Abstract
Objective
The purpose of this study is to: 1) describe a newly discovered mechanism of blood flow to the brain during CPR using the impedance threshold device (ITD) in a piglet model of cardiac arrest, and 2) describe the survival benefits in humans of applying all of the highly recommended changes in the 2005 Guidelines related to increasing circulation during CPR, including use of the ITD, from 6 EMS systems in the United States
Design
Animal studies prospective trial with each piglet serving as its own control. Historical controls were used for the human studies.
Subjects
Piglet and patients with out-of-hospital cardiac arrest
Interventions
Piglets (10-12 kg) were treated with an active (n=9) or sham (n=9) ITD after 6 minutes of ventricular fibrillation. Humans were treated with CPR per the AHA 2005 Guidelines and the ITD.
Measurements and Main Results
Animals
The primary endpoint in the piglet study was carotid blood flow which increased from 59 ml/min without an ITD to 91 ml/min (p=0.017) with ITD use. Airway pressures during the chest recoil phase decreased from -0.46 mmHg to -2.59 mmHg (p=0.0006) with the active ITD. Intracranial pressure (ICP) decreased more rapidly and to a greater degree during the decompression phase of CPR with the active ITD
Humans
Conglomerate quality assurance data were analyzed from 6 EMS systems in the United States serving a population of ~3 million people. There were 920 patients treated for cardiac arrest following implementation of the 2005 AHA Guidelines, including ITD use, and 1750 patients in the control group during the year prior to implementation. Demographics were similar between the two groups. Survival to hospital discharge was 9.3 % in the control group versus 13.6 % in the intervention group. The odds ratio, 95% confidence interval, and p values were, 1.54 (1.19, 1.99), and P = 0.0008, respectively. This survival advantage was conferred to patients with a presenting cardiac arrest rhythm of ventricular fibrillation (28.5% vs. 18.0%, P = 0.0008).
Conclusions
Use of the ITD in piglets increased carotid blood flow and coronary and cerebral perfusion pressures and reduced intracranial pressure during the decompression phase of CPR at a faster rate than controls, resulting in a greater duration of time when intracranial pressures are at their nadir. Patients in 6 EMS systems treated with the ITD together with the renewed emphasis on more compressions, fewer ventilations, and complete chest wall recoil had a nearly 50% increase in survival rates after out-of-hospital cardiac arrest compared with historical controls.
Keywords: Cardiac arrest, sudden death, impedance threshold device, cerebral perfusion pressure, intracranial pressure, CPR
Introduction
It has been nearly a half a decade since closed chest cardiopulmonary resuscitation (CPR), also known as standard CPR, was first described. (1) While this technique has helped to save hundreds of thousands of people worldwide, cardiac output during CPR remains less than 20% of baseline. (2,3) Even when applied correctly, nine out of every ten patients who suffer a cardiac arrest and receive standard CPR never survive an out-of-hospital cardiac arrest in most cities. (4-6) These dismal survival rates prompted a reassessment of the basic physiology of CPR beginning in the mid-1990’s. As a result, there have been a number of important breakthroughs in the science of resuscitation. (7-22) Many of these breakthroughs were incorporated into the American Heart Association Guidelines in 2005. (23) These advances include a greater emphasis on continuous chest compressions without interruptions (7-9), less frequent ventilations (10-13), chest compression immediately before and after defibrillation (14-16), complete chest wall recoil (17-19), and use of a new technology called the impedance threshold device (ITD) to harnesses the recoil energy of chest wall decompression to lower intrathoracic pressures, thereby improving the refilling process of the cardiac chambers (20-22). Since that time, research has continued on the mechanism of blood flow during CPR and ways to optimize clinical outcomes. (24-26) The purpose of this paper is to, a) describe a newly discovered mechanism of blood flow to the brain during CPR with the ITD in a piglet model of cardiac arrest, and b) describe the survival benefits in humans of applying all of the highly recommended changes to increase circulation during CPR in the 2005 Guidelines, including use of the ITD, from 6 EMS systems in the United States.
Materials and Methods
Animal Studies
The study was approved by the Institutional Animal Care Committee of the Minneapolis Medical Research Foundation at Hennepin County Medical Center. Anesthesia was used in all surgical interventions to avoid all unnecessary suffering. Experiments were performed by a qualified, experienced team. The study was performed on female farm piglets (10-12 kg).
Preparatory Phase
Initial sedation in each animal was achieved with 3 ml (100 mg/ml) of intramuscular ketamine HCl (Ketaset®, Fort Dodge Animal Health, Fort Dodge, IA). Propofol anesthesia (PropoFlo®, Abbott Laboratories, North Chicago, IL), (2.3 mg/kg), was also delivered as an intravenous (IV) bolus via the lateral ear vein. While spontaneously breathing, but sedated, the piglets were intubated with a 5.0 French endotracheal tube. Additional propofol (1 mg/kg) was then administered, followed by a propofol infusion of 160μg/kg/min. The method of anesthesia and the general surgical approach for the preparation was previously described (10,11).
While the animals were sedated and mechanically ventilated, under aseptic conditions, a hole was drilled through the skull for intracranial pressure recordings. After identifying the posterior bony prominence of the piglet’s cranium, a burr hole was made at the middle of the distance between the left eyebrow and the posterior bony prominence. A 3.5 French continuous recording micromanometer pressure transducer (Mikro-Tip® Transducer, Millar Instruments, Inc., Houston, TX) was inserted 1 cm into the parietal lobe of the animal and secured in place. The pressure transducer was connected to a signal amplifier (model No. 13-6615-50, Gould Instrument Systems, Inc. Valley View, OH) and then to a digital recording system (SuperScope II) providing real time intracranial pressure (ICP) tracings. A second hole was drilled in the same way on the right side for a second intracranial pressure transducer (Camino, Integra Life Sciences) which was used to calibrate the micromanometer pressure transducer.
