transfection of KHDC1A, which is significantly different from the basal level of apoptosis (<3%) for the control cells (Fig. 1B). In addition, we conducted Western blot analysis to examine the expression level of cleaved caspase-3 and cleaved PARP, which are other important mediators and markers of apoptosis. As expected, both apoptotic markers were readily detected in cells expressing KHDC1A but were undetectable in cells expressing KHDC1B, confirming that KHDC1A specifically induces apoptosis (Fig. 1C). This apoptotic activity occurred despite the fact that the KHDC1A protein appeared to be expressed at lower levels than KHDC1B (Fig. 1C). Real-time PCR results further revealed that the mRNA levels of Khdc1a and Khdc1b were comparable (Fig. 1D), indicating that the transfection efficiencies and transcription of KHDC1A and KHDC1B were similar. The reduced level of the KHDC1A protein could indicate differences in protein stability or translational efficiency.
FIG. 1.
Overexpression of KHDC1A induces apoptosis. (A) Detection of apoptosis by cleaved caspase-3 and DAPI staining. HeLa cells were transfected with Flag-tagged KHDC1A, KHDC1B, or Esg1 plasmids, and immunostaining was performed with antibodies against Flag (FITC, green) and cleaved caspase-3 (Cy5, red). The nucleus was counterstained with DAPI (blue). The scale bar was 100 μm. (B) Detection of apoptosis by Sub-G1 assay. HeLa cells were transfected with control vector, Flag-tagged KHDC1A, or KHDC1B plasmids. Cells were fixed and stained with propidium iodide. The apoptosis was analyzed by flow cytometry assay. The up panel showed the data summarized from three independent flow cytometry assays. The bottom panel showed the representative flow cytometry result. (C) Detection of apoptosis by Western blot analysis. HeLa cells were transfected with control vector, Flag-tagged KHDC1A, or KHDC1B plasmids. Cells were harvested, and Western blot analysis was performed with antibodies against β-tubulin, Flag, cleaved caspase-3, and cleaved PARP. (D) The mRNA level of transfected khdc1a and khdc1b was analyzed with real-time PCR. DAPI, 4′,6-diamidino-2-phenylindole; Esg1, embryonic stem cell-specific gene 1; FITC, fluorescein isothiocyanate; PARP, poly (ADP-ribose) polymerase; PCR, polymerase chain reaction.
KHDC1A inhibits translation
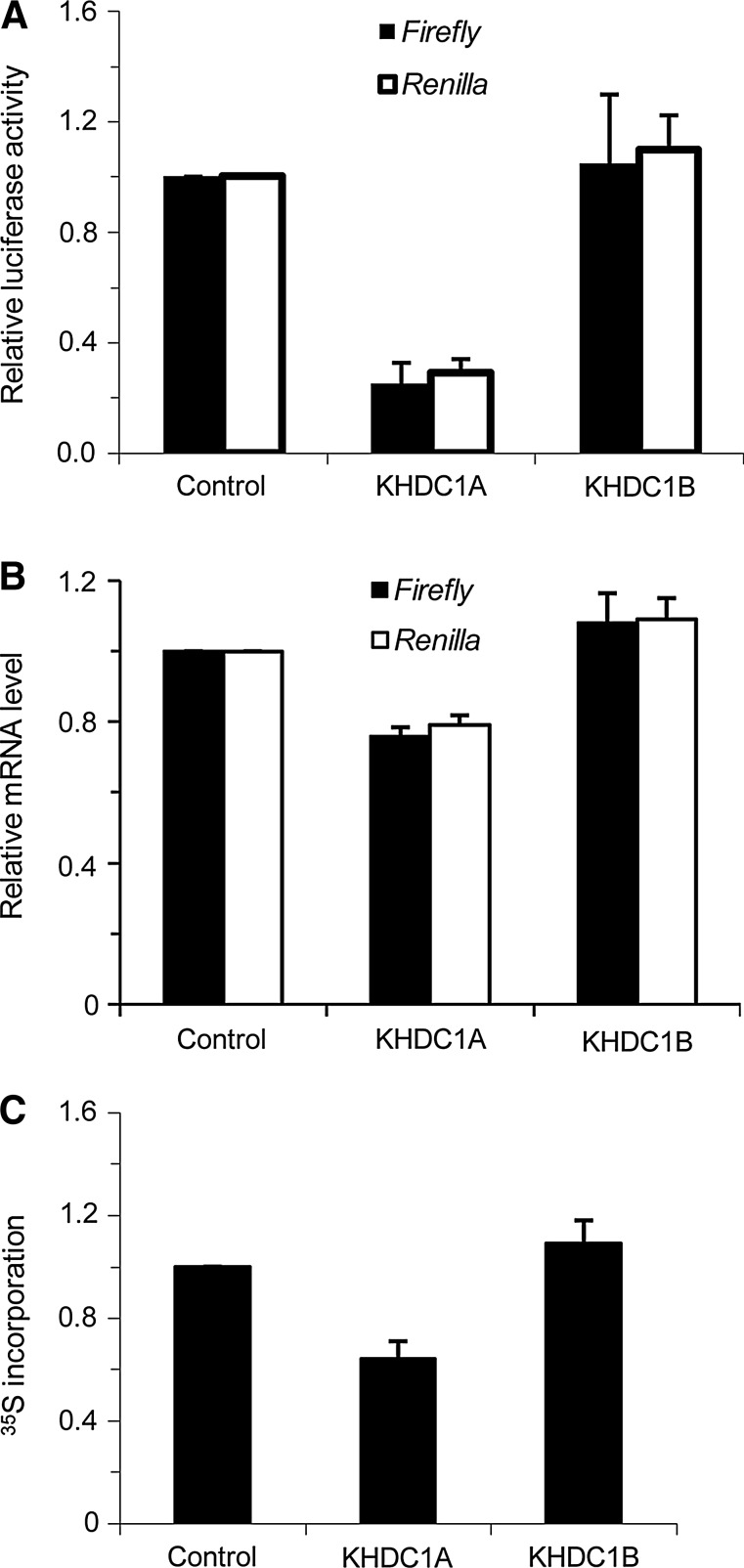
Many KH-domain containing proteins serve as either target-specific or general translational repressors (Zalfa et al., 2003; Mootz et al., 2004). Our previous data has shown that KHDC1 family members can interact with known translational regulators, CPEB (Cai et al., 2010). In addition, the consistently lower expression efficiency of KHDC1A protein could be the result of translational repression (Fig. 1C). To test whether KHDC1A can act as a translational repressor, we examined the influence of KHDC1A on the expression of co-transfected Firefly and Renilla luciferases, two reporters functionally irrelevant to the