Abstract
BACKGROUND
The effectiveness of surgery versus observation for men with localized prostate cancer detected by means of prostate-specific antigen (PSA) testing is not known.
METHODS
From November 1994 through January 2002, we randomly assigned 731 men with localized prostate cancer (mean age, 67 years; median PSA value, 7.8 ng per milliliter) to radical prostatectomy or observation and followed them through January 2010. The primary outcome was all-cause mortality; the secondary outcome was prostate-cancer mortality.
RESULTS
During the median follow-up of 10.0 years, 171 of 364 men (47.0%) assigned to radical prostatectomy died, as compared with 183 of 367 (49.9%) assigned to observation (hazard ratio, 0.88; 95% confidence interval [CI], 0.71 to 1.08; P = 0.22; absolute risk reduction, 2.9 percentage points). Among men assigned to radical prostatectomy, 21 (5.8%) died from prostate cancer or treatment, as compared with 31 men (8.4%) assigned to observation (hazard ratio, 0.63; 95% CI, 0.36 to 1.09; P = 0.09; absolute risk reduction, 2.6 percentage points). The effect of treatment on all-cause and prostate-cancer mortality did not differ according to age, race, coexisting conditions, self-reported performance status, or histologic features of the tumor. Radical prostatectomy was associated with reduced all-cause mortality among men with a PSA value greater than 10 ng per milliliter (P = 0.04 for interaction) and possibly among those with intermediate-risk or high-risk tumors (P = 0.07 for interaction). Adverse events within 30 days after surgery occurred in 21.4% of men, including one death.
CONCLUSIONS
Among men with localized prostate cancer detected during the early era of PSA testing, radical prostatectomy did not significantly reduce all-cause or prostate-cancer mortality, as compared with observation, through at least 12 years of follow-up. Absolute differences were less than 3 percentage points. (Funded by the Department of Veterans Affairs Cooperative Studies Program and others; PIVOT ClinicalTrials.gov number, NCT00007644.)
The treatment of early-stage prostate cancer remains controversial, especially for tumors detected by means of prostate-specific antigen (PSA) testing.1 Systematic reviews have provided inadequate information for assessing the comparative effectiveness of treatments and any associated harms.2 Although the lifetime risk of receiving a diagnosis of prostate cancer is about 17%, the risk of dying from the disease is approximately 3%, suggesting that conservative management may be appropriate for many men.3,4
Two randomized trials compared radical prostatectomy with observation but were conducted before PSA testing became widespread.5,6 One study failed to show a significant difference in overall mortality after more than 20 years.5 Another showed absolute differences in all-cause and prostate-cancer mortality at 15 years of 6.6 percentage points and 6.1 percentage points, respectively, in favor of surgery.6 Benefits were confined to men younger than 65 years of age. A randomized trial comparing external-beam radiotherapy with observation, also among men who received the diagnosis before PSA testing became widespread, showed no significant differences in mortality through at least 16 years.7 During the era of PSA testing, an observational study showed high 10-year survival rates among men treated conservatively.8 Despite excellent long-term, disease-specific survival with observation, this option is rarely used, in part because of a lack of evidence from randomized trials comparing observation with attempted curative treatment for prostate cancer detected since PSA testing became common practice. We conducted a randomized trial to compare radical prostatectomy with observation in 731 men who had received a diagnosis of clinically localized prostate cancer in the early era of PSA testing.
METHODS
STUDY DESIGN
We previously reported the baseline characteristics of the patients and the design of the Prostate Cancer Intervention versus Observation Trial (PIVOT).9 Enrollment began in November 1994 and ended in January 2002, with follow-up through January 2010. We recruited men from 44 Department of Veterans Affairs sites and 8 National Cancer Institute sites.
The research protocol was approved by the institutional review board at each site. All patients provided written informed consent. Randomization was stratified according to site and implemented by means of a central interactive telephone system.
Patients had to be medically fit for radical prostatectomy and to have histologically confirmed, clinically localized prostate cancer (stage T1-T2NxM0 in the tumor–node–metastasis classification system according to the American Joint Committee on Cancer10) of any grade diagnosed within the previous 12 months. Patients also had to have a PSA value of less than 50 ng per milliliter, an age of 75 years or less, negative results on a bone scan for metastatic disease, and a life expectancy of at least 10 years from the time of randomization. The study sites assessed eligibility on the basis of locally obtained PSA values and biopsy readings. After randomization, a central pathologist reviewed the biopsy and radical-prostatectomy specimens, and a central laboratory measured PSA.
TREATMENT PROTOCOL
The technique used for radical prostatectomy was at the surgeon’s discretion. Additional interventions were determined by each participant and his physician. Men randomly assigned to the observation group were offered palliative therapy or chemotherapy for symptomatic or metastatic progression.
FOLLOW-UP AND CLINICAL OUTCOMES
We scheduled study visits every 6 months for a minimum of 8 years and a maximum of 15 years or until the patient died. Bone scans were obtained at 5, 10, and 15 years or at the last visit for persons with less than 15 years of follow-up, with additional scans obtained at the clinician’s discretion. The primary outcome was all-cause mortality. Our secondary outcome was prostate-cancer mortality, which was defined as death that was definitely or probably due to prostate cancer or definitely or probably due to prostate-cancer treatment by a three-member end-points committee that was unaware of the study assignments. Bone metastases were documented on the basis of positive results of bone scanning or skeletal radiography. We assessed 30-day perioperative harms and the prevalence of urinary incontinence and erectile and bowel dysfunction at 2 years, which was based on self-reported dysfunction that was at least moderate in severity.
STUDY OVERSIGHT
The authors are responsible for the study design and oversight and the analysis and reporting of the data. All authors vouch for the accuracy of the data and the fidelity of the study to the protocol. The site investigators and assistants collected and transmitted data to the coordinating center for analysis. An independent data and safety monitoring board monitored the trial for safety and scientific integrity. Interim analyses were stipulated in the protocol.