Animals were then positioned supine and unilateral femoral artery cannulation was performed under aseptic conditions. Central aortic blood pressures were recorded continuously, with a micromanometer-tipped catheter (Mikro-Tip® Transducer, Millar Instruments, Inc., Houston, TX). A similar central venous catheter was placed in the right external jugular vein. All animals were treated with heparin bolus (100 units/kg IV), once catheters were in place. The left common carotid artery was then surgically exposed and a Doppler flow probe (Transonic, 420-Series Multi-channel, transonic Systems, Inc., Ithaca, NY) was placed to quantify common carotid blood flow. Animals were carefully maintained at 37 ± -0.5 °C using a warming blanket (Bair Hugger, Augustine Medical, Eden Prairie, MN) as needed.
During the preparatory phase, animals were ventilated with room air using a volume-control Narkomed 2A ventilator (North American Dräger, Telford, PA) with the tidal volume of 10 cc/Kg and rate adjusted to maintain an arterial CO2 at 40 mmHg and PaO2 of >80mmHg (oxygen saturation >95%), based upon analysis of arterial blood gases (IL Synthesis, Instrumentation Laboratory, Lexington, MA).
Electrocardiographic monitoring was recorded continuously. Intratracheal Pressure (ITP) was measured continuously using a micromanometer-tipped catheter positioned 2 cm above the trachea. All data were digitized by a digital recording system (Superscope II vl.295, GW Instruments, Somerville, MA) and a Power Macintosh G3® computer (Apple Computer, Inc., Cupertino, CA). End tidal CO2 (ETCO2) was measured with a CO2SMO Plus®, (Novametrix Medical Systems, Wallingford, CT.
All parameters (aortic, right atrial, intratracheal, intracranial, coronary perfusion and cerebral perfusion pressures) were analyzed as follows: discrete point measurements were obtained from the aortic, right atrial, intratracheal pressure (ITP) pressures measured at the level of the carina as a surrogate for intrathoracic pressure, and ICP recordings during both compression and decompression. Each minute of CPR was characterized by three sets of four measurements made just prior to a ventilation cycle evenly distributed throughout the minute (each set 20 seconds apart). Coronary perfusion pressures were calculated values: coronary perfusion pressure was calculated as the difference between aortic and right atrial pressures during decompression. Cerebral perfusion pressure was calculated as the difference between the mean values of aortic pressure and intracranial pressure using the mean value of digitalized aortic and intracranial pressure recordings. ITP was also measured as minute ITP (minute-ITP): electronic data of ITP values over one minute were transferred to Microsoft Excel and then averaged, resulting in the mean ITP value over the whole minute of intervention. . The slope of the ICP waveform was evaluated by analyzing ICP tracings from the same 4 compression/decompression cycles selected for calculating the cerebral perfusion pressure. The slope of the decompression phase was analyzed as follows: the local maximum value corresponding to compression and the local minimum corresponding to decompression of each cycle were identified. The slope was calculated for the linear portion of the decent of the ICP recording by first subtracting 10% of the delta between the local maximum and minimum values, since the peak and trough portions of the recordings were associated with a nonlinear rather than linear shape. The slope of the remaining linear portion curves was then calculated. By eliminating the upper and lower 10% of the y-component of the ICP waveform, the nonlinear portion of the waveform was removed allowing high fidelity between real and calculated slopes.
Experimental Protocol I
Once the surgical preparations were completed, oxygen saturation was >90% and ETCO2 stable between 35-42 mmHg for 5 minutes, ventricular fibrillation (VF) was induced by delivering direct electrical current via a temporary pacing wire (Daig Division, St Jude Medical, Minnetonka, MN) positioned in the right ventricle. At that time, a computer generated randomization list was used to determine the sequence of the interventions. Once VF was induced, the ventilator was disconnected from the ET tube and the dose of propofol was reduced to 100mcg/kg/min. After 8 min of untreated ventricular fibrillation, closed-chest standard CPR was performed with a pneumatically driven automatic piston device (Pneumatic Compression Controller, AMBU International, Glostrup, Denmark) as previously described (11,30) for 6 min in all piglets. The compression rate was 100/min, uninterrupted, with a 50% duty cycle, and a depth of 25% of the anterior-posterior diameter of the chest wall. The chest wall was allowed to recoil passively but completely with this device as the compression piston was actively pulled upward to a point 0,1 cm off the chest after each compression. Pressure-controlled ventilation was performed with a Smart Bag (O2 Systems, Toronto, CA) using 100% Oxygen at a constant flow rate (10 L/min). Ventilations were initiated synchronously with the decompression phase of CPR: approximately 10 cc/kg were delivered with each breath. After 6 minutes of CPR an active ITD (ResQPOD, Advanced Circulatory Systems, Inc., resistance of safety check valve -10 cm H2O) was added and CPR was performed for another 6 minutes. After a total of 12 minutes of CPR, defibrillation was attempted with 3 successive 100 joule shocks using a defibrillator, (Zoll M Series, Manchester, NH). No additional supportive therapy was provided, but if the pig redeveloped ventricular fibrillation spontaneously after an initially successful return of spontaneous circulation, additional defibrillation shocks were used. If VF persisted after the initial 3 shocks, then epinephrine was administered at a dose of 45mcg/kg and then 3 more shocks (100 joules) were delivered. If VF still persisted thereafter, all resuscitation efforts were terminated. When resuscitation was successful, animals were again ventilated with a Narkomed 2A machine with a tidal volume of 10 cc/Kg. No further interventions were performed after restoration of spontaneous circulation. Thirty minutes after resuscitation the animals were sacrificed using an intravenous bolus of propofol 60 mg and then 10M KCl.
