Abstract

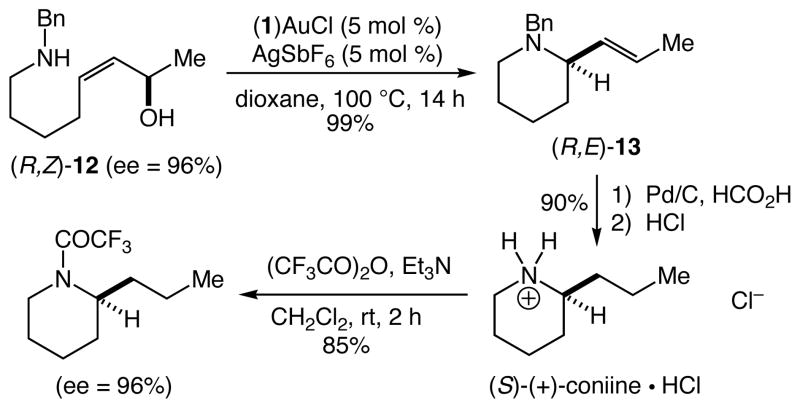
A 1:1 mixture of (1)AuCl [1 = P(t-Bu)2o-biphenyl] and AgSbF6 catalyzes the intramolecular amination of allylic alcohols with alkylamines to form substituted pyrrolidine and piperidine derivatives. Gold(I)-catalyzed cyclization of (R,Z)-8-(N-benzylamino)-3-octen-2-ol (96% ee, 95% de) led to isolation of (R,E)-1-benzyl-2-(1-propenyl)piperidine in 99% yield with 96% ee, consistent with the net syn addition of the amine relative to the departing hydroxyl group.
In 2002, Ozawa first demonstrated the intermolecular amination of underivatized allylic alcohols without the need for a Lewis acid co-catalyst through employment of cationic palladium(II) bis(phosphine) complexes.1 Since this time, a number of transition metal complexes including Pd(0),2,3 Pt(II),4 Mo(VI),5 Bi(III),6 Au(I),7 and Au(III)7 have been shown to catalyze the intermolecular amination of allylic alcohols in the absence of Lewis acid activators.8 Included in this family of transformations are a subset that employ simple alkylamines as nucleophiles.2,4 The intramolecular amination of underivatized allylic alcohols has also attracted attention as a route to functionalized nitrogen heterocycles and methods employing Au(III),9 Bi(III),10 and Pd(II)11,12,13 catalysts in combination with carbamate or sulfonamide nucleophiles have been documented. Of these, the Pd(II)-catalyzed methods are most highly developed and have been applied to the synthesis of a number of naturally-occurring nitrogen heterocycles.12,13 However, in contrast to the intermolecular amination of allylic alcohols, metal-catalyzed intramolecular amination of allylic alcohols with simple alkylamines has not been demonstrated.14
We have recently reported the intermolecular amination of underivatized allylic alcohols with imidazolidin-2-ones catalyzed by a mixture of (1)AuCl [1 = P(t-Bu)2o-biphenyl] and AgSbF6, a transformation that was distinguished by the high γ-regioselectivity of addition in the case of unhindered allylic alcohols.15 Concurrent with our efforts, Bandini and Aponick have developed effective gold(I)-catalyzed methods for the intramolecular arylation16 and alkoxylation17 of allylic alcohols, and these results pointed to the feasibility of gold(I)-catalyzed intramolecular allylic amination. Furthermore, Aponick’s demonstration of gold(I)-catalyzed intramolecular amination of propargylic alcohols with secondary alkylamines suggested that alkylamines might also be suitable nucleophiles for gold(I)-catalyzed intramolecular allylic amination.18,19 Indeed, here we report the regio- and stereoselective gold(I)-catalyzed intramolecular amination of underivatized allylic alcohols with alkylamines.