STATISTICAL ANALYSIS
We carried out analyses according to the intention-to-treat principle. Recruitment difficulties prevented the attainment of our original goal of enrolling 2000 men. We revised our sample on the basis of estimates that 740 men enrolled over a period of 7 years, with an additional 8 years of follow-up, would provide 91% power to detect a 25% relative reduction in all-cause mortality, assuming a median survival of 10 years. The data and safety monitoring board reviewed and approved this revision. For assessment of the secondary end point (death from prostate cancer or treatment), a survival analysis was performed in which the data from surviving patients were censored at the end of the study and the data from patients who died from causes other than prostate cancer were censored at the date of death from that other cause.11
We analyzed death from any cause, death from prostate cancer (with death from other causes treated as a competing risk), and bone metastases. Outcomes were analyzed with the use of a proportional-hazards model, which provided hazard ratios and corresponding 95% confidence intervals. Cumulative incidence and between-group differences were assessed at 4, 8, and 12 years and at the end of the study. P values of less than 0.05 (two-sided) were considered to indicate statistical significance.
Mortality and bone metastases were estimated for each study group with the Kaplan–Meier method. Seven subgroups defined according to baseline characteristics were prespecified for assessment of overall and prostate-cancer mortality and were specified post hoc for assessment of bone metastases: age (<65 years vs. ≥65 years), race (white, black, or other), coexisting conditions (Charlson comorbidity index score, 0 vs. ≥1),12 self-reported performance status (0 [fully active] vs. 1 to 4, with higher scores indicating poorer functional status), PSA level (≤10 vs. >10 ng per milliliter), score on the Gleason histologic scale (<7 vs. ≥7 on a scale of 2 to 10, with 10 indicating the most poorly differentiated tumors),10,13 and D’Amico tumor risk score (low, intermediate, or high), which was based on tumor stage, the histologic score assigned by the local study site, and the PSA level.14
To determine whether the treatment effect varied according to subgroup, we performed tests of interaction between group assignment and risk-factor category. Modification of the effect of radical prostatectomy according to subgroup was assessed by means of a Cox proportional-hazards model that included an interaction term between subgroup category and study group. A P value of less than 0.05 was considered to indicate statistical significance, with no correction for multiple comparisons. We performed sensitivity analyses using centrally assessed histopathological findings and PSA values. We used SAS software, version 9.2 (SAS Institute), for all analyses.11 The protocol, including the statistical analysis plan, is available with the full text of this article at NEJM.org.
RESULTS
CHARACTERISTICS OF THE PARTICIPANTS
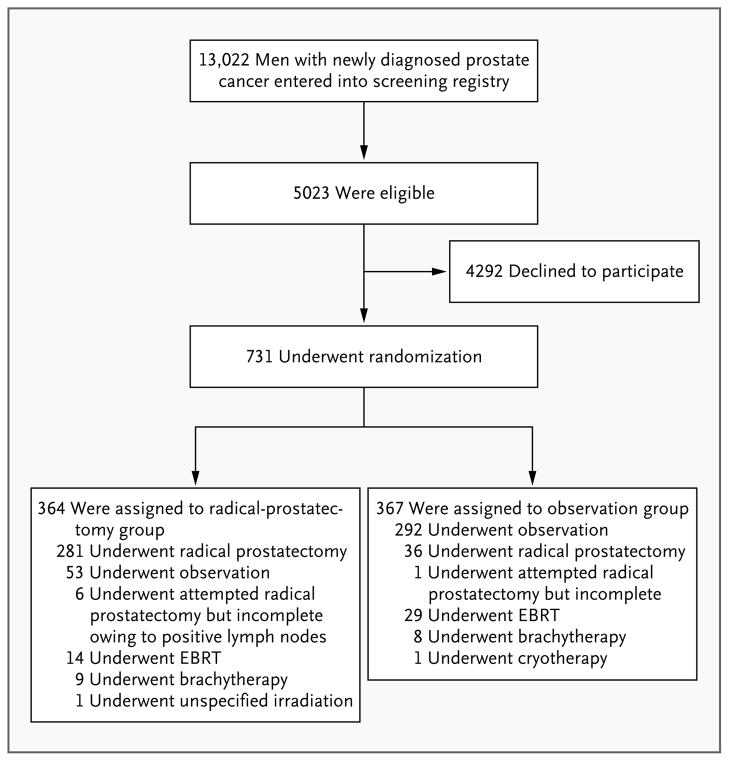
Among 13,022 men with prostate cancer (Fig. 1), 5023 were eligible for enrollment. A total of 731 men (14.6%) agreed to participate and underwent randomization to radical prostatectomy (364 men) or observation (367). The mean age was 67 years (see Table 1 in the Supplementary Appendix, available at NEJM.org). Nearly one third of the patients were black; 85% reported full independence in activities of daily living. The median PSA value was 7.8 ng per milliliter (mean, 10.1). About 50% of the men had stage T1c disease (not palpable, detected by means of PSA testing), and about 25% had histologic scores of 7 or higher on the Gleason scale; 40% of the men had low-risk, 34% intermediate-risk, and 21% high-risk prostate cancer (about 5% had missing data). On the basis of central pathological review, 48% of the patients had histologic scores of 7 or higher on the Gleason scale, and 66% had tumors in the intermediate-risk or high-risk categories.
Figure 1. Study Enrollment and Treatment.
Of a total of 13,022 men who were screened for participation, 5023 were eligible for enrollment; of these, 731 were randomly assigned to radical prostatectomy or observation. Of the 364 men in the radical-prostatectomy group, 287 underwent attempted surgery, as did 37 of the 367 men in the observation group. EBRT denotes external-beam radiotherapy.
TREATMENT ADHERENCE
During follow-up, 287 of the 364 men (78.8%) who were randomly assigned to radical prostatectomy underwent an attempted radical prostatectomy (median time from randomization to surgery, 35 days; interquartile range, 24 to 50), and 311 (85.4%) received definitive therapy (median time from randomization to definitive therapy, 36 days; inter-quartile range, 24 to 59). Among men assigned to the observation group, 37 (10.1%) underwent an attempted radical prostatectomy (median time from randomization to surgery, 61 days; interquartile range, 30 to 624) (Fig. 1), and 75 (20.4%) received definitive therapy (median time from randomization to initiation of treatment, 652 days; interquartile range, 61 to 1502). Median follow-up from randomization until death or the end of the study was 10.0 years (interquartile range, 7.3 to 12.6).