Experimental Protocol II
Following an analysis of the initial animal study results, a second series of piglet studies were performed, but instead of using an active ITD after 8 minutes of untreated ventricular fibrillation and 6 minutes of CPR, a sham ITD was used instead. Similar to the first series of studies, efforts were made to defibrillate the piglets after a total of 12 minutes of CPR.
The analysis of all the parameters during the two different intervention protocols was performed after averaging data from all animals and calculating the mean. With this approach, sequence effects and potential changes in key parameters over time were minimized. CPR was performed continuously for a total of 12 minutes. Aortic pressure, right atrial pressure, intracranial pressure, intratracheal pressure, ETCO2 and O2 saturation were measured continuously. Arterial blood gases were sampled before ventricular fibrillation was induced and after 5.5 and 11.5 minutes of CPR.
Statistical analysis
Values are expressed as mean ± SEM. The primary endpoint was carotid blood flow. Additional measurements included mean arterial pressures, intracranial pressure, coronary and cerebral perfusion pressures, the slope of the ICP curves, arterial oxygen saturation and arterial partial pressure of oxygen. A paired t-test was used to determine statistical significance between different interventions. A p-value of < 0.05 was considered statistically significant.
Human Studies
Analysis and publication of conglomerate, de-identified quality assurance data described in this evaluation was approved by Institutional Review Board of the Medical College of Wisconsin. Six Emergency Medical Services (EMS) systems contributed data to this study. They were Anoka County, Minnesota; Harris County, Texas; Pinellas County, Florida; Wake County, North Carolina; and the cities of Milwaukee, Wisconsin; and Omaha, Nebraska. The combined sites have a population of approximately 3million people and include urban, suburban, and rural settings. Each site deployed the impedance threshold device (ITD) (ResQPOD™ Advanced Circulatory Systems, Inc., Minneapolis, MN) for a minimum of 3 months duration concurrently with manual CPR that emphasized more compressions and fewer ventilations, a compression rate of 100/min, complete chest wall recoil, minimum interruptions in compressions for pulse checks, a continuously tight face mask seal during compressions and ventilations when the ITD was used, ventilation duration of 1 second/ breath, a compression/ventilation ratio of 30:2 prior to an advanced airway, and continuous chest compression without interruption for ventilation (10 breaths/minute with a duration of 1 second/breath) when an advanced airway was placed. The ITD was deployed on both the face mask and an endotracheal tube, or other means to secure the airway including a CombiTube™ or Laryngeal Mask Airway (LMA™). For the purposes of this paper, this physiologically-based approach to optimizing circulation during CPR will be referred to as ‘new’ CPR. The key aspects of ‘new’ CPR, including use of the ITD, are highly recommended (level I or IIa Recommendations) in the 2005 American Heart Association Guidelines. The EMS systems selected for this report had the most extensive clinical experience with the ITD and ‘new’ CPR at the time the data were pooled.
Patients were included in this analysis if they presented with a cardiac arrest, received CPR by EMS personnel, and met local criteria for ITD use. Criteria for ITD use varied: it was applied in patients ≥ 12 years of age and deployed on a face mask in 5/6 systems. Patients who presented in cardiac arrest secondary to a presumed non-cardiac etiology (e.g. blunt or penetrating trauma) were excluded from this analysis. For the purpose of this study, patients who presented with burns or electrocution were included in the analysis.
Historical or concurrent control data were used for comparison in this evaluation. Historical controls were derived from patients using matching local criteria for ITD use during a one year period prior to ITD implementation Hospital discharge data were available from all sites.
Pooled conglomerate data were analyzed for statistically significant differences between the historical control and study population. Odds ratios, 95% confidence intervals, and p values were calculated.
Results
Animal studies
To determine the potential for the ITD to increase forward blood flow to the brain and to lower intracranial pressure (ICP) during the decompression phase of CPR, carotid blood flow and ICP were measured in 9 piglets undergoing continuous chest compressions in the absence and then the presence of an ITD with a resistance of -10 cm H2O. Application of the ITD resulted in a decrease in airway pressures (in mmHg) from -0.71 +/- 0.23 (SEM) to -2.76 +/- 0.45 and in a rapid increase in carotid blood flow, as shown in Figure 1, compared with flow during the first 6 minute epoch without the ITD. A comparison of the effect of an active versus sham ITD on carotid blood flow comparing protocols I and II is shown in Figure 2. There was no change in airway pressures or carotid flow with the application of the sham ITD.
Figure 1.
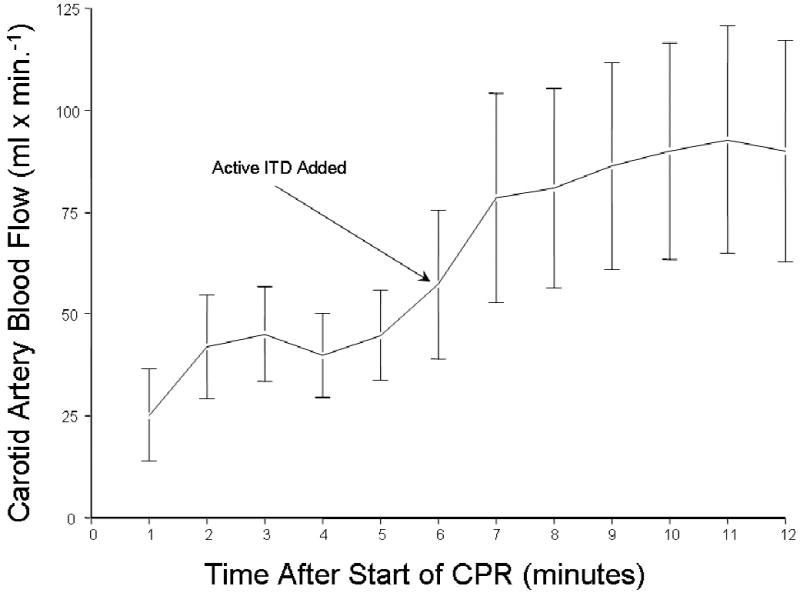
The means +/- SEM carotid blood flow increased when the ITD was added to the respiratory circuit after 8 minutes of untreated ventricular fibrillation and 6 minutes of CPR without the ITD. The increase was rapid and resulted in a 50% increase in carotid blood flow.