KHDC1A protein. As shown in Figure 2A, the co-expression of KHDC1A with Firefly luciferase significantly reduced the protein level of the reporter, as monitored by the luciferase activity. Compared with the vector or KHDC1B control, a reduction of 75% of Firefly luciferase activity was observed in KHDC1A-expressing samples, indicating that KHDC1A interferes with the expression of the Firefly luciferase when co-expressed. Results from the Renilla luciferase reporter appeared consistent with those of Firefly luciferase, indicating that the repression by KHDC1A is general. To examine whether this repression occurs at the translational level, we assayed the mRNA levels of the luciferase reporters. Results showed that the mRNA levels were only slightly lower in the KHDC1A-expressing sample, with a 21% reduction compared with the control samples (Fig. 2B). Hence, the repression mediated by KHDC1A occurs mainly at the level of translation, although the transcription efficiency and/or mRNA stability may also contribute to the repression. To further confirm that the repression is general and occurs at the translational level, we conducted a 35S labeling experiment to examine the rate of total protein synthesis in KHDC1A cells. As shown in Figure 2C, KHDC1A significantly inhibited the incorporation of 35S labeled methionine/cysteine, with a reduction of more than 40% compared with KHDC1B. Taken together, these results indicate that KHDC1A functions as a general translational repressor.
FIG. 2.
KHDC1A is a general translational repressor. (A) HeLa cells were co-transfected with Firefly or Renilla luciferase reporters and either control, KHDC1A, or KHDC1B expressing plasmids. Twenty-four hours after transfection, the cells were harvested, and luciferase assays were performed to examine the protein level of reporters. Data were summarized from three independent experiments. (B) HeLa cells were transfected and harvested as just described. The RNAs were prepared, and the mRNA level of reporters was examined by real-time PCR. The luciferase mRNA level was normalized to the level of β-actin. Data were summarized from three independent experiments. (C) HeLa cells were transfected with control vector, KHDC1A, or KHDC1B expressing plasmids, and 35S labeling analysis was used to determine translational efficiency. Data were summarized from three independent experiments.
KHDC1A-induced translational repression is independent of apoptosis
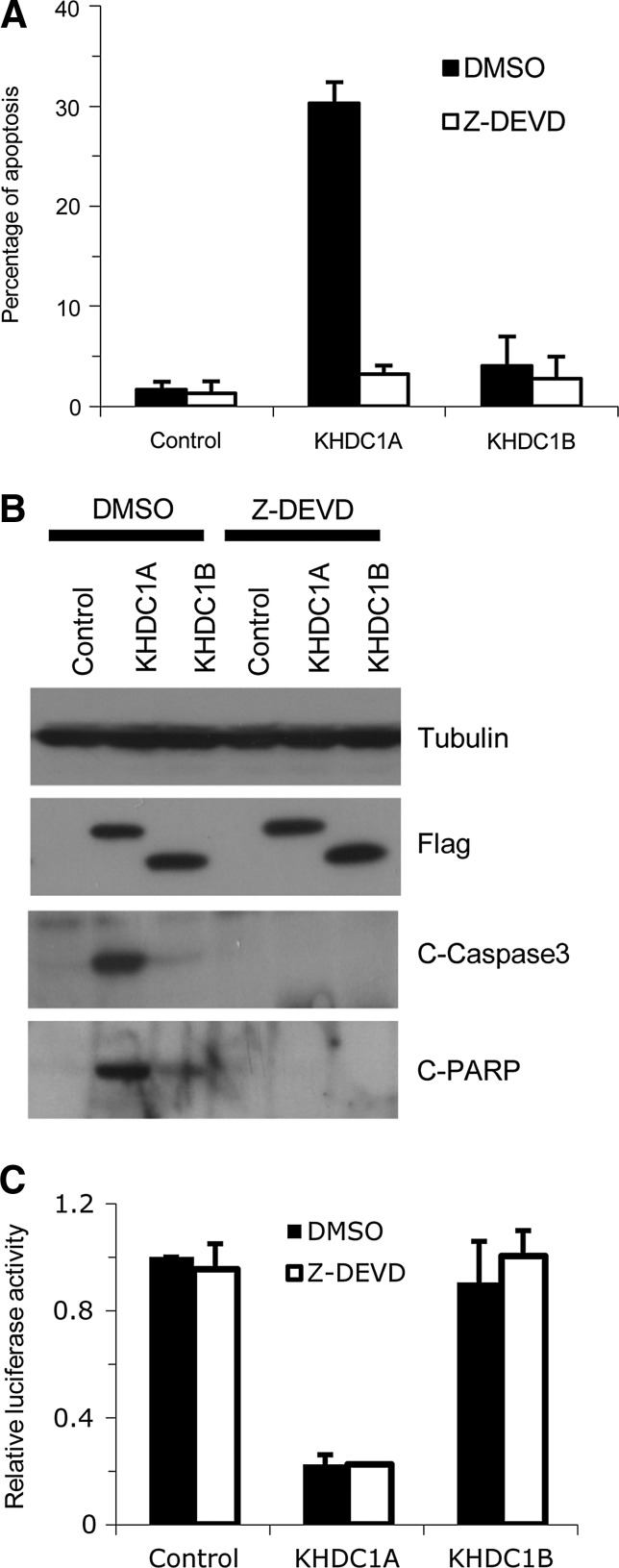
Translational repression and cellular apoptosis are functionally interwoven. Translational repression may induce apoptosis, and, conversely, apoptosis may cause a considerable and rapid reduction in the global rate of translation (Clemens et al., 2000). To characterize the interdependence of the translational repression and apoptosis mediated by KHDC1A, we treated KHDC1A-expressing cells with the caspase-3 inhibitor Z-DEVD. When the KHDC1A expressing cells were mock treated with