SURGICAL TECHNIQUE AND PATHOLOGICAL FINDINGS
Of the 281 radical-prostatectomy procedures performed in men in the radical-prostatectomy group (Fig. 1), nerve-sparing surgery was used in 108 (38.4%) (Table 2 in the Supplementary Appendix). On the basis of local pathological findings, the tumor was confined to the prostate in 150 men (53.4%), including 65.8% of those with low-risk prostate cancer (75 of 114 men) and 35.6% of those with high-risk disease (21 of 59). Capsular invasion was noted in 28 men (10.0%) and capsular penetration in 16 (5.7%). Surgical margins were positive for tumor in 64 men (22.8%).
ALL-CAUSE MORTALITY
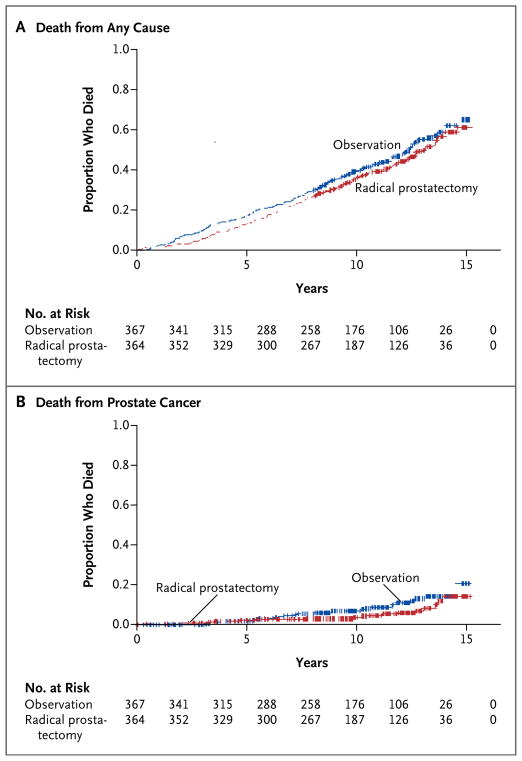
By the end of the study, 354 men (48.4%) had died (Table 3 in the Supplementary Appendix). Among men in the radical-prostatectomy group, 171 (47.0%) died, as compared with 183 (49.9%) in the observation group (hazard ratio, 0.88; 95% confidence interval [CI], 0.71 to 1.08; P = 0.22; absolute risk reduction, 2.9 percentage points; 95% CI, −4.1 to 10.3) (Fig. 2A, and Table 4 in the Supplementary Appendix).
Figure 2. Kaplan–Meier Plots of Mortality.
By the end of the study, 354 men (48.4%) had died from any cause (Panel A). Death attributed to prostate cancer or treatment occurred in 52 men (7.1%) (Panel B). Data from the radical-prostatectomy group are shown in red, and data from the observation group in blue.
Median survival was 13.0 years (95% CI, 12.2 to 13.7) in the radical-prostatectomy group and 12.4 years (95% CI, 11.4 to 13.1) in the observation group. At 12 years, 40.9% of men assigned to radical prostatectomy and 43.9% of those assigned to observation had died. The absolute reduction in mortality with radical prostatectomy was not significant at any interval and declined over time, from 4.6 percentage points (95% CI, −0.2 to 9.3) at 4 years to 2.9 percentage points (95% CI, −4.2 to 10.0) at 12 years.
PROSTATE-CANCER MORTALITY
Death attributed to prostate cancer or treatment occurred in 52 men (7.1%) (Table 3 in the Supplementary Appendix). In the radical-prostatectomy group, 21 of 364 men (5.8%) died from prostate cancer or treatment, as compared with 31 of 367 (8.4%) in the observation group (hazard ratio, 0.63; 95% CI, 0.36 to 1.09; P = 0.09; absolute risk reduction, 2.6 percentage points; 95% CI, −1.1 to 6.5) (Fig. 2B). Two thirds of the deaths due to prostate cancer (34 of 52 deaths, accounting for 4.7% of all patients) were considered to be definitely due to prostate cancer or treatment, with no significant difference between the groups: 16 men (4.4%) in the radical-prostatectomy group and 18 (4.9%) in the observation group. Prostate-cancer mortality was identical in the observation and radical-pros-tatectomy groups at 4 years. At 12 years, radical prostatectomy was associated with a nonsignificant absolute reduction in mortality of 3.0 percentage points, as compared with observation (4.4 vs. 7.4 percentage points; relative risk, 0.60; 95% CI, 0.33 to 1.09), declining slightly at the end of the study (Fig. 2B, and Table 5 in the Supplementary Appendix).
BONE METASTASES
Bone metastases occurred in 17 men assigned to radical prostatectomy (4.7%), as compared with 39 (10.6%) assigned to observation (hazard ratio, 0.40; 95% CI, 0.22 to 0.70; P<0.001) (Fig. 1 and Table 6 in the Supplementary Appendix). Differences in the cumulative incidence between the radical-prostatectomy and observation groups changed little after 8 years of follow-up.