Toward the development of a gold(I)-catalyzed protocol for the intramolecular amination of underivatized allylic alcohols with alkylamines, we targeted 6-(N-benzylamino)-5,5-diphenyl-2-hexenol (2a) in combination with the catalyst system employed for intermolecular amination. This combination proved highly efficient and treatment of 2a with a catalytic 1:1 mixture of (1)AuCl and AgSbF6 (5 mol %) in dioxane at room temperature for 2 h led to isolation of 2-vinyl pyrrolidine 3a in 97% yield (Table 1, entry 1).20 In addition to N-benzylamine, an n-butylamine (2b), tert-butylamine (2c), or primary amine (2d) moiety also functioned as an effective nucleophile for gold(I)-catalyzed intramolecular allylic amination, although the primary amine required 48 h to reach completion (Table 1, entries 2–4). In comparison, gold(I)-catalyzed cyclization of benzyl carbamate 2e at room temperature for 72 h formed pyrrolidine 3e in only 48% yield (Table 1, entry 5). Substitution of AgClO4 for AgSbF6 led to a marked increase in the efficiency of the conversion of 2e to 3e (Table 1, entry 6), although this transformation was still considerably slower than was the gold(I)-catalyzed cyclization of substrates 2a–2c.
Table 1.
Effect of Nucleophile on the Gold(I)-Catalyzed Intramolecular Amination of Allylic Alcohols 2.
| |||||
|---|---|---|---|---|---|
| entry | R | substrate | time (h) | pyrrolidine | yield (%)a |
| 1 | Bn | 2a | 2 | 3a | 97 |
| 2 | n-Bu | 2b | 2 | 3b | 96 |
| 3 | t-Bu | 2c | 2 | 3c | 87 |
| 4 | H | 2d | 48 | 3d | 91 |
| 5 | Cbz | 2e | 72 | 3e | 48 |
| 6b | Cbz | 2e | 48 | 3e | 97 |
Isolated yield in >95% purity.
AgClO4 used in place of AgSbF6
We next investigated the effects of substitution, ring size, and alkene configuration on the efficiency and diastereoselectivity of gold(I)-catalyzed intramolecular allylic amination (Table 2). Cyclohexyl-substituted allylic alcohols 4a and 4b that possessed a benzylamine or α-methylbenzyl amine nucleophile underwent intramolecular allylic amination to form pyrrolidines 5a and 5b, respectively, in high yield and in the case of 4b with modest (2:1) diastereoselectivity (Table 2, entries 1 and 2). gem-Dialkyl substitution along the alkyl chain that tethered the amino group to the allylic alcohol moiety facilitated intramolecular allylic amination, but was not required (Table 2, entries 3–5). For example, 6-amino-2-hexenol derivative 6a that possessed a C5 phenyl group underwent cyclization at 60 °C to form a separable 1.3:1 mixture of cis-7a and trans-7a in 95% combined yield (Table 2, entry 3). Similarly, unsubstituted 6-(N-benzylamino)-2-hexenol (8; Z/E = 7.7:1) underwent gold(I)-catalyzed cyclization at 60 °C to form 1-benzyl-2-vinylpyrrolidine (9) in 86% yield (Table 2, entry 5). Periodic analysis of the conversion of 8 to 9 by GC revealed no significant difference in the rates of cyclization of (E)-8 and (Z)-8.
Table 2.
Substrate Scope of the Intramolecular Amination of Allylic Alcohols Catalyzed by a 1:1 Mixture of (1)AuCl and AgSbF6 (5 mol %) in Dioxane.
| entry | substrate | temp (°C) | time (h) | heterocycle | yield (%)a | drb |
|---|---|---|---|---|---|---|
| 1 |
 4a (R = Bn) |
23 | 2 |
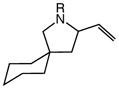 5a |
94 | – |
| 2 | 4b [R = CH(Me)Ph] | 23 | 2 | 5b | 99 | 2:1 |
| 3 |
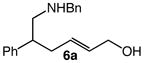 6a |
60 | 8 |
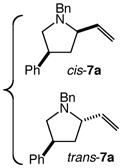
|
54 | – |
| 41 | – | |||||
| 4 |
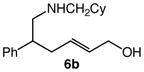 6b |
60 | 8 |
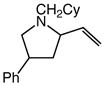 7b |
99 | 1.5:1 |
| 5 |
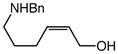 8 (Z/E = 7.7:1) |
60 | 12 |
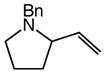 9 |
86 | – |
| 6 |
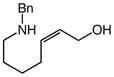 10 |
60 | 16 |
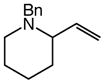 11 |
91 | – |
| 7 |
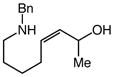 12 |
100 | 14 |
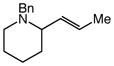 13 |
99 | ≥50:1 |
| 8 |
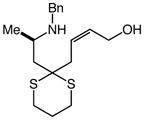 14 |
100 | 48 |
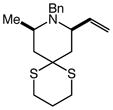 15 |
91 | 25:1 |
Isolated yield in >95% purity.