dimethyl sulfoxide (DMSO), 30% of the cells underwent apoptosis; however, when the cells were treated with Z-DEVD, apoptosis was significantly blocked (Fig. 3A). Western blot analysis of apoptotic markers further confirmed that KHDC1A-induced apoptosis was blocked by inhibiting the activity of caspase-3 (Fig. 3B). Although Z-DEVD treatment rescued KHDC1A-induced apoptosis, it failed to alleviate the translational repression induced by KHDC1A, as assayed by measuring the activity of a cotransfected luciferase reporter (Fig. 3C). These results indicate that the translational repression mediated by KHDC1A is independent of the KHDC1A-induced apoptosis.
FIG. 3.
The translational repression activity of KHDC1A is independent of apoptosis. (A, B) Caspase-3 inhibitor Z-DEVD rescues KHDC1A-induced apoptosis. (C) The repression activity of KHDC1A is not changed by Z-DEVD treatment. HeLa cells were co-transfected with luciferase reporter along with the control vector, Flag-KHDC1A, or Flag-KHDC1B plasmids. The transfected cells were treated with 20 μM caspase-3 inhibitor Z-DEVD or DMSO as the control. Twenty-four hours after transfection, cells were harvested. Flow cytometry and Western blot analysis were used to detect the apoptosis (A, B). Luciferase assay was performed to determine the translational repression (C). All the data were summarized from three independent experiments. DMSO, dimethyl sulfoxide.
Both the N-terminus and C-terminus of KHDC1A are essential for its biological activities
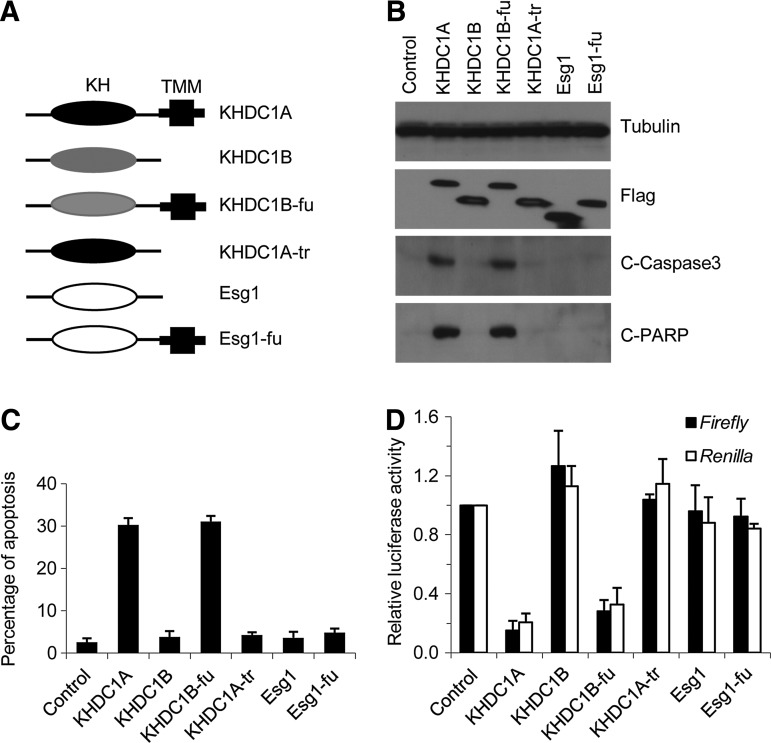
KHDC1A and KHDC1B are nearly identical at their C-termini; however, KHDC1A has a unique 40-amino-acid C-terminal extension. To test the function of this unique C-terminal extension, we grafted it onto the C-termini of both KHDC1B and Esg1 (Fig. 4A). As shown in Figure 4B, when the C-terminus was removed from KHDC1A, the truncated protein (KHDC1A-tr) failed to activate the apoptotic markers of caspase-3 and PARP. When the C-terminus of KHDC1A was swapped to KHDC1B, the fusion protein (KHDC1B-fu) activated the markers. However, when the C-terminus of KHDC1A was fused with Esg1, the resulting fusion protein (Esg1-fu) did not possess the pro-apoptotic activity. Flow cytometry analyses further verified these results, suggesting that the C-terminus of KHDC1A is essential but not sufficient for its pro-apoptotic activity (Fig. 4C). Subsequently, we asked whether the C-terminus was required to inhibit translation. As shown in Figure 4D, the truncated KHDC1A (KHDC1A-tr) failed to repress the activity of luciferases, but the fused KHDC1B protein (KHDC1B-fu) gained the inhibitory activity. When the C-terminus of KHDC1A was fused to Esg1, however, the resulting fusion protein (Esg1-fu) did not repress translation. These results demonstrate that the C-terminus of KHDC1A is necessary but not sufficient to inhibit translation. Therefore, both the N-terminus and C-terminus of KHDC1A are essential for its biological activities.
