Abstract
Analysis of neural oscillations in the electroencephalogram (EEG) during cognitive tasks provides valuable information about underlying neuronal processing not accessible by other methods such as event-related potentials (ERPs) and the BOLD signal in fMRI. We investigated neural substrates of motor preparation and expectancy by analyzing neural oscillations of healthy subjects performing the AX continuous performance task (AX-CPT), a task widely used to evaluate processes such as cognitive control, motor preparation and anticipatory and sustained attention. The task consists of letters presented sequentially on a monitor, and subjects are required to respond only when they see the letter A (cue) followed by the letter X (target). In this study, to emphasize expectation and motor preparation, three versions of AX-CPT were used in which the overall propensity to respond was differentially modulated, by changing the probability of the letter sequences. Neural activity was investigated in three time windows following presentation of the cue: sensory, evaluation and preparation. Alpha power was reduced following cue onset similarly in all versions of the task in both the sensory and evaluation periods, but in the later preparation period there were task dependent modulations. Alpha was decreased when an infrequent cue increased the chance of a response, and increased when a propensity to respond had to be overcome, possibly reflecting an anticipatory attentional mechanism to gate visuo-motor processing. Beta power was modulated by task and cue in both evaluation and preparation periods. In the latter, beta power reflected the propensity to respond and correlated both with amplitude of the contingent negative variation (CNV), an ERP that reflects response preparation, and with reaction time. Some clinical populations such as patients with schizophrenia or attention-deficit disorder show specific deficits when performing the AX-CPT. These results provide a basis for investigating the differential neural underpinnings of oscillatory cognitive control deficits observed in various patient populations.
Keywords: Oscillations, Alpha, Beta, AX-CPT, Cognitive, Motor Preparation
1. Introduction
During cortical processing, brain electrical activity is manifest as an evolving spatiotemporal profile of activity that forms the surface electroencephalogram (EEG). The study of neural oscillations in the EEG during cognitive tasks provides new information about brain processing, adding to that obtained by other approaches such as event-related potentials (ERPs) and the BOLD signal in fMRI.
Oscillatory approaches have been extensively utilized over recent years, initially to evaluate processes such as visual integration (Gray et al., 1989), and more recently have expanded into studies of cognitive processing, including working memory (Sarnthein et al., 1998), attention (Lakatos et al., 2008), and sensory-motor coordination (Schoffelen et al., 2005). An increasing body of evidence indicates the importance of neural oscillations in various frequency bands for anticipatory biasing of information processing. For example, selective attention has been most commonly associated with modulation of oscillations in the alpha and gamma frequency ranges (Fries et al., 2001; Kelly et al., 2006), whereas preparation for action in motor brain areas are reflected in changes in oscillations in the beta frequency range (Farmer, 1998). The AX-type continuous performance task (AX-CPT), is a widely used executive processing and cognitive control task first developed in 1956 (Rosvold et al., 1956), that can be manipulated to emphasize specific aspects of response preparation and stimulus expectation (Barch et al., 2001; Cohen et al., 1999; Dias et al., 2011; Dias et al., 2003). The task has also been widely employed for the study of neurocognitive disorders such as schizophrenia (Barch et al., 2001; Cohen et al., 1999; Dias et al., 2011; Holmes et al., 2005; Javitt et al., 2000) and ADHD (Banaschewski et al., 2008). The present study evaluates patterns of oscillatory modulation observed during execution of the AX-CPT in order to assess relationship of oscillatory modulations to specific cognitive operation in normal volunteers, as well as to provide a template for future investigations extending present findings to the study of clinical populations
In the AX-CPT, letters are sequentially presented on a screen and subjects have to press a button whenever a letter “A” (cue stimulus) is followed by the letter “X” (target probe) and withhold response to any other cue-probe pair (AY, BX, BY, where B and Y represent any letters other than A and X). Thus, the instructive cue sets the context (local expectancy) in each trial, i.e. whether the subject should prepare for a response. Besides the local expectancy, a global expectancy to respond is set by the probability of correct cue-target pairs in a testing block. In order to study effects of expectancy and preparation, three versions of the task were recorded differing in overall propensity to respond (global expectancy) by changing contingency of cue-probe trials between blocks (Dias et al., 2003). For example, the most commonly used version of the AX-CPT consists of 70% AX cue-probe pairs and 10% of each of the other pairs, generating a high overall contingency to respond (Servan-Schreiber et al., 1996). If a B cue appears in this version of the task, the overall propensity to respond has to be overcome in order to avoid a false alarm if it is followed by the target X. On the other hand, a task variation with 70% AY pairs results in the global expectancy to not prepare a response.