Diastereomeric ratio determined by 1H NMR analysis of the crude reaction mixture.
Gold(I)-catalyzed intramolecular allylic amination was also effective for the formation of piperidine derivatives (Table 2, entries 6–8). For example, gold(I)-catalyzed cyclization of (Z)-6-(N-benzylamino)-2-heptenol (10; Z/E ≥50:1) at 60 °C for 16 h led to isolation of 1-benzyl-2-vinyl piperidine (11) in 91% yield (Table 2, entry 6). Similarly, gold(I)-catalyzed cyclization of (Z)-8-(N-benzylamino)-3-octen-2-ol (12) at 100 °C led to isolation of 2-(1-propenyl)piperidine 13 in 99% yield with ≥50:1 diastereoselectivity (Table 2, entry 7). Gold(I)-catalyzed cyclization of dithiane derivative 14 that possessed a stereogenic center α-to the amino group at 100 °C for 48 h formed cis-2,6-disubsituted piperidine 15 in 91% yield with 25:1 diastereoselectivity (Table 2, entry 8). Piperidine 15 represents an advanced intermediate in the synthesis of a range of naturally-occurring piperidine alkaloids.21
To evaluate the selectivity of 1,3-chirality transfer in the gold(I)-catalyzed intramolecular amination of allylic alcohols with secondary alkylamines, we employed enantiomerically and diastereomerically enriched (R,Z)-12 (96% ee, 95% de). Treatment of (R,Z)-12 with a catalytic 1:1 mixture of (1)AuCl and AgSbF6 led to isolation of (R,E)-13 in 99% yield as a single diastereomer (≥50:1) (Scheme 1). Piperidine (R,E)-13 represents a direct precursor to the naturally occurring alkaloid (S)-(+)-coniine, and this connection was exploited to determine both the enantiopurity and absolute configuration of (R,E)-13 generated via gold(I)-catalyzed cyclization of (R,Z)-12. To this end, one-pot hydrogenative cleavage of the benzyl group and reduction of the exocyclic C=C bond (Pd/C, MeOH, HCOOH) followed by acidification with aqueous HCl gave (S)-(+)-coniine hydrochloride in 90% isolated yield as a white microcrystalline solid {[α]20D +6.9° (c 0.1, EtOH); lit. [α]21D +7.1° (c 1.0, EtOH)}.13,22 Chiral phase GC analysis of the corresponding (S)-(+)-coniine trifluoroacetamide (TFAA, Et3N, 85%) revealed an enantiomeric purity of 96% ee. Together, these results established complete transfer of chirality in the conversion of (R,Z)-12 to (R,E)-13 and net syn addition of the amine relative to the departing hydroxyl group.
Scheme 1.