FIG. 4.
Both the N-terminus and C-terminus of KHDC1A are necessary for its function. (A) Diagram of KHDC1A, KHDC1B, Esg1, and related constructs used for analysis. (B) HeLa cells were transfected with the constructs indicated. Twenty-four hours after transfection, cells were harvested. The expression of the constructs and the apoptosis were examined by Western blot analysis with indicated antibodies. (C) The cells were transfected and harvested as just described. The cells were fixed, and the apoptosis was quantified by sub-G1 assay. (D) HeLa cells were co-transfected with luciferase reporters as well as the constructs indicated. Twenty-four hours after transfection, the cells were harvested, and luciferase assay was performed to determine the protein level of the reporters.
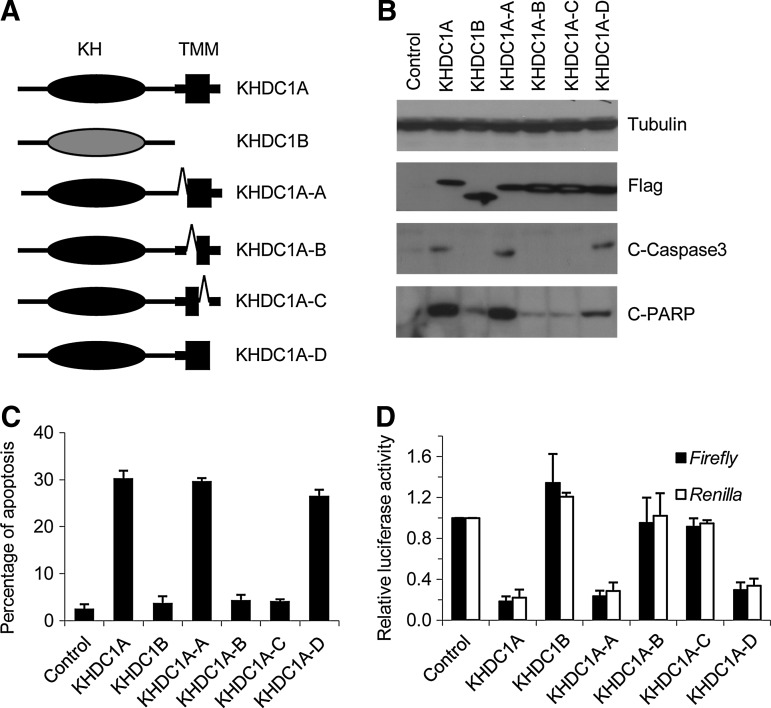
The putative TMM is critical for the biological activities of KHDC1A
In the C-terminus of KHDC1A, a 21-amino-acid TMM was predicted by InterProScan analysis, based on its hydrophobicity (Cai et al., 2010). Given that the C-terminus of KHDC1A is required for its function, we decided to test whether TMM is essential for its biological activities. To answer this question, we generated a series of deletions within or flanking the TMM region (Fig. 5A). Compared with the full-length KHDC1A, truncated proteins with intact TMM (KHDC1A-A and KHDC1A-D) retained their ability to induce apoptosis. However, proteins carrying deletions within the TMM (KHDC1A-B and KHDC1A-C) failed to induce apoptosis, suggesting that the TMM in KHDC1A is critical for its apoptotic activity (Fig. 5B, C). In addition, the TMM region was important for KHDC1A to repress translation (Fig. 5D). Compared with the full-length KHDC1A, the truncated proteins with intact TMM (KHDC1A-A and KHDC1A-D) maintained the ability to repress translation, while proteins with deletion disrupting TMM (KHDC1A-B and KHDC1A-C) lost this activity. These results suggest that the TMM region is critical for the biological activities of KHDC1A.
FIG. 5.
Trans-membrane motif is critical for the function of KHDC1A. (A) Diagram of KHDC1A, KHDC1B, and KHDC1A-derived truncated constructs used for analysis. (B) HeLa cells were transfected with the constructs indicated. Twenty-four hours after transfection, cells were harvested. The expression of the constructs and the apoptosis were examined by Western blot analysis with indicated antibodies. (C) The cells were transfected and harvested as just described. The cells were fixed, and the apoptosis was quantified by sub-G1 assay. (D) HeLa cells were co-transfected with luciferase reporters and the constructs as indicated. Twenty-four hours after transfection, the cells were harvested, and luciferase assay was performed to determine the protein level of the reporters.