SUBGROUP AND SENSITIVITY ANALYSES
All-Cause Mortality
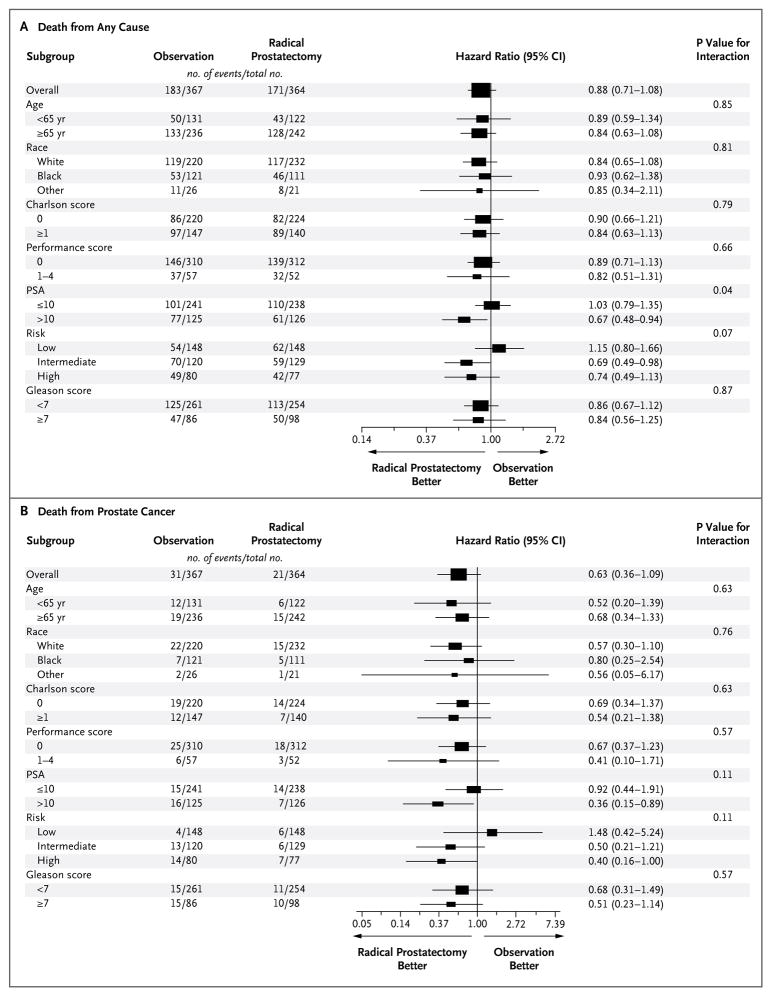
The effect of radical prostatectomy, as compared with observation, on all-cause mortality did not differ significantly according to age, score on the Gleason histologic scale, race, self-reported performance status, or score on the Charlson comorbidity index. We identified a significant interaction between study group and baseline PSA value (P = 0.04 for interaction) and a borderline interaction (P = 0.07) for tumor risk category (Fig. 3A, and Table 3 in the Supplementary Appendix). As compared with observation, surgery did not reduce all-cause mortality among men with a PSA value of 10 ng per milliliter or less (median, 6.0) (hazard ratio, 1.03; 95% CI, 0.79 to 1.35). Among men with a PSA value greater than 10 ng per milliliter (median, 15.0), surgery reduced all-cause mortality by 13.2% (hazard ratio, 0.67; 95% CI, 0.48 to 0.94) (Fig. 2A and 2B and Table 4 in the Supplementary Appendix).
Figure 3. Forest Plots for Primary and Secondary Outcomes.
There were no significant between-group differences in all-cause mortality according to age, score on the Gleason histologic scale (<7 vs. ≥7 on a scale of 2 to 10, with 10 indicating the most poorly differentiated tumors),13 self-reported race, self-reported performance status (0 [fully active] vs. 1 to 4, with higher scores indicating poorer functional status), or score on the Charlson co-morbidity index12 (Panel A), but there was a significant interaction between study group and baseline PSA value (P = 0.04 for interaction) and a borderline interaction (P = 0.07) for tumor risk (D’Amico tumor risk score [low, intermediate, or high], which was based on tumor stage, histologic score, and PSA level14). Prostate-cancer mortality did not differ significantly between the study groups according to age, race, score on the Charlson comorbidity index, or self-reported performance status (Panel B), although there was borderline evidence of an interaction for PSA value and tumor-risk category (P = 0.11 for interaction for both comparisons). The bars indicate 95% confidence intervals, and the size of the symbol indicates the weight of the estimate.
Among men with intermediate-risk tumors (as determined by a PSA value of 10.1 to 20.0 ng per milliliter, a score of 7 on the Gleason scale, or a stage T2b tumor), those who were randomly assigned to surgery had a 31% relative reduction in all-cause mortality, as compared with those assigned to observation (hazard ratio, 0.69; 95% CI, 0.49 to 0.98; absolute risk reduction, 12.6 percentage points). Among men with high-risk tumors, surgery resulted in a nonsignificant absolute reduction in mortality of 6.7 percentage points, as compared with observation (P = 0.16) (Fig. 2C, 2D, and 2E in the Supplementary Appendix). In contrast, among men with low-risk cancers (as determined by a PSA value ≤10 ng per milliliter, a score of 6 or less on the Gleason scale, and a stage T1a–c or T2a tumor), there was a 15% non-significant increase in mortality among men randomly assigned to radical prostatectomy, as compared with those assigned to observation (hazard ratio, 1.15; 95% CI, 0.80 to 1.66). The absolute difference at 12 years was 5.4 percentage points, in favor of observation over surgery (37.2% vs. 31.8%). Sensitivity analyses performed with the use of central biopsy readings showed no significant differences in all-cause mortality between radical prostatectomy and observation according to scores on the Gleason scale or tumor risk categories (P>0.13 for all categories). When local histologic findings for men with intermediate-risk disease and those with high-risk disease were pooled, radical prostatectomy was associated with an absolute reduction in all-cause mortality of 10.5 percentage points (hazard ratio, 0.71; 95% CI, 0.54 to 0.92; P = 0.01). The reduction in mortality was smaller and was not significant when the pooled data were assessed on the basis of central pathological review (hazard ratio, 0.81; 95% CI, 0.63 to 1.0; P = 0.10; absolute risk reduction, 4.7 percentage points).
Prostate-Cancer Mortality
As compared with observation, the effect of radical prostatectomy on prostate-cancer mortality did not differ significantly according to age, race, score on the Charlson comorbidity index, or self-reported performance status (Fig. 3B). We found some evidence of treatment interaction for subgroups defined by PSA value and tumor risk category (P = 0.11 for interaction for both comparisons). Prostate-cancer mortality was lower in the radical-prostatectomy group than in the observation group among men with a PSA value of more than 10 ng per milliliter (5.6% vs. 12.8%, P = 0.02) and among men with high-risk prostate cancer (9.1% vs. 17.5%, P = 0.04). However, prostate-cancer mortality was not significantly lower in the radical-prostatectomy group among men with a PSA level of 10 ng per milliliter or less (P = 0.82) or among those with low-risk tumors (P = 0.54) or intermediate-risk tumors (P = 0.12) (Fig. 3A through 3E and Table 5 in the Supplementary Appendix).