Figure 2.
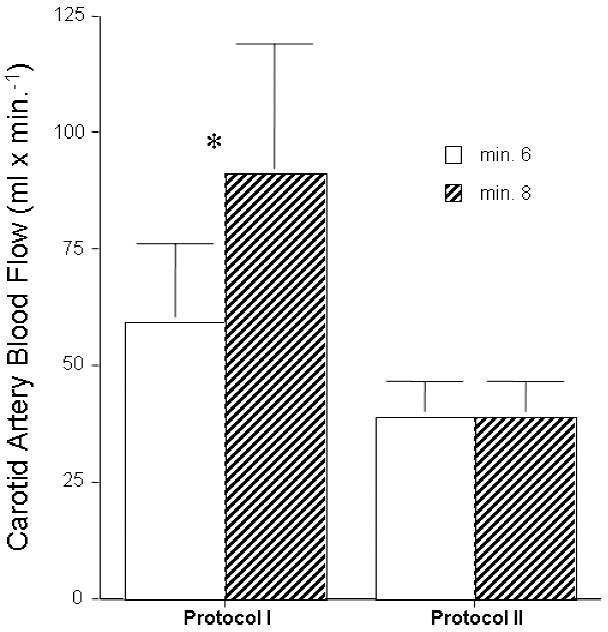
Two groups of piglets were subjected to 8 minutes of untreated ventricular fibrillation followed by 6 minutes of CPR alone and then CPR with either an active or a sham ITD. Carotid blood flow increased after addition of an active ITD in Protocol I but did not change after adding a sham ITD in Protocol II.
To further explore the mechanism of ITD effect on blood flow to the brain, ICP was measured along with the change in ICP during the decompression phase of CPR. ICP, measured with a pressure transducer located in the parietal lobe, decreased more rapidly and to a greater degree during the decompression phase of CPR when the ITD was used compared with no ITD, as shown in a representative graph in Figure 3. The slope of the ICP pressure curves during the decompression phase of CPR was, on average 19% steeper resulting in a significantly longer period of time that the cerebral perfusion pressures were higher compared with no ITD. The mean slope values (+/- SEM) during the decompression phase of CPR before and after addition of the active ITD in Protocol I was -0.95 +/- .09 and -1.14 +/- 0.12, P = 0.001), respectively. By comparison, the mean slope values were similar before (-.85 +/- .08) and after the sham ITD (.88 +/-.08) (P=NS) was used in Protocol II.
Figure 3.
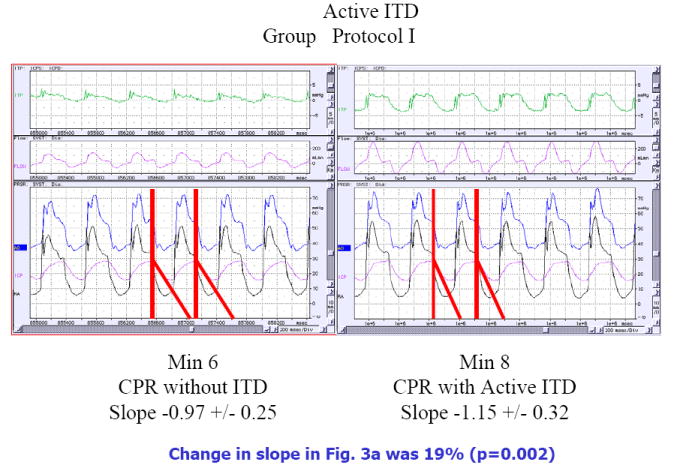

Piglets were subjected to 8 minutes of untreated ventricular fibrillation followed by 6 minutes of CPR alone and then CPR with either an active or a sham ITD. The effect of adding an active (Fig 3a) or a sham (Fig 3b) ITD on changes in airway pressures (ITP), carotid blood flow (flow in pink), aortic pressure (Ao in blue), right atrial pressure (RA in black), and intracranial pressure (ICP in pink) are shown in these representative figures. The data is shown after 6 minute of CPR alone and then at minute 8, 2 minutes after addition of the active ITD (Figure 3a) or a sham ITD (Fig 3b). The change in the slope of the ICP curves are highlighted by the red line and the actual values are shown below each figure. When the active ITD is added to the respiratory circuit there is a more rapid decrease in the slope of the ICP curve during the decompression phase of CPR resulting in less resistance to forward blood flow for a longer period of time (Fig 3a). This effect was not observed with the addition of the sham ITD in Fig 3b). These figures also highlight the decreases observed during the chest wall recoil phase of the airway pressure curves with the addition of the active ITD (labeled ITP for intrathoracic pressure in green) in the top panels. This effect is absent with the sham ITD.
Coronary and cerebral perfusion pressures were calculated to further demonstrate the ITD effect in the piglets: both were significantly higher with the active ITD compared with no ITD, as shown in Figure 4a and Figure 4b, respectively.
Figure 4.