KHDC1A induces apoptosis through an ER-dependent pathway
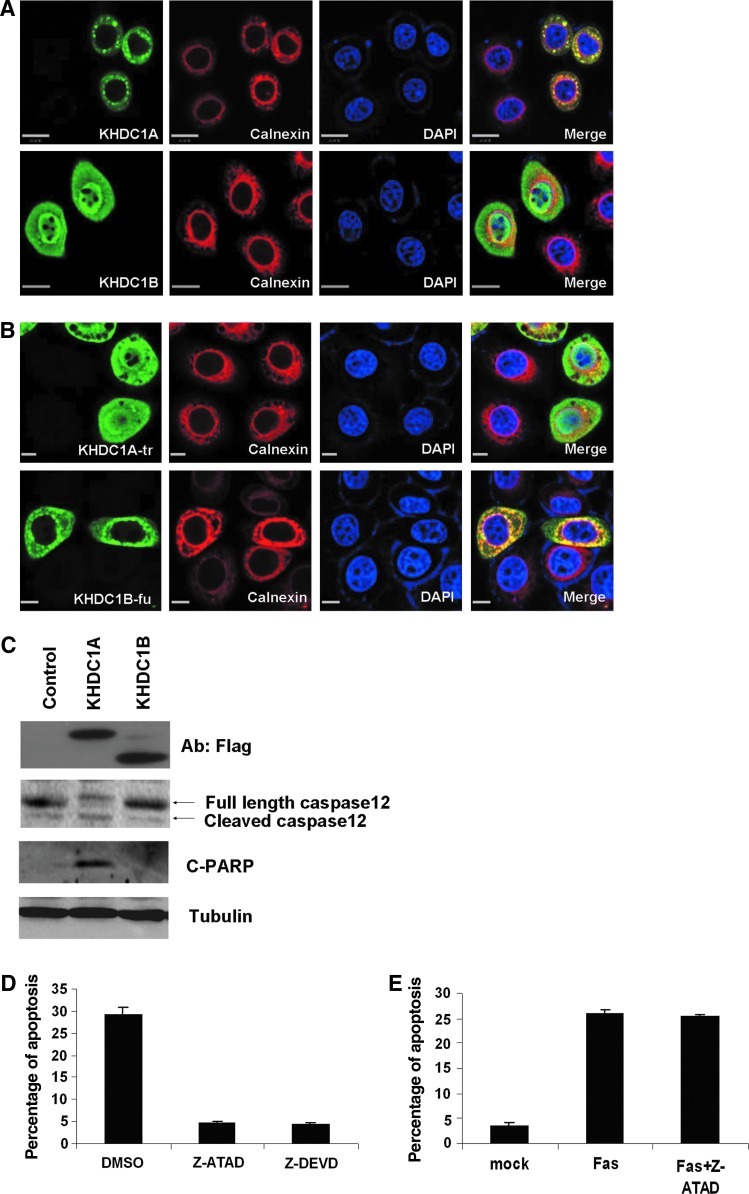
The ER is a primary site for mRNA translation, and ER stress is known to result in apoptosis. In addition, the fact that a putative TMM was shown to be required for KHDC1A's apoptotic activity and translational repression activity raises the possibility that the anchoring of KHDC1A to intracellular membranes such as the ER might be vital for its physiological functions. To test this possibility, we utilized dual immunostaining to see whether KHDC1A colocalizes with the ER biomarker Calnexin. As shown in Figure 6A, both KHDC1A and Calnexin were visualized in the cytoplasm, and merged images showed that the two proteins were colocalized. In contrast, KHDC1B did not exhibit an obvious colocalization with Calnexin (Fig. 6A). Furthermore, compared with the nontransfected cells, KHDC1A also induced the expression of Calnexin and the formation of granule structure, indicating that it might affect the function of the ER. To further test whether the C-terminus of KHDC1A is indispensible for its subcellular localization, we examined the subcellular localization of KHDC1A-tr and KHDC1B-fu. As shown in Figure 6B, KHDC1A-tr was detected in both the cytoplasm and the nucleus, a pattern distinct from the ER-specific localization for the full length of KHDC1A. When fused with the C-terminus of KHDC1A, however, KHDC1B-fu was primarily co-localized with ER-specific markers, a pattern distinct from the subcellular localization of KHDC1B.
FIG. 6.
ER is involved in KHDC1A-induced apoptosis. (A) Overexpressed KHDC1A is colocalized with ER. HeLa cells were transfected with KHDC1A or KHDC1B. Immunostaining was performed to determine the expression of KHDC1A, KHDC1B (FITC, green), and ER marker Calnexin (Cy5, red). Scale bar was 16 μm. (B) C-terminus of KHDC1A determines the ER localization. HeLa cells were transfected with Flag-tagged KHDC1A-tr or KHDC1B-fu. Immunostaining was performed to determine the expression of KHDC1A-tr, KHDC1B-fu (FITC, green), and ER marker Calnexin (Cy5, red). Scale bar was 16 μm. (C) The cleavage of caspase-12 is detected in Flag-tagged KHDC1A-transfected Hela cells. HeLa cells were transfected with control vector, Flag-tagged KHDC1A, or KHDC1B plasmids. The cells were harvested, and Western blot analysis was performed with antibodies against Flag, caspase-12, cleaved PARP, and β-tubulin. (D) Caspase-12 specific inhibitor Z-ATAD rescues KHDC1A-induced apoptosis. HeLa cells were transfected with KHDC1A. Transfected cells were treated with 20 μM Z-ATAD, Z-DEVD, or DMSO as control. Twenty-four hours after transfection, the cells were harvested, and apoptosis was assayed by flow cytometry. Data were summarized from three independent experiments. (E) Caspase-12 specific inhibitor Z-ATAD cannot rescue Fas-induced apoptosis. Hela cells were pretreated with mock or 1 μg/mL of anti-Fas/Apo1 antibodies (Biovision) for 6 h and then exposed to 20 μM of caspase-12 inhibitors Z-ATAD FMK for another 48 h. Cells were then harvested, and apoptosis was assayed by flow cytometry. Data were summarized from three independent experiments. ER, endoplasmic reticulum.