The results for prostate-cancer mortality were generally consistent when we substituted central for local PSA measures and histopathological findings. However, among men with intermediate-risk prostate cancer, the absolute risk difference of 4.6 percentage points in favor of radical pros-tatectomy on the basis of local histologic findings changed to 1.3 percentage points in favor of observation, on the basis of central histologic findings. Bone metastases were not reduced among men with PSA values of 10 ng per milliliter or less or among those with low-risk disease. Among men with PSA levels that were greater than 10 ng per milliliter or with intermediate-risk or high-risk disease, absolute reductions of approximately 9.0 to 11.0 percentage points occurred. Subgroup differences in cumulative incidence remained stable after about 8 years (Table 6 in the Supplementary Appendix).
SURGICAL MORBIDITY
Perioperative complications during the first 30 days after surgery occurred in 21.4% of men in the radical-prostatectomy group who underwent radical prostatectomy and included one death. The most common complication was wound infection, in 4.3% of the men (Table 1). Complications occurring in more than 2% of the men included urinary tract infection, surgical repair, bleeding requiring transfusion, and urinary catheterization more than 30 days after surgery. At 2 years, patient-reported urinary incontinence and erectile dysfunction, but not bowel dysfunction, were significantly more common among men who were randomly assigned to radical prostatectomy than among those randomly assigned to observation (Table 2).
Table 1.
Adverse Events Occurring within 30 Days after Surgery.*
| Event | Patients (N=280) |
|---|---|
| no. (%) | |
| Any | 60 (21.4) |
|
| |
| Pneumonia | 2 (0.7) |
|
| |
| Wound infection | 12 (4.3) |
|
| |
| Urinary tract infection | 7 (2.5) |
|
| |
| Sepsis | 3 (1.1) |
|
| |
| Deep-vein thrombosis | 2 (0.7) |
|
| |
| Stroke | 1 (0.4) |
|
| |
| Pulmonary embolism | 2 (0.7) |
|
| |
| Myocardial infarction | 3 (1.1) |
|
| |
| Renal failure or dialysis | 1 (0.4) |
|
| |
| Bowel injury requiring surgical repair | 3 (1.1) |
|
| |
| Additional surgical repair | 7 (2.5) |
|
| |
| Bleeding requiring transfusion | 6 (2.1) |
|
| |
| Urinary catheter present >30 days after surgery | 6 (2.1) |
|
| |
| Death | 1 (0.4) |
|
| |
| Other | 28 (10.0) |
Of the 364 men randomly assigned to the radical-prostatectomy group, radical prostatectomy was completed in 280. Multiple events may have occurred in a single patient.
Table 2.
Patient-Reported Urinary, Erectile, and Bowel Dysfunction at 2 Years, According to Study Group.*
| Dysfunction | Radical Prostatectomy | Observation | P Value |
|---|---|---|---|
| no./total no. (%) | |||
| Urinary incontinence† | 49/287 (17.1) | 18/284 (6.3) | <0.001 |
|
| |||
| Erectile dysfunction‡ | 231/285 (81.1) | 124/281 (44.1) | <0.001 |
|
| |||
| Bowel dysfunction§ | 35/286 (12.2) | 32/282 (11.3) | 0.74 |
The values reported are the number of men reporting the dysfunction and the total number of men who responded to the question.
Urinary incontinence was defined by patient reports (“have a lot of problems with urinary dribbling,” “lose larger amounts of urine than dribbling but not all day,” “have no control over urine,” or “have an indwelling catheter”).
Erectile dysfunction was defined as the inability to have an erection or an erection sufficient for vaginal penetration.
Bowel dysfunction was defined by patient reports that it was a “moderate” or “big” problem.
Discussion
Among men with clinically localized prostate cancer that had been diagnosed after PSA testing came into practice, our study showed that radical prostatectomy did not reduce all-cause or prostate-cancer mortality, as compared with observation, through at least 12 years of follow-up. Confidence intervals for the effect size indicated that surgery did not reduce all-cause mortality by more than 10% and might have increased mortality by as much as 4%. Differences in all-cause mortality decreased over time, suggesting that longer follow-up would not alter these findings. Only 10% of patients were younger than 60 years of age. Longer follow-up may be important for the minority of men with prostate cancer who were younger than 60 years of age. However, the nonsignificant between-group difference in prostate-cancer mortality and the significant 6% reduction in bone metastases with radical prostatectomy remained fairly constant after 8 years. Our findings add to evidence supporting observation, and possibly active surveillance, for most men who receive a diagnosis of localized prostate cancer, especially those with a low PSA value or low-risk disease.2,3,6,8,15–24
Death due to prostate cancer or treatment occurred infrequently, in 7.1% of patients. Any differences in prostate-cancer mortality between surgery and observation occurred primarily among men whose death was judged as probably due to prostate cancer or treatment. Among men whose death was considered to be definitely due to prostate cancer or treatment, we found almost no difference between surgery and observation. Between-group differences in the time of death due to prostate cancer or treatment did not explain the differences in all-cause mortality in the entire cohort or the subgroups. These findings highlight the limitations of using prostate-cancer mortality as an outcome, even with the use of adjudication committees whose members are unaware of treatment assignments and who are following standardized protocols.25–27
The effect of radical prostatectomy on mortality did not vary according to age, race, self-reported performance status, or coexisting conditions, but our findings suggest that it may vary according to PSA value and possibly tumor risk. Positive results were from multiple subgroup comparisons; the tests of interaction typically approached but did not reach significance and may therefore be due to chance. Among men with PSA levels of 10 ng per milliliter or less, all-cause mortality was slightly lower at 12 years in the observation group than in the radical-prostatectomy group; prostate-cancer mortality in the observation group was 6%, with a nonsignificant absolute reduction of less than 1.0 percentage point in the radical-prostatectomy group. Among men with low-risk disease, observation was associated with a non-significant reduction in all-cause and prostate cancer mortality, with no significant between-group difference in bone metastases. Among men with a PSA value that was greater than 10 ng per milliliter and possibly among those with intermediate-risk or high-risk prostate cancer (as determined according to the PSA value, local histologic findings, and stage), absolute reductions in all-cause mortality with radical prostatectomy ranged from 6.7 to 13.2 percentage points. Reductions were smaller and not significant when central histopathological findings were used, and we found no significant reductions with radical prostatectomy in categories that were derived solely on the basis of higher scores on the Gleason histologic scale or tumor stage. Reductions in prostate-cancer mortality in the radical-prostatectomy group were limited to men with a PSA value that was greater than 10 ng per milliliter and to those with high-risk disease, with absolute reductions of 7.2 to 8.4 percentage points. Absolute reductions in bone metastases of 10.4 and 8.6 percentage points occurred, respectively, in men with a PSA value of 10 ng per milliliter or higher and in those with high-risk disease.