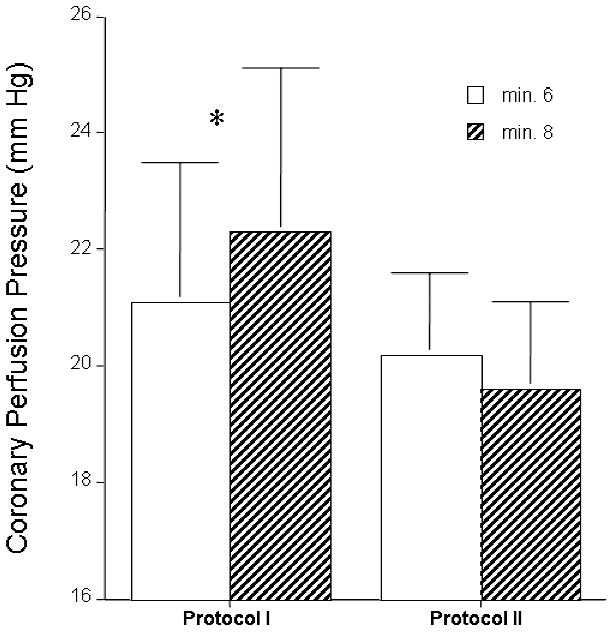

Piglets were subjected to 8 minutes of untreated ventricular fibrillation followed by 6 minutes of CPR alone and then CPR with either an active or a sham ITD and the coronary (Fig 4a) and cerebral (Fig 4b) perfusion pressures were compared. Adding an active ITD (Fig 4a) resulted in a statistically significant increase in coronary perfusion pressures (calculated by subtracting the right atrial pressure from the aortic pressure during the decompression phase of CPR). By contrast, coronary perfusion pressures trended lower after adding a sham ITD (Fig 4a). Similarly, adding an active ITD resulted in a statistically significant increase in cerebral perfusion pressures (Fig 4b) (difference between mean arterial pressure and intracranial pressure) whereas addition of the sham ITD had no benefit.
Human Studies
There were 1750 patients who had a cardiac arrest in the control group during the year prior to implementation of new CPR and the ITD. During new CPR and the ITD a total of 920 patients were evaluated. The ratio of male to female patients was similar in both groups, 65 % of the patients were male. The average age was also similar in both groups at 64 years of age). Survival to hospital discharge was 9.3 % in the control group versus 13.6 % in the intervention group. The odds ratio, 95% confidence interval, and p values were, 1.54 (1.19, 1.99), and P = 0.0008, respectively.
Survival to hospital discharge was also evaluated based on presenting rhythm. For patients with an initial rhythm of ventricular fibrillation, survival to hospital discharge rates were 18.0 % (81/450) in the control group and 28.5 % (84/295) in the new CPR and ITD group (P=0.0008). For patients with an initial rhythm of either asystole or pulseless electrical activity, hospital discharge rates were 6.2 % (81/1300) in the control group and 6.6 % (41/635) in the new CPR and ITD group (P = NS).
Discussion
The physiology underlying the beneficial effects, as well as the clinical role, of the ITD have continued to evolve since the concept of lowering intrathoracic pressures during chest wall decompression to augment vital organ perfusion was first described over a decade ago. (27-29) The ITD was initially thought to increase circulation to the heart and brain by augmenting venous return to the heart. (20, 21) This mechanism was previously supported by a number of animal studies performed with both standard manual CPR and active compression decompression (ACD) CPR. (29, 30)
New data from the current study as well as data from ongoing animal and clinical studies provide support of an additional mechanism of action: an active ITD lowers intracranial pressures faster and to a greater magnitude with each chest wall recoil when compared to sham controls. More specifically, there is an instantaneous reduction in intracranial pressures at a faster rate with the active ITD than with a sham ITD, thereby allowing for a greater duration of time when intracranial pressures are at their nadir. Since intracranial pressure is a major determinant of resistance to forward flow to the brain, the more rapid reduction in intracranial pressure by the ITD further enhances cerebral circulation.
The results of these piglet studies support a dual mechanism for the increase in circulation previously reported by using the ITD in animals. The first is the augmentation in cardiac output by greater refilling of the cardiac chambers during CPR. The second is a reduction in intracranial pressure secondary to thoraco-cranial interactions. These data help explain the marked improvement in neurological outcomes previously reported in pigs treated with an active ITD compared with sham-treated controls after cardiac arrest. (29) The mechanism of pressure transference is most likely via the spinal column as shown by Guerci et al in studies on how positive pressure ventilation causes an instantaneous increase in intracranial pressure. (31) The current studies are the first demonstration of how a reduction in intrathoracic pressure generated with the ITD result in a more rapid reduction in intracranial pressures. These relationships are fundamental for understanding how to optimize blood flow to the brain during CPR and how to reduce errors which may cause an increase in intrathoracic pressure and compromise blood flow to the brain. (10,17-19)
Just as the mechanism of action of the ITD has continued to become clearer, so is the role of the ITD as it continues to become integrated into the care of patients in cardiac arrest. An initial, double-blind, randomized clinical trial demonstrated that the ITD doubled systolic blood pressure in cardiac arrest patients. (21) That trial was equally significant for identifying some of the common and lethal errors associated with the performance of CPR. (10,17) The first error was that patients are frequently ventilated at excessive rates, which in turn cause higher intrathoracic pressures for a greater duration of the CPR cycles. The higher intrathoracic pressures reduce cardiac filling, increase intracranial pressures, and result in a decrease in coronary and cerebral perfusion pressures, which in turn markedly reduce the likelihood of survival. (10) The second major error, which has an equally detrimental effect, is that rescuers often fail to allow the chest to fully recoil after each compression. (17) This also results in higher intrathoracic pressures during the decompression phase of CPR which, in turn, reduces venous blood flow back to the heart and increases intracranial pressures. As with excessive ventilation rates, incomplete chest wall recoil reduces vital organ blood flow and reduces the likelihood of survival. (17-19) In pigs, the combination of incomplete chest wall recoil and positive pressure ventilation effectively reduces cerebral perfusion pressure to zero. (18)
The correction of these life-threatening errors has been an important contribution to the field of CPR and has helped to reshape to the 2005 American Heart Association Guidelines.(23) Furthermore, the ITD was redesigned to incorporate a timing light that flashes 10 times/min at 1 second/flash to provide rescue personnel with a visual cue when to provide a positive pressure ventilation, an appropriate ventilation duration, and how often to compress the chest (10 compression/flash). (25) Given the adverse effects of these common errors in CPR performance on the efficacy of the ITD in patients undergoing CPR, implementation of the ITD in the United States was performed concurrently with a strong emphasis on how to correctly perform CPR. This implementation process incorporated re-teaching rescue personnel how to perform CPR including: 1) ventilating at the proper compression/ventilation frequency of 30:2 for Basic Life Support when using a face mask and the ITD, 2) maintaining a continuously tight facemask seal during compressions and ventilations using a two-handed technique throughout the entire BLS phase, 3) allowing for full chest wall recoil by a slight alteration in the hand position so that the palm of the hand lifts slightly but completely off the chest each time the chest recoils, 4) continuous chest compressions without interruption for ventilation once an advanced airway was placed, and 5) CPR for 90-180 seconds before delivery of a shock. Implementing CPR immediately after a shock was also emphasized. When the ITD was introduced for clinical use, these key elements of proper CPR were highlighted during the training sessions with first responders and Advanced Life Support personnel.