It has been reported that caspase-12 could be specifically activated by apoptotic signals with an ER stress component, but not by apoptotic signals that do not induce ER stress (Nakagawa et al., 2000). To test whether the ER is directly involved in KHDC1A-induced apoptosis, first, we examined the cleavage of caspase-12 by Western blot analysis. As shown in Figure 6C, we found that the cleavage of caspase-12 was only detected in Flag-tagged KHDC1A-transfected Hela cells, but not in vector- or KHDC1B-transfected cells (Fig. 6C), indicating that the ER may be involved in KHDC1A-induced apoptosis. Furthermore, we also blocked the activity of caspase-12 by treating KHDC1A-transfected cells with or without Z-ATAD, a well-characterized caspase-12 specific inhibitor. As shown in Figure 6D, the mimicry control DMSO failed to rescue KHDC1A-induced apoptosis; however, Z-ATAD prevented apoptosis as efficient as Z-DEVD; by contrast, the same amount of Z-ATAD could not rescue the Fas-induced apoptosis (Fig. 6E). Together with the subcellular localization data, these results strongly suggest that KHDC1A expression induces apoptosis via an ER-dependent pathway. This is consistent with the previous observation that KHDC1A-induced apoptosis is independent of the death receptor or Bcl-2 (Rajpal et al., 2003).
Discussion and Conclusion
In this study, we explored the biological function of the KH domain-containing protein KHDC1A. We demonstrate that KHDC1A specifically induces apoptosis (Fig. 1) and globally represses translation (Fig. 2). Inhibition of the caspase-3 activity successfully rescues the KHDC1A-mediated apoptosis but not the translational repression, leading to the conclusion that KHDC1A-mediated translational repression is independent of KHDC1A-induced apoptosis (Fig. 3). We also demonstrate that the C-terminus, particularly the TMM region, is a major determinant for the biological activities of KHDC1A. Disruption of the TMM completely abolished the ability of KHDC1A to induce apoptosis and to inhibit translation (Figs. 4 and 5). Finally, we show that KHDC1A induces apoptosis through an ER dependent pathway (Fig. 6).
Many KH domain-containing proteins are known to induce apoptosis under certain physiological conditions. Several reports indicate that these proteins induce apoptosis by regulating the key apoptosis regulators such as caspases or Bcl-2 family members (Schwerk and Schulze-Osthoff, 2005). For example, the KH domain-containing protein Kep1 induces apoptosis in fly and mammalian cells. When expressed, Kep1 binds to the transcript of the caspase-8 like gene dredd and regulates alternative splicing of this apoptosis controller (Di Fruscio et al., 1998, 2003). Similarly, Sam68 and hnRNP F/H proteins promote apoptosis by changing the ratio of Bcl-x(L) and Bcl-x(S) (Garneau et al., 2005; Paronetto et al., 2007). The KH domain-containing protein KHDC1A has also been reported to induce apoptosis. However, the mechanism is unclear (Rajpal et al., 2003). In this article, we provide several lines of evidence which support that KHDC1A may induce apoptosis via its interaction with the ER. First, KHDC1A is located in the cytoplasm but not the nucleus, suggesting that this protein may not directly regulate transcription or splicing. Second, KHDC1A is localized to the ER but not to the mitochondria (Fig. 6 and data not shown), suggesting that this protein may not directly target mitochondria for apoptosis. Third, deletion of the TMM in KHDC1A, which disrupts the ER-specific localization, also disrupts the ability of KHDC1A to induce apoptosis. Fourth, adding the C-terminus of KHDC1A to KHDC1B enables the fusion protein to localize to ER and accordingly gain the ability to induce apoptosis. Lastly, the inhibition of caspase-12, the caspase specific to ER-related apoptosis, rescues the KHDC1A-induced apoptosis. Taken together, these results clearly demonstrate that KHDC1A works through the ER to induce apoptosis. However, little is known about the underlying mechanism. One possibility is that the overexpression of KHDC1A directly triggers ER stress and activates the unfolded protein response, as reported for Calnexin and Nogo-B/ASY proteins (Li et al., 2001; Kuang et al., 2006; Guerin et al., 2008). Intriguingly, similar to KHDC1A, both Calnexin and Nogo/ASY are located in the ER, and the TMM in these two proteins are critical for their apoptotic activity.
A close relationship has been well established between translational inhibition and apoptosis. On the one hand, the inhibition of general translation or the translation of specific genes induces or sensitizes cells to apoptosis (Herbert et al., 2000). For example, eIF4E is a critical regulator of cap-dependent translation initiation. 4E-BP1, the binding protein of eIF4E, promotes apoptosis by inhibiting translation initiation (Li et al., 2002). On the other hand, the induction of apoptosis leads to a substantial inhibition of protein synthesis (Clemens et al., 2000; Bushell et al., 2004). Our data indicate that the translation repression induced by KHDC1A may be upstream of the apoptosis, given that inhibition of the activity of caspase-3 successfully prevents the apoptosis but fails for the translational repression. Considering that KHDC1A targets ER for apoptosis, it appears reasonable that the KHDC1A-mediated translation inhibition may functionally couple the ER stress and apoptosis.