As compared with the Scandinavian Prostate Cancer Group 4 (SPCG-4) trial of radical prostatectomy versus watchful waiting in men with prostate cancer detected before widespread PSA testing,6 PIVOT enrolled a higher percentage of men with nonpalpable tumors (stage T1c, 50% vs. 12%) and with PSA values of 10 ng per milliliter or lower. Treatment adherence was similar in the two trials.6,28 In contrast to the SPCG-4 trial, we did not find a significant reduction in all-cause or prostate-cancer mortality with radical prostatectomy. Our findings are particularly robust among men with a PSA value of 10 ng per milliliter or less, including men with a score of 7 or higher on the Gleason histologic scale, and low-risk tumor — categories that were underrepresented in the SPCG-4 trial. Unlike the SPCG-4 trial, our study did not show that the effect of surgery, as compared with observation, varied according to age. Although hazard ratios indicated that the relative effect of radical prostatectomy on prostate-cancer mortality was similar in PIVOT and the SPCG-4 trial (37% and 38% reduction, respectively), the relative reduction in all-cause mortality in our study was less than half that in the SPCG-4 trial (12% vs. 25%), as were the absolute reductions in all-cause mortality (2.9 percentage points vs. 6.6 percentage points) and prostate-cancer mortality (2.6 percentage points vs. 6.1 percentage points); the overall percentage of men who died from prostate cancer was also lower in our study (7.1% vs. 19.6%). The mortality reductions in our study were not significant and probably reflect the more favorable prognosis for patients with tumors detected by means of PSA testing.
Our study has strengths that enhance the clinical applicability of the findings. The age, health status, PSA value, and tumor-risk characteristics of the men enrolled in this study were similar to those of both men who were eligible but declined to undergo randomization9 and men in the general population who have received a diagnosis of prostate cancer.1–3,8,29 Perioperative morbidity and mortality were similar to those previously reported.28,30 The percentage of men with positive surgical margins was similar to that in earlier studies and lower than that in the SPCG-4 trial.27 The tumor volumes and PSA values in our study population, although higher than in some contemporary series,31–35 are probably representative of those in the general population of men who received a diagnosis of prostate cancer at the time the study was being conducted. Our choice of all-cause mortality as the primary outcome underscores the importance of improving life expectancy with cancer treatment and eliminates the possibility of biased cause-of-death ascertainment.25–27
Our study was conducted in the early era of PSA testing. The current practices of performing repeated PSA testing, using a lower PSA threshold for biopsy, obtaining more tissue-biopsy cores, and performing a repeat biopsy after initially negative findings increase the detection of smaller-volume indolent cancers.15,16 Along with systematically higher assignment of tumor grades (upgrading), these factors increase the likelihood of overdiagnosis and overtreatment.36–38 Among men with a current diagnosis of prostate cancer who undergo radical prostatectomy, the absolute reductions in the risks of metastasis and death will probably be smaller, and the time required to identify a reduction will probably be longer than reported in our study or in the SPCG-4 trial.
Our findings support observation for men with localized prostate cancer, especially those who have a low PSA value and those who have low-risk disease. Up to two thirds of men who have received a diagnosis of prostate cancer have a low PSA value or low-risk disease, but nearly 90% receive early intervention — typically surgery or ra-diotherapy.1,15,16,24 In contrast to observation, active surveillance initiates therapy with curative intent if disease progression is suspected on the basis of repeat PSA testing, digital rectal examinations, and prostate biopsies.3,24 Active surveillance is being compared with surgery or radiotherapy in a randomized trial.39 Informing men of the favorable long-term effects of observation on mortality, bone metastases, urinary and erectile function, and quality of life40–42 and increasing the use of observation may avert the harms of unnecessary biopsies43 and interventions2,3,6 while maintaining excellent long-term disease-specific survival.
In conclusion, our study showed that, as compared with observation, radical prostatectomy did not significantly reduce all-cause or prostate-cancer mortality through at least 12 years among men with clinically localized prostate cancer that had been diagnosed in the era of PSA testing. Absolute differences in mortality between the study groups were less than 3 percentage points. Subgroup analyses suggested that surgery might reduce mortality among men with higher PSA values and possibly among men with higher-risk tumors, but not among men with PSA levels of 10 ng per milliliter or less or among men with low-risk tumors.
Supplementary Material
Acknowledgments
Supported by grants from the Department of Veterans Affairs Cooperative Studies Program, the National Cancer Institute, and the Agency for Healthcare Research and Quality.