This study, comprising a population base of greater than 3 million people, is the first to demonstrate that when CPR is performed correctly and the ITD is simultaneously deployed, there is a >50% increase in survival to hospital discharge rates when compared with historical controls (13.6 % vs 9.3%, P = 0.0008 %)(Table 1). This survival advantage was conferred to patients with a presenting cardiac arrest rhythm of ventricular fibrillation (28.5% vs. 18.0%, P = 0.0008). Differences for patients with non-VF rhythms were not statistically significant (6.6% vs. 6.2%, P = NS).
Table 1.
Hospital discharge rates in control and intervention groups
| Parameter | Control Group | Intervention (‘new CPR’ +ITD) Group | P Value | OR (95% CI) |
|---|---|---|---|---|
| Hospital Discharge | 9.3% (n=1750) | 13.6% (n=920) | 0.0008 | 1.541 (1.192, 1.990) |
| Hospital Discharge (VF) | 81/450(18.0%) | 84/295 (28.5%) | 0.0008 | 1.814 (1.259, 2.610) |
| Hospital Discharge (Non-VF) | 81/1300 (6.2%) | 41/635 (6.6%) | 0.7655 | 1.057 (0.698, 1.579) |
The findings demonstrate potential for increasing survival rates after cardiac arrest by augmenting vital organ blood flow during CPR. While this is only one step in the overall sequence of care essential to long-term success, it is the most fundamental first step. Treating cardiac arrest is like treating other complex and lethal diseases; the treatment requires multiple synergistic therapies. For example, optimizing hospital-based care of the resuscitated patient is equally important, but not yet widely applied. Two of the sites in this clinical evaluation began to use therapeutic hypothermia during the course of implementing “new CPR” and the ITD, and, as such, one third of the survivors from Anoka County, Minnesota and Wake County, North Carolina received the added benefits of therapeutic hypothermia. The authors believe therapeutic hypothermia is an additional essential therapy for those patients who remain comatose after a return of spontaneous circulation, and represents incompletely utilized potential to even further improve outcome. (32,33)
There are several limitations to these animal and human investigations. The animal protocols are limited in that no microphere or long-term survival studies were performed. As such, the correlation between improved cerebral blood flow with ITD use and survival is lacking. However, both of these parameters have been previously evaluated in 30 kg pigs. (27-30) Those studies showed a doubling of blood flow to the brain with the ITD and a dramatic improvement in neurologically intact survival rates. (27-30) The human studies are limited in that historical controls were used and neurological outcome data was not available at the time of this writing for most of the sites. In addition, the human studies have the inherent limitation that the beneficial effects of the ITD cannot be attributed to any single intervention but only the combination of interventions. This was intentional, and follows advances in other deadly disease states requiring multiple synergistic therapies. This new approach is important conceptually, and suggests that while different interventions may have benefit in and by themselves, the greatest clinical benefit will come from combining multiple therapies together in a physiological sound manner. No new circulatory technology (including the ITD) will be of maximum benefit unless it is used in a system that optimizes the engine (e.g. high quality CPR) that drives circulation in the first place. Finally, differences from site to site in both the number of patients evaluated and the impact of new CPR plus the ITD were particularly notable in patients with non-VF rhythms. In some sites there was a consistent increase in the number of patients who survived to hospital discharge with an initial non-VF rhythm but this effect was not observed in the site with the largest number of patients evaluated. Consequently there was no increase in survival to hospital discharge for non-VF patients when the data were analyzed in aggregate.
The authors believe that as we continue to better understand the physiology of CPR and how to maximize post-resuscitation care, the approaches described herein have the potential for even greater improvements in outcomes. While only a few patients in the current study were treated in the post-resuscitation phase with therapeutic hypothermia, we believe this advance will help to reduce the striking mortality rates that occur for patients after they have been admitted to the hospital. Thus, the results presented herein represent an important first step in overhauling how CPR and post-resuscitation care can and should be provided for all patients in cardiac arrest. The sequence of care, including use of “new CPR” and the ITD represent the best care available today to optimize circulation and the chance for survival. These data provide strong support for physiological and clinical advances of this new approach to the treatment of patients in cardiac arrest.
Conclusions
These studies identify a new mechanism for improving cerebral perfusion during CPR. Use of the ITD reduces intracranial pressure during the decompression phase of CPR at a faster rate, resulting in a greater duration of time when intracranial pressures are at their nadir, thereby enhancing cerebral circulation. We also provide the first evidence that consistently implementing a series of interventions (including use of the ITD), as recommended by the AHA Guidelines, can result in a nearly 50% increase in survival to hospital discharge rate.