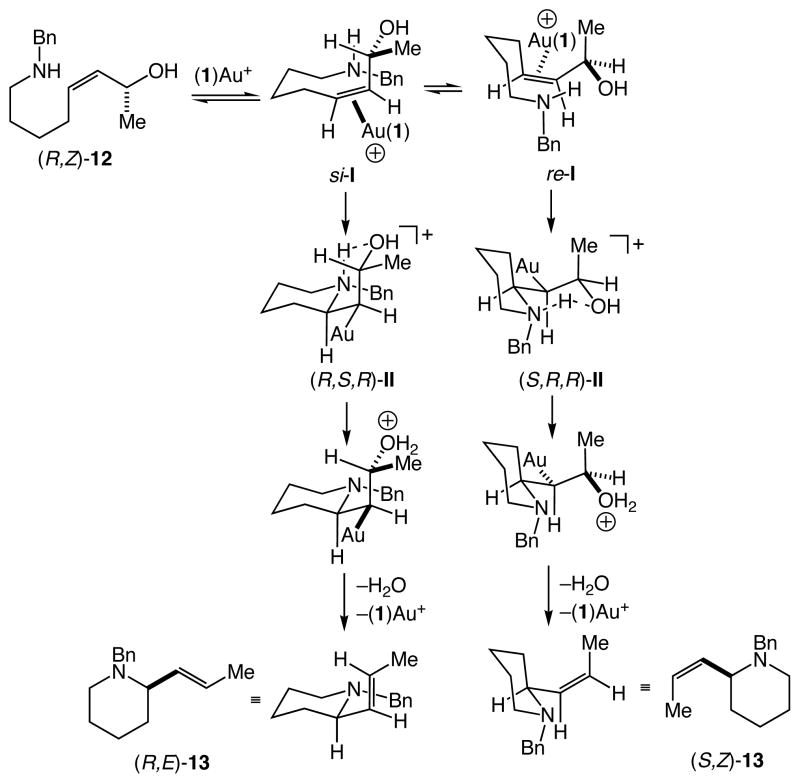
Although the syn substitution observed in the conversion of (R,Z)-12 to (R,E)-13 is consistent with a concerted SN2′ pathway,23 a mechanism involving σ-activation of the hydroxyl group appears unlikely given the low oxophilicity of gold(I). Rather, the pronounced π-acidity of cationic gold(I) complexes points to a mechanism involving nucleophilic addition/OH-elimination initiated by π-activation of the allylic C=C bond.24,25 Within this framework, the net syn-substitution in the conversion of (R,Z)-12 to (R,E)-13 is consistent with either a syn-addition/syn-elimination or an anti-addition/anti-elimination sequence. Uenishi and Kawai have proposed π-activation mechanisms involving hydroxyl group-directed syn-addition/syn-elimination to account for the syn-substitution observed for the Pd(II)-catalyzed alkoxylation26 and amination13 of allylic alcohols. However, owing to the strong preference of cationic gold(I) complexes to form two-coordinate, as opposed to three-coordinate complexes,27 a mechanism involving hydroxyl group-directed syn-addition/syn-elimination appears unlikely. Alternatively, Maseras has provided computational evidence for an anti-addition/anti-elimination sequence for the gold(I)-catalyzed isomerization of allylic ethers in the presence of alcohol.28 A key component of this pathway was the formation of a six-membered chelate structure involving hydrogen-bonding between the nucleophile and allylic alkoxy group that both facilitates nucleophilic addition and stabilizes the initially formed cationic intermediate.29
Guided by the hypotheses of Maseras, we propose a mechanism involving anti-addition/anti-elimination to account for the gold(I)-catalyzed conversion of (R,Z)-12 to (R,E)-13. Within this construct, two diasteromeric reaction manifolds are feasible resulting from cyclization of the diasteromeric gold(I) π-alkene complexes si-I and re-I (Scheme 2). In the former manifold, outer-sphere addition of the pendant amine to the complexed C=C bond of si-I would form the cyclic, hydrogen-bonded gold alkyl intermediate (R,S,R)-II. Proton transfer to the hydroxyl group followed by anti-elimination of water and displacement of gold would form (R,E)-13. In the latter reaction manifold, outer-sphere addition of the pendant amine to the complexed C=C bond of re-I to form (S,R,R)-II followed by proton transfer, anti-elimination, and alkene displacement would form (S,Z)-13 (Scheme 2). A rationale for selective formation of (R,E)-13 in preference to (S,Z)-13 can be found in the consideration of the potential chair-like conformations of the initially formed hydrogen-bonded bicyclic structures (R,S,R)-II and (S,R,R)-II. Whereas intermediate (R,S,R)-II can adopt a fully equatorial-substituted cis-decalin type conformation, intermediate (S,R,R)-II possesses a pseudo-axial methyl substituent. This latter species should experience unfavorable 1,3-diaxial interaction with the piperidine methylene group, thereby disfavoring cyclization of gold π-alkene complex re-I leading to selective formation of (R,E)-13.
Scheme 2.
In summary, we have developed a gold(I)-catalyzed protocol for the intramolecular amination of allylic alcohols with secondary alkylamines. The procedure tolerated a number of alkylamines and substitution patterns, was effective for the synthesis of both pyrrolidine and piperidine derivatives, and occurred with selective 1,3-chirality transfer in the conversion of (R,Z)-12 to (R,E)-13. This latter observation established the net syn addition of the amine relative to the departing hydroxyl group.