Dr. Barry reports being employed by and serving as a board member of the Foundation for Informed Medical Decision Making, which receives royalties from Health Dialog. Dr. Wei reports serving on the board for Envisioneering, receiving consulting fees and grant support from Sanofi-Aventis, providing expert testimony for Genprobe concerning prostate-cancer detection, and serving as proctor for benign prostatic hyperplasia laser surgery for American Medical Systems. Dr. Andriole reports receiving consulting fees, payment for the development of presentations, and payment for travel, accommodation, and meeting expenses from Amgen; consulting fees, stock options, and payment for travel, accommodation, and meeting expenses from Augmenix; consulting fees and payment for travel, accommodation, and meeting expenses from Bayer; consulting fees and payment for travel, accommodation, and meeting expenses from Bristol-Myers Squibb; consulting fees, stock options, and payment for travel, accommodation, and meeting expenses from Cambridge Endo; consulting fees and payment for travel, accommodation, and meeting expenses from Caris; consulting fees and payment for travel, accommodation, and meeting expenses from GlaxoSmithKline; consulting fees and payment for travel, accommodation, and meeting expenses from Janssen Biotech; consulting fees and payment for travel, accommodation, and meeting expenses from Myriad Genetics; consulting fees and payment for travel, accommodation, and meeting expenses from Steba Biotech; consulting fees and payment for travel, accommodation, and meeting expenses from Ortho Clinical Diagnostics; consulting fees and stock options from Viking Medical; stock options from Envisioneering Medical; and grant support to his institution from Johnson & Johnson, Medivation, and Wilex; and being a member of an independent data monitoring committee for Amarex. Dr. Wheeler reports serving as a board member of Medscape; receiving consulting fees from Glaxo-SmithKline; providing expert testimony for various law firms regarding medical malpractice, product liability, and toxic tort; receiving royalties from Metabolon; and receiving stock options from Digipath.
Footnotes
No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010;28:1117–23. doi: 10.1200/JCO.2009.26.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilt TJ, MacDonald R, Rutks I, Shamliyan TA, Taylor BC, Kane RL. Systematic review: comparative effectiveness and harms of treatments for clinically localized prostate cancer. Ann Intern Med. 2008;148:435–48. doi: 10.7326/0003-4819-148-6-200803180-00209. [Erratum, Ann Intern Med 2008;148:888.] [DOI] [PubMed] [Google Scholar]
- 3.Thompson I, Thrasher JB, Aus G, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. Linthicum, MD: American Urological Association Education and Research; 2007. ( http://www.auanet.org/content/clinical-practice-guidelines/clinical-guidelines/main-reports/proscan07/content.pdf) [Google Scholar]
- 4.SEER cancer statistics review 1975–2004. Bethesda, MD: National Cancer Institute; 2007. ( http://seer.cancer.gov/csr/1975_2004) [Google Scholar]
- 5.Iversen P, Madsen PO, Corle DK. Radical prostatectomy versus expectant treatment for early carcinoma of the prostate: twenty-three year follow-up of a prospective randomized study. Scand J Urol Nephrol Suppl. 1995;172:65–72. [PubMed] [Google Scholar]
- 6.Bill-Axelson A, Holmberg L, Ruutu M, et al. Radical prostatectomy versus watchful waiting in localized prostate cancer. N Engl J Med. 2011;364:1708–17. doi: 10.1056/NEJMoa1011967. [DOI] [PubMed] [Google Scholar]
- 7.Widmark A, Tomic R, Modig H, et al. Prospective randomized trial comparing external beam radiotherapy versus watchful waiting in early prostate cancer (T1b-T2, pN0, grade 1–2, M0). Presented at the 53rd Annual ASTRO Meeting; Miami Beach, FL. October 2–6, 2011; abstract. [Google Scholar]
- 8.Lu-Yao GL, Albertsen PC, Moore DF, et al. Outcomes of localized prostate cancer following conservative management. JAMA. 2009;302:1202–9. doi: 10.1001/jama.2009.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilt TJ, Brawer MK, Barry MJ, et al. The Prostate cancer Intervention Versus Observation Trial: VA/NCI/AHRQ Cooperative Studies Program 407 (PIVOT): design and baseline results of a randomized controlled trial comparing radical prostatectomy to watchful waiting for men with clinically localized prostate cancer. Contemp Clin Trials. 2009;30:81–7. doi: 10.1016/j.cct.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Fleming ID, Cooper JS, Henson DE, et al., editors. AJCC cancer staging manual. 5. Philadelphia: Lippincott-Raven; 1997. [Google Scholar]
- 11.Allison PD. Survival analysis using the SAS system: a practical guide. Cary, NC: SAS Institute; 1995. p. 292. [Google Scholar]
- 12.Charlson ME, Pompei P, Ales K, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 13.Gleason DF. The Veteran’s Administration Cooperative Urologic Research Group: histologic grading and clinical staging of prostatic carcinoma. In: Tannenbaum M, editor. Urologic pathology: the prostate. Philadelphia: Lea & Febiger; 1977. pp. 171–98. [Google Scholar]
- 14.D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–74. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 15.Welch HG, Albertsen P. Prostate cancer diagnosis and treatment after the introduction of prostate-specific antigen testing. J Natl Cancer Inst. 2009;101:1325–9. doi: 10.1093/jnci/djp278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson IM, Klotz L. Active surveillance for prostate cancer. JAMA. 2010;304:2411–2. doi: 10.1001/jama.2010.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayes JH, Ollendorf DA, Pearson SD, et al. Active surveillance compared with initial treatment for men with low-risk prostate cancer: a decision analysis. JAMA. 2010;304:2373–80. doi: 10.1001/jama.2010.1720. [Erratum, JAMA 2011; 305:1862.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooperberg MR, Broering JM, Kantoff PW, Carroll PR. Contemporary trends in low risk prostate cancer: risk assessment and treatment. J Urol. 2007;178:S14–S19. doi: 10.1016/j.juro.2007.03.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleming C, Wasson JH, Albertsen PC, Barry MJ, Wennberg JE. A decision analysis of alternative treatment strategies for clinically localized prostate cancer. JAMA. 1993;269:2650–8. [PubMed] [Google Scholar]