KHDC1A, together with KHDC1B, is highly expressed in mouse oocytes and early embryos. Although both KHDC1A and KHDC1B interact with the key translational regulator CPEB, the physiological function of KHDC1A in oocyte maturation and early embryonic development is not clear (Cai et al., 2010). The current study provides new clues, and it is desirable to further study its function by loss-of-function assay in vivo. In addition to oocytes, KHDC1A can be induced to express in other cells such as T cells. It is well established that negative selection is important for T-cell development. Nur77 has been shown to be vital for negative selection, although the mechanism is not fully understood. Khdc1a is up-regulated by Nur77; so, it is reasonable to propose that Khdc1a and the ER pathway maybe involved in Nur77-mediated apoptosis (Rajpal et al., 2003). The finding that Nur77 is located on ER during the ER stress-induced apoptosis is consistent with this hypothesis (Liang et al., 2007).
In conclusion, in this article, we present evidence that KHDC1A but not KHDC1B specifically targets ER for pro-apoptosis and translational inhibition activities. Undoubtedly, further identifying downstream targets of KHDC1A will advance our understanding of the physiological function of KHDC1A as well as the underlying molecular mechanism of KHDC1A-induced apoptosis. Although our data indicated that KHDC1A represses translation, the mechanism of this activity needs to be further studied. As an RNA binding protein, it is also valuable to study whether the pro-apoptotic activity and translational repression activity of KHDC1A are dependent on its RNA binding activity.
Supplementary Material
Acknowledgments
Financial support for this project was provided by the Special Fund for Basic Scientific Research of Central Colleges, South-Central University for Nationalities to Jinhua Shen (CZZ11010), and the National Natural Science Foundation of China to Jinhua Shen (Grant No. 81170227).
Disclosure Statement
No competing financial interests exist, and there was no conflict of interest in the study.
References
- Adams J.M. Ways of dying: multiple pathways to apoptosis. Genes Dev. 2003;17:2481–2495. doi: 10.1101/gad.1126903. [DOI] [PubMed] [Google Scholar]
- Amano H. Itakura K. Maruyama M. Ichisaka T. Nakagawa M. Yamanaka S. Identification and targeted disruption of the mouse gene encoding ESG1 (PH34/ECAT2/DPPA5) BMC Dev Biol. 2006;6:11. doi: 10.1186/1471-213X-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budihardjo I. Oliver H. Lutter M. Luo X. Wang X. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol. 1999;15:269–290. doi: 10.1146/annurev.cellbio.15.1.269. [DOI] [PubMed] [Google Scholar]
- Bushell M. Stoneley M. Sarnow P. Willis A.E. Translation inhibition during the induction of apoptosis: RNA or protein degradation? Biochem Soc Trans. 2004;32:606–610. doi: 10.1042/BST0320606. [DOI] [PubMed] [Google Scholar]
- Cai C. Tamai K. Molyneaux K. KHDC1B is a novel CPEB binding partner specifically expressed in mouse oocytes and early embryos. Mol Biol Cell. 2010;21:3137–3148. doi: 10.1091/mbc.E10-03-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens M.J. Bushell M. Jeffrey I.W. Pain V.M. Morley S.J. Translation initiation factor modifications and the regulation of protein synthesis in apoptotic cells. Cell Death Differ. 2000;7:603–615. doi: 10.1038/sj.cdd.4400695. [DOI] [PubMed] [Google Scholar]
- Di Fruscio M. Chen T. Bonyadi S. Lasko P. Richard S. The identification of two Drosophila K homology domain proteins. Kep1 and SAM are members of the Sam68 family of GSG domain proteins. J Biol Chem. 1998;273:30122–30130. doi: 10.1074/jbc.273.46.30122. [DOI] [PubMed] [Google Scholar]
- Di Fruscio M. Styhler S. Wikholm E. Boulanger M.C. Lasko P. Richard S. Kep1 interacts genetically with dredd/caspase-8, and kep1 mutants alter the balance of dredd isoforms. Proc Natl Acad Sci U S A. 2003;100:1814–1819. doi: 10.1073/pnas.0236048100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garneau D. Revil T. Fisette J.F. Chabot B. Heterogeneous nuclear ribonucleoprotein F/H proteins modulate the alternative splicing of the apoptotic mediator Bcl-x. J Biol Chem. 2005;280:22641–22650. doi: 10.1074/jbc.M501070200. [DOI] [PubMed] [Google Scholar]
- Guerin R. Arseneault G. Dumont S. Rokeach L.A. Calnexin is involved in apoptosis induced by endoplasmic reticulum stress in the fission yeast. Mol Biol Cell. 2008;19:4404–4420. doi: 10.1091/mbc.E08-02-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert T.P. Fahraeus R. Prescott A. Lane D.P. Proud C.G. Rapid induction of apoptosis mediated by peptides that bind initiation factor eIF4E. Curr Biol. 2000;10:793–796. doi: 10.1016/s0960-9822(00)00567-4. [DOI] [PubMed] [Google Scholar]
- Kuang E. Wan Q. Li X. Xu H. Zou T. Qi Y. ER stress triggers apoptosis induced by Nogo-B/ASY overexpression. Exp Cell Res. 2006;312:1983–1988. doi: 10.1016/j.yexcr.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Li Q. Qi B. Oka K. Shimakage M. Yoshioka N. Inoue H. Hakura A. Kodama K. Stanbridge E.J. Yutsudo M. Link of a new type of apoptosis-inducing gene ASY/Nogo-B to human cancer. Oncogene. 2001;20:3929–3936. doi: 10.1038/sj.onc.1204536. [DOI] [PubMed] [Google Scholar]