Supplementary Material
Acknowledgments
We thank the NIH (GM-080422) for support of this research and Prof. J. Hong (Duke University) for enlightening discussions and for disclosing to us his efforts in the area of stereoselective piperidine formation prior to publication.
Footnotes
Supporting Information Available: Experimental procedures, analytical and spectroscopic data, and copies of HPLC traces and NMR spectra for new compounds (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Ozawa F, Okamoto H, Kawagishi S, Yamamoto S, Minami T, Yoshifuji M. J Am Chem Soc. 2002;124:10968. doi: 10.1021/ja0274406. [DOI] [PubMed] [Google Scholar]
- 2.(a) Muzart J. Eur J Org Chem. 2007:3077. [Google Scholar]; (b) Kinoshita H, Shinokubo H, Oshima K. Org Lett. 2004;6:4085. doi: 10.1021/ol048207a. [DOI] [PubMed] [Google Scholar]; (c) Piechaczyk O, Doux M, Ricard L, le Floch P. Organometallics. 2005;24:1204. [Google Scholar]; (d) Thoumazet C, Grützmacher H, Deschamps B, Ricard L, le Floch P. Eur J Inorg Chem. 2006:3911. [Google Scholar]; (e) Piechaczyk O, Thoumazet C, Jean Y, le Floch P. J Am Chem Soc. 2006;128:14306. doi: 10.1021/ja0621997. [DOI] [PubMed] [Google Scholar]
- 3.(a) Ozawa F, Ishiyama T, Yamamoto S, Kawagishi S, Murakami H, Yoshifuji M. Organometallics. 2004;23:1698. [Google Scholar]; (b) Kayaki Y, Koda T, Ikariya T. J Org Chem. 2004;69:2595. doi: 10.1021/jo030370g. [DOI] [PubMed] [Google Scholar]; (c) Mora G, Deschamps B, van Zutphen S, Le Goff XF, Ricard L, le Floch P. Organometallics. 2007;26:1846. [Google Scholar]; (d) Usui I, Schmidt S, Keller M, Breit B. Org Lett. 2008;10:1207. doi: 10.1021/ol800073v. [DOI] [PubMed] [Google Scholar]
- 4.(a) Ohshima T, Miyamoto Y, Ipposhi J, Nakahara Y, Utsunomiya M, Mashima K. J Am Chem Soc. 2009;131:14317. doi: 10.1021/ja9046075. [DOI] [PubMed] [Google Scholar]; (b) Utsunomiya M, Miyamoto Y, Ipposhi J, Ohshima T, Mashima K. Org Lett. 2007;9:3371. doi: 10.1021/ol071365s. [DOI] [PubMed] [Google Scholar]; (c) Mora G, Piechaczyk O, Houdard R, Mezailles N, Le Goff XF, le Floch P. Chem Eur J. 2008;14:10047. doi: 10.1002/chem.200801197. [DOI] [PubMed] [Google Scholar]
- 5.Yang H, Fang L, Zhang M, Zhu C. Eur J Org Chem. 2009:666. [Google Scholar]
- 6.Qin HB, Yamagiwa N, Matsunaga S, Shibasaki M. Angew Chem Int Ed. 2007;46:409. doi: 10.1002/anie.200602909. [DOI] [PubMed] [Google Scholar]
- 7.Guo S, Song F, Liu Y. Synlett. 2007:964. [Google Scholar]
- 8.See also: Defieber C, Ariger MA, Moriel P, Carreira EM. Angew Chem Int Ed. 2007;46:3139. doi: 10.1002/anie.200700159.Roggen M, Carreira EM. J Am Chem Soc. 2010;132:11917. doi: 10.1021/ja105271z.