- 20.Djulbegovic M, Beyth RJ, Neuberger MM, et al. Screening for prostate cancer: systematic review and metaanalysis of randomised controlled trials. BMJ. 2010;341:c4543. doi: 10.1136/bmj.c4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andriole GL, Crawford ED, Grubb RL, 3rd, et al. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104:125–32. doi: 10.1093/jnci/djr500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schröder F, Hugosson J, Roobol MJ, et al. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med. 2012;366:981–90. doi: 10.1056/NEJMoa1113135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coen JJ, Feldman AS, Smith MR, Zietman AL. Watchful waiting for localized prostate cancer in the PSA era: what have been the triggers for intervention? BJU Int. 2011;107:1582–6. doi: 10.1111/j.1464-410X.2010.09652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ganz PA, Barry JM, Burke W, et al. National Institutes of Health State-of-the-Science Conference: role of active surveillance in the management of men with localized prostate cancer. Ann Intern Med. 2012;156:591–5. doi: 10.7326/0003-4819-156-8-201204170-00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dubben HH. Trials of prostate-cancer screening are not worthwhile. Lancet Oncol. 2009;10:294–8. doi: 10.1016/S1470-2045(09)70066-X. [DOI] [PubMed] [Google Scholar]
- 26.Schellhammer P, Cockett A, Boccon-Gibod L, et al. Assessment of endpoints for clinical trials for localized prostate cancer. Urology. 1997;49:27–38. doi: 10.1016/s0090-4295(99)80321-5. [DOI] [PubMed] [Google Scholar]
- 27.Newschaffer CJ, Otani K, McDonald MK, Penberthy LT. Causes of death in elderly prostate cancer patients and in a comparison nonprostate cancer cohort. J Natl Cancer Inst. 2000;92:613–21. doi: 10.1093/jnci/92.8.613. [DOI] [PubMed] [Google Scholar]
- 28.Bill-Axelson A, Holmberg L, Filén F, et al. Radical prostatectomy versus watchful waiting in localized prostate cancer: the Scandinavian Prostate Cancer Group-4 randomized trial. J Natl Cancer Inst. 2008;100:1144–54. doi: 10.1093/jnci/djn255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shao Y-H, Demissie K, Shih W, et al. Contemporary risk profile of prostate cancer in the United States. J Natl Cancer Inst. 2009;101:1280–3. doi: 10.1093/jnci/djp262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilt TJ, Shamliyan T, Taylor B, et al. Comparative effectiveness of therapies for clinically localized prostate cancer: comparative effectiveness review number 13. Rockville, MD: Agency for Healthcare Research and Quality; Feb, 2008. ( http://www.effectivehealthcare.ahrq.gov/repFiles/2008_0204ProstateCancerFinal.pdf) [PubMed] [Google Scholar]
- 31.Eastham JA, Kattan MW, Riedel E, et al. Variations among individual surgeons in the rate of positive surgical margins in radical prostatectomy specimens. J Urol. 2003;170:2292–5. doi: 10.1097/01.ju.0000091100.83725.51. [DOI] [PubMed] [Google Scholar]
- 32.Obek C, Sadek S, Lai S, Civantos F, Rubinowicz D, Soloway MS. Positive surgical margins with radical retropubic prostatectomy: anatomic site-specific pathologic analysis and impact on prognosis. Urology. 1999;54:682–8. doi: 10.1016/s0090-4295(99)00204-6. [DOI] [PubMed] [Google Scholar]
- 33.Ohori M, Wheeler TM, Kattan MW, Goto Y, Scardino PT. Prognostic significance of positive surgical margins in radical prostatectomy specimens. J Urol. 1995;154:1818–24. [PubMed] [Google Scholar]
- 34.Swindle P, Eastham JA, Ohori M, et al. Do margins matter? The prognostic significance of positive surgical margins in radical prostatectomy specimens. J Urol. 2005;174:903–7. doi: 10.1097/01.ju.0000169475.00949.78. [DOI] [PubMed] [Google Scholar]
- 35.Vis AN, Schröder FH, van der Kwast TH. The actual value of the surgical margin status as a predictor of disease progression in men with early prostate cancer. Eur Urol. 2006;50:258–65. doi: 10.1016/j.eururo.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 36.Albertsen PC, Hanley JA, Barrows GH, et al. Prostate cancer and the Will Rogers phenomenon. J Natl Cancer Inst. 2005;97:1248–53. doi: 10.1093/jnci/dji248. [DOI] [PubMed] [Google Scholar]
- 37.Ghani KR, Grigor K, Tulloch DN, Bollina PR, McNeill SA. Trends in reporting Gleason score 1991 to 2001: changes in the pathologist’s practice. Eur Urol. 2005;47:196–201. doi: 10.1016/j.eururo.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 38.Stamey TA, Johnstone IM, McNeal JE, Lu AY, Yemoto CM. Preoperative serum prostate specific antigen levels between 2 and 22 ng/ml correlate poorly with post-radical prostatectomy cancer morphology: prostate-specific antigen cure rates appear constant between 2 and 9 ng/ml. J Urol. 2002;167:103–11. [PubMed] [Google Scholar]
- 39.Donovan J, Mills N, Smith M, et al. Improving design and conduct of randomized trials by embedding them in qualitative research: ProtecT (prostate testing for cancer and treatment) study. BMJ. 2002;325:766–70. doi: 10.1136/bmj.325.7367.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250–61. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 41.Johansson E, Steineck G, Holmberg L, et al. Long-term quality-of-life outcomes after radical prostatectomy or watchful waiting: the Scandinavian Prostate Cancer Group-4 randomised trial. Lancet Oncol. 2011;12:891–9. doi: 10.1016/S1470-2045(11)70162-0. [DOI] [PubMed] [Google Scholar]
- 42.Fransson P, Damber JE, Tomic R, et al. Quality of life and symptoms in a randomized trial of radiotherapy versus deferred treatment of localized prostate carcinoma. Cancer. 2001;92:3111–9. doi: 10.1002/1097-0142(20011215)92:12<3111::aid-cncr10160>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 43.Rosario DJ, Lane JA, Metcalfe C, et al. Short term outcomes of prostate biopsy in men tested for cancer by prostate specific antigen: prospective evaluation within ProtecT study. BMJ. 2012;344:d7894. doi: 10.1136/bmj.d7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.