Acknowledgments
Funding support: National Institute of Health: SBIR Grant Number: 2-R44-HL65851-02 to Advanced Circulatory Systems, Inc.
Footnotes
Dr. Lurie is an inventor of the impedance threshold device and founded Advanced Circulating Systems to bring new resuscitation technology into clinical use; he owns a patent and stock.
References
- 1.Kouwenhoven WB, Jude JR, Knickerbocker GG. Closed chest cardiac massage. JAMA. 1960;173:1064–1067. doi: 10.1001/jama.1960.03020280004002. [DOI] [PubMed] [Google Scholar]
- 2.Duggal C, Weil MH, Gazmuri RJ, Tang W, Sun S, O’Connell F, Ali M. Regional blood flow during closed-chest cardiac resuscitation in rats. J Appl Physiol. 1993 Jan;74(1):147–52. doi: 10.1152/jappl.1993.74.1.147. [DOI] [PubMed] [Google Scholar]
- 3.Andreka P, Frenneaux MP. Haemodynamics of cardiac arrest and resuscitation. Curr Opin Crit Care. 2006 Jun;12(3):198–203. doi: 10.1097/01.ccx.0000224861.70958.59. [DOI] [PubMed] [Google Scholar]
- 4.Niemann JT. Cardiopulmonary resuscitation. N Engl J Med. 1992;327:1075–1090. doi: 10.1056/NEJM199210083271507. [DOI] [PubMed] [Google Scholar]
- 5.Eisenberg MS, Horwood BT, Cummins RO, et al. Cardiac arrest and resuscitation: a tale of 29 cities. Ann Emerg Med. 1990;19:179–186. doi: 10.1016/s0196-0644(05)81805-0. [DOI] [PubMed] [Google Scholar]
- 6.Becker L, Ostrander M, Barrett J, et al. Outcome of cardiopulmonary resuscitation in a large metropolitan area: where are the survivors? Ann Emerg Med. 1991;20:355–361. doi: 10.1016/s0196-0644(05)81654-3. [DOI] [PubMed] [Google Scholar]
- 7.Wik L, Kramer-Johansen J, Myklebust H, Sørebø H, Svensson L, Fellows B, Steen PA. Quality of cardiopulmonary resuscitation during out-of-hospital cardiac arrest. JAMA. 2005 Jan 19;293(3):299–304. doi: 10.1001/jama.293.3.299. [DOI] [PubMed] [Google Scholar]
- 8.Abella BS, Alvarado JP, Myklebust H, Edelson DP, Barry A, O’Hearn N, Vanden Hoek TL, Becker LB. Quality of cardiopulmonary resuscitation during in-hospital cardiac arrest. JAMA. 2005 Jan 19;293(3):305–10. doi: 10.1001/jama.293.3.305. [DOI] [PubMed] [Google Scholar]
- 9.Iwami T, Kawamura T, Hiraide A, Berg RA, Hayashi Y, Nishiuchi T, Kajino K, Yonemoto N, Yukioka H, Sugimoto H, Kakuchi H, Sase K, Yokoyama H, Nonogi H. Effectiveness of bystander-initiated cardiac-only resuscitation for patients with out-of-hospital cardiac arrest. Circulation. 2007 Dec 18;116(25):2900–7. doi: 10.1161/CIRCULATIONAHA.107.723411. [DOI] [PubMed] [Google Scholar]
- 10.Aufderheide TP, Sigurdsson G, Pirrallo RG, Yannopoulos D, McKnite S, von Briesen C, et al. Hyperventilation-induced hypotension during cardiopulmonary resuscitation. Circulation. 2004 Apr 27;109(16):1960–5. doi: 10.1161/01.CIR.0000126594.79136.61. [DOI] [PubMed] [Google Scholar]
- 11.Yannopoulos D**, McKnite SH, Tang W, Zook M, Roussos C, Aufderheide TP, Idris AH, Lurie KG. Reducing ventilation frequency during cardiopulmonary resuscitation in a porcine model of cardiac arrest. Respiratory Care. 2005 May;50(5):628–635. [PubMed] [Google Scholar]
- 12.Yannopoulos D**, Aufderheide TP, Gabrielli A, Beiser DG, McKnite SH, Pirrallo RG, Wigginton J, Becker L, Vanden Hoek T, Tang W, Nadkarni VM, Klein JP, Idris AH, Lurie KG. Clinical and hemodynamic comparison of 15: 2 and 30:2 compression-to-ventilation ratios for cardiopulmonary resuscitation. Crit Care Med. 2006 May;34(5):1444–1449. doi: 10.1097/01.CCM.0000216705.83305.99. [DOI] [PubMed] [Google Scholar]
- 13.Yannopoulos D, Sigurdsson G, McKnite S, Benditt D, Lurie KG. Reducing ventilation frequency combined with an inspiratory impedance device improves CPR efficiency in swine model of cardiac arrest. Resuscitation. 2004 Apr;61(1):75–82. doi: 10.1016/j.resuscitation.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Cobb LA, Fahrenbruch CE, Walsh TR, Copass MK, Olsufka M, Breskin M, et al. Influence of cardiopulmonary resuscitation prior to defibrillation in patients with out-of-hospital ventricular fibrillation. Jama. 1999;281:1182–8. doi: 10.1001/jama.281.13.1182. [DOI] [PubMed] [Google Scholar]
- 15.Wik L, Hansen TB, Fylling F, Steen T, Vaagenes P, Auestad BH, et al. Delaying defibrillation to give basic cardiopulmonary resuscitation to patients with out-of-hospital ventricular fibrillation: a randomized trial. Jama. 2003 Mar 19;289(11):1389–95. doi: 10.1001/jama.289.11.1389. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs IG, Finn JC, Oxer HF, Jelinek GA. CPR before defibrillation in out-of-hospital cardiac arrest: a randomized trial. Emerg Med Australas. 2005 Feb;17(1):39–45. doi: 10.1111/j.1742-6723.2005.00694.x. [DOI] [PubMed] [Google Scholar]