- Li S. Sonenberg N. Gingras A.C. Peterson M. Avdulov S. Polunovsky V.A. Bitterman P.B. Translational control of cell fate: availability of phosphorylation sites on translational repressor 4E-BP1 governs its proapoptotic potency. Mol Cell Biol. 2002;22:2853–2861. doi: 10.1128/MCB.22.8.2853-2861.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang B. Song X. Liu G. Li R. Xie J. Xiao L. Du M. Zhang Q. Xu X. Gan X., et al. Involvement of TR3/Nur77 translocation to the endoplasmic reticulum in ER stress-induced apoptosis. Exp Cell Res. 2007;313:2833–2844. doi: 10.1016/j.yexcr.2007.04.032. [DOI] [PubMed] [Google Scholar]
- Meier P. Finch A. Evan G. Apoptosis in development. Nature. 2000;407:796–801. doi: 10.1038/35037734. [DOI] [PubMed] [Google Scholar]
- Mootz D. Ho D.M. Hunter C.P. The STAR/Maxi-KH domain protein GLD-1 mediates a developmental switch in the translational control of C. elegans PAL-1. Development. 2004;131:3263–3272. doi: 10.1242/dev.01196. [DOI] [PubMed] [Google Scholar]
- Nakagawa T. Zhu H. Morishima N. Li E. Xu J. Yankner B.A. Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- Paronetto M.P. Achsel T. Massiello A. Chalfant C.E. Sette C. The RNA-binding protein Sam68 modulates the alternative splicing of Bcl-x. J Cell Biol. 2007;176:929–939. doi: 10.1083/jcb.200701005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre A. Gautier M. Callebaut I. Bontoux M. Jeanpierre E. Pontarotti P. Monget P. Atypical structure and phylogenomic evolution of the new eutherian oocyte- and embryo-expressed KHDC1/DPPA5/ECAT1/OOEP gene family. Genomics. 2007;90:583–594. doi: 10.1016/j.ygeno.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Pilotte J. Larocque D. Richard S. Nuclear translocation controlled by alternatively spliced isoforms inactivates the QUAKING apoptotic inducer. Genes Dev. 2001;15:845–858. doi: 10.1101/gad.860301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthalakath H. O'Reilly L.A. Gunn P. Lee L. Kelly P.N. Huntington N.D. Hughes P.D. Michalak E.M. McKimm-Breschkin J. Motoyama N., et al. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129:1337–1349. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Rajpal A. Cho Y.A. Yelent B. Koza-Taylor P.H. Li D. Chen E. Whang M. Kang C. Turi T.G. Winoto A. Transcriptional activation of known and novel apoptotic pathways by Nur77 orphan steroid receptor. EMBO J. 2003;22:6526–6536. doi: 10.1093/emboj/cdg620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwerk C. Schulze-Osthoff K. Regulation of apoptosis by alternative pre-mRNA splicing. Mol Cell. 2005;19:1–13. doi: 10.1016/j.molcel.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Shiraishi H. Okamoto H. Yoshimura A. Yoshida H. ER stress-induced apoptosis and caspase-12 activation occurs downstream of mitochondrial apoptosis involving Apaf-1. J Cell Sci. 2006;119:3958–3966. doi: 10.1242/jcs.03160. [DOI] [PubMed] [Google Scholar]
- Szegezdi E. Fitzgerald U. Samali A. Caspase-12 and ER-stress-mediated apoptosis: the story so far. Ann N Y Acad Sci. 2003;1010:186–194. doi: 10.1196/annals.1299.032. [DOI] [PubMed] [Google Scholar]
- Tanaka T.S. Lopez de Silanes I. Sharova L.V. Akutsu H. Yoshikawa T. Amano H. Yamanaka S. Gorospe M. Ko M.S. Esg1, expressed exclusively in preimplantation embryos, germline, and embryonic stem cells, is a putative RNA-binding protein with broad RNA targets. Dev Growth Differ. 2006;48:381–390. doi: 10.1111/j.1440-169X.2006.00875.x. [DOI] [PubMed] [Google Scholar]
- Taylor S.J. Resnick R.J. Shalloway D. Sam68 exerts separable effects on cell cycle progression and apoptosis. BMC Cell Biol. 2004;5:5. doi: 10.1186/1471-2121-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiede B. Dimmler C. Siejak F. Rudel T. Predominant identification of RNA-binding proteins in Fas-induced apoptosis by proteome analysis. J Biol Chem. 2001;276:26044–26050. doi: 10.1074/jbc.M101062200. [DOI] [PubMed] [Google Scholar]
- Thompson C.B. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- Valverde R. Edwards L. Regan L. Structure and function of KH domains. FEBS J. 2008;275:2712–2726. doi: 10.1111/j.1742-4658.2008.06411.x. [DOI] [PubMed] [Google Scholar]
- Vaux D.L. Korsmeyer S.J. Cell death in development. Cell. 1999;96:245–254. doi: 10.1016/s0092-8674(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Wan L. Dockendorff T.C. Jongens T.A. Dreyfuss G. Characterization of dFMR1, a Drosophila melanogaster homolog of the fragile X mental retardation protein. Mol Cell Biol. 2000;20:8536–8547. doi: 10.1128/mcb.20.22.8536-8547.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalfa F. Giorgi M. Primerano B. Moro A. Di Penta A. Reis S. Oostra B. Bagni C. The fragile X syndrome protein FMRP associates with BC1 RNA and regulates the translation of specific mRNAs at synapses. Cell. 2003;112:317–327. doi: 10.1016/s0092-8674(03)00079-5. [DOI] [PubMed] [Google Scholar]
- Zhu J. Chen X. MCG10, a novel p53 target gene that encodes a KH domain RNA-binding protein, is capable of inducing apoptosis and cell cycle arrest in G(2)-M. Mol Cell Biol. 2000;20:5602–5618. doi: 10.1128/mcb.20.15.5602-5618.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.