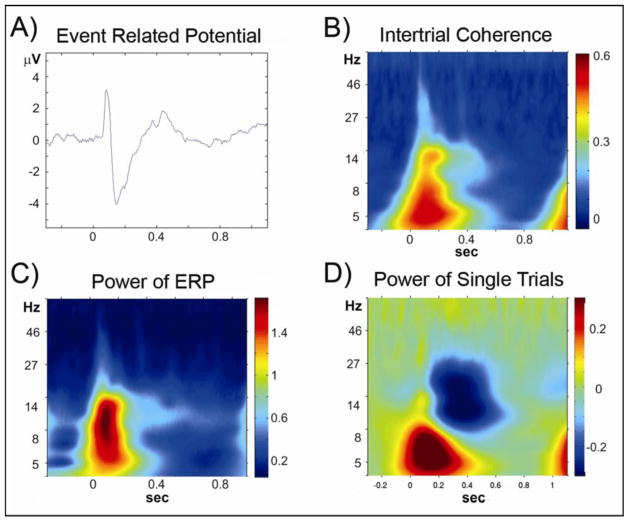
A cascade of processes can be studied in the time window following the instructive cue, including sensory processing, cue evaluation, cue maintenance, motor planning and anticipation. A previous analysis of this dataset in the temporal domain showed differential ERP effects following both the cue and probe presentations (Dias et al., 2003). Analysis of the cue-probe interval in the time-frequency domain can provide valuable additional information elusive to the other approaches (Figure 1).
Figure 1. Sensory window.
Analyses of data from the period following Cue A in the AX70 task variation, from the average of electrodes PO3 and PO4, which showed the largest sensory event-related potential amplitudes. The graphs show activity time locked to cue stimulus onset (time = 0). A) Group averaged event related potential to cue onset; B) Time-frequency plot of the intertrial coherence; C) Time-frequency plot of the power of ERP; D) Time-frequency plot of the power of single trials. Most of the activity in the theta and alpha frequency ranges, in the first 200 ms after stimulus onset, represents the evoked potential (A–C); in addition, there was modulation in alpha and beta frequency range not phase locked to stimulus presentation (D).
Investigating the cue-probe interval in this task is of particular clinical interest since previous studies indicate that deficient AX-CPT performance in schizophrenia is mainly determined by impaired processing of local context (Cohen et al., 1999; Javitt et al., 2000). The present study describes the modulation of oscillatory neural activity in three time epochs, sensory, evaluation, and preparation, following instructive cues in variations of the AX-CPT. These epochs were chosen based on results from the previous analysis of this dataset in the time domain (Dias et al., 2003). This study provides a framework for the investigation of neural processes underlying impaired AX-CPT performance in clinical populations.
2. Methods
2.1. Subjects
Eleven healthy, paid volunteers (seven males, mean age = 25.7 years (18–37 years),) participated in the study after signing informed consent. All but one were right handed as assessed with the Edinburgh Handedness Inventory (Oldfield, 1971). The Nathan Kline Institute Internal Review Board approved all procedures.