- 9.Kothandaraman P, Foo SJ, Chan PWH. J Org Chem. 2009;74:5947. doi: 10.1021/jo900917q. [DOI] [PubMed] [Google Scholar]
- 10.Kawai N, Abe R, Uenishi J. Tetrahedron Lett. 2009;50:6580. [Google Scholar]
- 11.(a) Banfi L, Basso A, Cerulli V, Guanti G, Riva R. J Org Chem. 2008;73:1608. doi: 10.1021/jo702087x. [DOI] [PubMed] [Google Scholar]; (b) Eustache J, de Weghe PV, Le Nouen D, Uyehara H, Kabuto C, Yamamoto Y. J Org Chem. 2005;70:4043. doi: 10.1021/jo0501543. [DOI] [PubMed] [Google Scholar]
- 12.(a) Yokoyama H, Ejiri H, Miyazawa M, Yamaguchi S, Hirai Y. Tetrahedron: Asymm. 2007;18:852. [Google Scholar]; (b) Yokoyama H, Hirai Y. Heterocycles. 2008;75:2133. [Google Scholar]; (c) Yokoyama H, Otaya K, Kobayashi H, Miyazawa M, Yamaguchi S, Hirai Y. Org Lett. 2000;2:2427. doi: 10.1021/ol0060432. [DOI] [PubMed] [Google Scholar]; (d) Hirai Y, Watanabe J, Nozaki T, Yokoyama H, Yamaguchi S. J Org Chem. 1997;62:776. [Google Scholar]; (e) Hirai Y, Nagatsu M. Chem Lett. 1994:21. [Google Scholar]; (f) Hirai Y, Shibuya K, Fukuda Y, Yokoyama H, Yamaguchi S. Chem Lett. 1997:221. [Google Scholar]; (g) Yokoyama H, Otaya K, Yamaguchi S, Hirai Y. Tetrahedron Lett. 1998;39:5971. [Google Scholar]; (h) Makabe H, Kong LK, Hirota M. Org Lett. 2003;5:27. doi: 10.1021/ol0201916. [DOI] [PubMed] [Google Scholar]; (j) Harrington PJ, Hegedus LS, McDaniel KF. J Am Chem Soc. 1987;109:4335. [Google Scholar]; (i) Jin-Mo K, Byeong-Seon J, Sang-Sup J, Hyeung-Geun P. J Org Chem. 2007;72:8115. [Google Scholar]
- 13.Hande SM, Kawai N, Uenishi J. J Org Chem. 2009;74:244. doi: 10.1021/jo801926g. [DOI] [PubMed] [Google Scholar]
- 14.A single example of the acid-catalyzed intramolecular amination of a tertiary allylic alcohol with an alkylamine *has been documented. Yokoyama Y, Hikawa H, Mitsuhashi M, Uyama A, Murakami Y. Tetrahedron Lett. 1999;40:7803.
- 15.Mukherjee P, Widenhoefer RA. Org Lett. 2010;12:1184. doi: 10.1021/ol902923e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.(a) Bandini M, Eichholzer A. Angew Chem Int Ed. 2009;48:9533. doi: 10.1002/anie.200904388. [DOI] [PubMed] [Google Scholar]; (b) Bandini M, Eichholzer A, Gualandi A, Quinto T, Savoia D. ChemCatChem. 2010;2:661. [Google Scholar]
- 17.(a) Aponick A, Biannic B. Synthesis. 2008:3356. [Google Scholar]; (b) Aponick A, Li C-Y, Palmes JA. Org Lett. 2009;11:1121. doi: 10.1021/ol802491m. [DOI] [PubMed] [Google Scholar]; (c) Aponick A, Li C-Y, Biannic B. Org Lett. 2008;10:669. doi: 10.1021/ol703002p. [DOI] [PubMed] [Google Scholar]; (d) Aponick A, Biannic B, Jong MR. Chem Commun. 2010;46:6849. doi: 10.1039/c0cc01961e. [DOI] [PubMed] [Google Scholar]; (e) Bandini M, Monari M, Romaniello A, Tragni A. Chem Eur J. 2010;16:14272. doi: 10.1002/chem.201002606. [DOI] [PubMed] [Google Scholar]
- 18.Aponick A, Li CY, Malinge J, Marques EF. Org Lett. 2009;11:4624. doi: 10.1021/ol901901m. [DOI] [PubMed] [Google Scholar]
- 19.For other examples of gold-catalyzed amination of benzylic or propargylic alcohols see: Georgy M, Boucard V, Debleds O, Dal Zotto C, Campagne J-M. Tetrahedron. 2009;65:1758.Terrasson V, Marque S, Georgy M, Campagne JM, Prim D. Adv Synth Catal. 2006;348:2063.