- 17.Aufderheide TP, Pirrallo RG, Yannopoulos D, Klein JP, von Briesen C, Sparks CW, et al. Incomplete chest wall decompression: a clinical evaluation of CPR performance by EMS personnel and assessment of alternative manual chest compression-decompression techniques. Resuscitation. 2005 Mar;64(3):353–62. doi: 10.1016/j.resuscitation.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Yannopoulos D**, McKnite S, Aufderheide TP, Sigurdsson G, Pirrallo RG, Benditt D, Lurie KG. Effects of incomplete chest wall decompression during cardiopulmonary resuscitation on coronary and cerebral perfusion pressures in a porcine model of cardiac arrest. Resuscitation. 2005 March;64(3):363–372. doi: 10.1016/j.resuscitation.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Aufderheide TP, Pirrallo RG, Yannopoulos D**, Klein JP, von Briesen C, Sparks CW, Deja KA, Kitscha DJ, Provo TA, Lurie KG. Incomplete chest wall decompression: a clinical evaluation of CPR performance by trained laypersons and assessment of alternative manual chest compression-decompression techniques. Resuscitation. 2006 December;71(3):341–351. doi: 10.1016/j.resuscitation.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 20.Aufderheide TP, Pirrallo RG, Provo TA, Lurie KG. Clinical evaluation of an inspiratory impedance threshold device during standard cardiopulmonary resuscitation in patients with out-of-hospital cardiac arrest. Crit Care Med. 2005 April;33(4):734–740. doi: 10.1097/01.ccm.0000155909.09061.12. [DOI] [PubMed] [Google Scholar]
- 21.Pirrallo RG, Aufderheide TP, Provo TA, Lurie KG. Effect of an inspiratory impedance threshold device on hemodynamics during conventional manual cardiopulmonary resuscitation. Resuscitation. 2005 July;66(1):13–20. doi: 10.1016/j.resuscitation.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 22.Thayne RC, Thomas DC, Neville JD, van Dellen A. Use of an impedance threshold device improves short-term outcomes following out-of-hospital cardiac arrest. Resuscitation. 2005 Oct;67(1):103–8. doi: 10.1016/j.resuscitation.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 23.2005 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Circulation. 2005;112(Suppl I):IV-1–IV-203. doi: 10.1161/CIRCULATIONAHA.105.166550. [DOI] [PubMed] [Google Scholar]
- 24.Aufderheide TP. The problem with and benefit of ventilations: should our approach be the same in cardiac and respiratory arrest? Current Opinion in Critical Care. 2006 June;12(3):207–212. doi: 10.1097/01.ccx.0000224863.55711.56. [DOI] [PubMed] [Google Scholar]
- 25.Aufderheide TP, Frascone RJ, Pirrallo RG. Resuscitation in 2005: New ways to optimize manual CPR. EMS Magazine. 2005 September;34(9):42–45. [PubMed] [Google Scholar]
- 26.Yannopoulos D**, Nadkarni V, Aufderheide TP, McKnite S, Benditt DG, Lurie KG. Intrathoracic pressure regulation improves vital organ perfusion pressures in normovolemic and hypovolemic pigs. Resuscitation. 2006 September;70(3):445–453. doi: 10.1016/j.resuscitation.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Lurie KG, Lindner KH. Recent advances in cardiopulmonary resuscitation. J Cardiovasc Electrophysiol. 1997 May;8:584–600. doi: 10.1111/j.1540-8167.1997.tb00827.x. [DOI] [PubMed] [Google Scholar]
- 28.Lurie KG, Mulligan KA, McKnite S, Detloff B, Lindstrom P, Lindner KH. Optimizing standard cardiopulmonary resuscitation with an inspiratory impedance threshold valve. Chest. 1998 April;113(4):1084–1090. doi: 10.1378/chest.113.4.1084. [DOI] [PubMed] [Google Scholar]
- 29.Lurie KG, Zielinski T, McKnite S, Aufderheide TP, Voelckel W. Use of an inspiratory impedance valve improves neurologically intact survival in a porcine model of ventricular fibrillation. Circulation. 2002 January 1/8;105(1):124–129. doi: 10.1161/hc0102.101391. [DOI] [PubMed] [Google Scholar]
- 30.Lurie KG, Coffeen P, Shultz J, McKnite S, Detloff B, Mulligan K. Improving active compression-decompression cardiopulmonary resuscitation with an inspiratory impedance valve. Circulation. 1995;91:1629–1632. doi: 10.1161/01.cir.91.6.1629. [DOI] [PubMed] [Google Scholar]
- 31.Guerci AD, Shi AY, Levin H, Tsitlik J, Weisfeldt ML, Chandra N. Transmission of intrathoracic pressure to the intracranial space during cardiopulmonary resuscitation in dogs. Circ Res. 1985 Jan;56(1):20–30. doi: 10.1161/01.res.56.1.20. [DOI] [PubMed] [Google Scholar]
- 32.Yannopoulos D, Kotsifas K, Aufderheide TP, Lurie KG. Cardiac arrest, mild therapeutic hypothermia, and unanticipated cerebral recovery. Neurologist. 2007 Nov;13(6):369–75. doi: 10.1097/NRL.0b013e3180de4dc3. [DOI] [PubMed] [Google Scholar]
- 33.Lurie KG, Holbomb J, Idris A. Level I Cardiac Arrest Centers: Learning from the Trauma Surgeons. Acad Emerg Med. 2005;12(1):80–81. doi: 10.1197/j.aem.2004.11.010. [DOI] [PubMed] [Google Scholar]