- 20.Control experiments established that both (1)AuCl and AgSbF6 are required for efficient conversion of 2a to 3a and provided strong evidence against a Brønsted acid-catalyzed pathway: Treatment of 2a with either (1)AuCl (10 mol %) or HOTf (10 mol %) in dioxane at 23 °C for 24 h led to no detectable consumption of 2a and no detectable formation of 3a; treatment of 2a with AgSbF6 (10 mol %) in dioxane at 23 °C for 24 h led to <10% conversion to 3a.
- 21.Ying Y, Kim H, Hong J. Org Lett. doi: 10.1020/ol103064f. ASAP. [DOI] [PubMed] [Google Scholar]
- 22.For recent examples of the enantioselective synthesis of (S)-(+)-coniine see: Lebrun S, Couture A, Deniau E, Grandclaudon P. Org Lett. 2007;9:2473. doi: 10.1021/ol070757w.Girard N, Pouchain L, Hurvois JP, Moinet C. Synlett. 2006:1679.Nagata K, Nishimura K, Yokoya M, Itoh T. Heterocycles. 2006;70:335.Itoh T, Nishimura K, Nagata K, Yokoya M. Synlett. 2006:2207.Xu X, Lu J, Li R, Ge Z, Dong Y, Hu Y. Synthesis. 2004:122.Gommermann N, Knochel P. J Chem Soc Chem Commun. 2004:2324. doi: 10.1039/b409951f.Passarella D, Barilli A, Belinghieri F, Fassi P, Riva S, Sacchetti A, Silvani A, Danieli B. Tetrahedron: Asymmetry. 2005;16:2225.
- 23.(a) Magid RM. Tetrahedron. 1980;36:1901. [Google Scholar]; (b) Paquette LA, Stirling CJM. Tetrahedron. 1992;48:7383. [Google Scholar]
- 24.For a recent review on the mechanisms of gold(I)-catalyzed transformations see: Hashmi ASK. Angew Chem Int Ed. 2010;49:5232. doi: 10.1002/anie.200907078.
- 25.For recent examples of cationic, two-coordinate gold π-alkene complexes see: Brown TJ, Dickens MG, Widenhoefer RA. J Am Chem Soc. 2009;131:6350. doi: 10.1021/ja9015827.Brown TJ, Dickens MG, Widenhoefer RA. Chem Commun. 2009:6451. doi: 10.1039/b914632f.Hooper TN, Green M, Mcgrady JE, Patel JR, Russell CA. Chem Commun. 2009:3877. doi: 10.1039/b908109g.Zuccaccia D, Belpassi L, Tarantelli F, Macchioni A. J Am Chem Soc. 2009;131:3170. doi: 10.1021/ja809998y.de Frémont P, Marion N, Nolan SP. J Organomet Chem. 2009;694:551.
- 26.(a) Kawai N, Lagrange JM, Ohmi M, Uenishi J. J Org Chem. 2006;71:4530. doi: 10.1021/jo060415o. [DOI] [PubMed] [Google Scholar]; (b) Kawai N, Lagrange JM, Uenishi J. Eur J Org Chem. 2007:2808. doi: 10.1021/jo060415o. [DOI] [PubMed] [Google Scholar]; (c) Uenishi J, Vikhe YS, Kawai N. Chem Asian J. 2008;3:473. doi: 10.1002/asia.200700390. [DOI] [PubMed] [Google Scholar]
- 27.(a) Gimeno MC, Laguna A. Chem Rev. 1997;97:511. doi: 10.1021/cr960361q. [DOI] [PubMed] [Google Scholar]; (b) Carvajal MA, Novoa JJ, Alvarez S. J Am Chem Soc. 2004;126:1465. doi: 10.1021/ja038416a. [DOI] [PubMed] [Google Scholar]; (c) Schwerdtfeger P, Hermann HL, Schmidbaur H. Inorg Chem. 2003;42:1334. doi: 10.1021/ic026098v. [DOI] [PubMed] [Google Scholar]
- 28.Paton RS, Maseras F. Org Lett. 2009;11:2237. doi: 10.1021/ol9004646. [DOI] [PubMed] [Google Scholar]
- 29.Hashmi has recently highlighted the importance of hydrogen-bonding interactions in the proton transfer steps in gold(I)-catalyzed hydration of alkynes. Krauter CM, Hashmi ASK, Pernpointner M. ChemCatChem. 2010;2:1226.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.