2.2. Paradigm
The tasks have been previously described in more detail (Dias et al., 2003). White letters (~2 degrees visual angle) on black background were sequentially presented for 250 ms on a screen 137 cm in front of the subjects’ eyes. Subjects were instructed to press a button with the right index finger whenever the target letter X followed the cue letter A. They were instructed to withhold response to any other combination (B followed by X or Y, A followed by Y). The collectively called letter B could be any letter other than X or A. The cue-probe interval (stimulus onset asynchrony, SOA, onset to onset of stimuli) was 1.1 seconds and the SOA between target and next cue onset was 1.25 seconds. Three variants of the task were presented differing in the probability of cue-target pair presentations. As in the most widely used variation of the task, the base version, subsequently called “AX70”, had 70% of the cue target pairs consisting of the correct cue-probe sequence (A followed by X). The other three possible sequences (A-Y, B-X, B-Y) were presented with 10% each. Thus, task variation AX70 induced a strong global, as well as local, prepotency to respond. On the contrary, in task variations “BX70” and “AY70”, 70% of the cue-probe sequences consisted of B-X or A-Y sequences respectively, with other trial types appearing in 10% of the trials. Thus, in both task variations, BX70 and AY70, the global prepotency was to not prepare a response. However, the local prepotency to respond upon presentation of A cues was high in task variation BX70, but low in task variation AY70.
2.3. Data Acquisition and Signal Processing
Electroencephalographic data was acquired with Neuroscan Synamps from 64 scalp locations, referenced to an electrode on the nose, with a pass-band of 0.05 to 100 Hz, and digitized at 512 Hz. Data was analyzed with Matlab (Mathworks, Natick, MA), using the toolbox Fieldtrip (http://www.ru.nl/fcdonders/fieldtrip/) and custom written scripts. Noisy channels were identified by visual inspection and statistical criteria (kurtosis of amplitude values across trials), followed by interpolation using data from neighbouring channels within a 4 cm radius. The continuous data was epoched from −1.5 to 2.5 seconds relative to the onset of the cues. Relatively long time epochs were chosen to avoid edge effects of the time-frequency decomposition. For subsequent steps we only used data from correct trials. Thus, A cue trials were used when they either were followed by a target (X) that subject correctly identified by button press or when they were followed by the invalid probe (Y) and the subject withheld the response. B cues were used when subjects correctly withheld response following subsequent invalid probes (X or Y). Trials exceeding +/− 120 μV were discarded and trials above a statistical criterion were inspected. Instantaneous phase and power was computed on each trial using complex Morlet wavelets on 82 scales in a logarithmic increase between 0.95 to 85 Hz.
While both intertrial coherence (ITC) and power across single trials were computed for all epochs, the effects of interest were not stimulus locked and we therefore focused our analysis on power across single trials (Figure 1).
To account for variability of absolute power values across subjects and since most previous reports in the EEG literature relevant to this study use baseline corrected results, we described the spectral response as relative change compared to pre-stimulus epoch (−0.3 to −0.1 s). However, to test for possible baseline effects due to the different number of trials for the different cues, a result of the different percentages of sequences in the task, we computed repeated measures ANOVA of wavelet spectrograms for the baseline period with factors “task” and “stimulus” for each frequency band, and report such differences when they occurred. To obtain the contingent negative variation (CNV) potential in the time domain, the data was low pass filtered at 35 Hz (6th order Butterworth filter) and baseline corrected (−0.1 – 0 s) prior to averaging.
2.4. Statistical analysis
We chose the frequency boundaries, theta (4–8 Hz), alpha (8–14 Hz), beta (18–26 Hz), and gamma (30–80 Hz) based on the EEG literature in humans and also by identifying the presence of activity in these frequency bands in the data. Specifically, the frequency range of alpha was chosen as 8–14Hz given extensive prior literature using this frequency band to investigate preparatory alpha modulation in attention tasks (for examples see (Kelly et al., 2009; Rihs et al., 2007; Thut et al., 2006; Worden et al., 2000)). Similarly the frequency range of beta was chosen based upon extensive literature showing oscillatory modulations related to motor preparation centered around 20 Hz (Farmer, 1998; Pfurtscheller, 1981).
We focused our analysis on three time windows, subsequently referred to as sensory, evaluation and preparation windows. Based on the ERP literature, the sensory time window was defined as 0 to 0.2 s from cue onset, to include the sensory ERPs P1 and N1. The evaluation time window was defined as 0.2 – 0.5 s from cue onset, and was chosen to span the stimulus induced alpha event-related desynchronization (ERD), and the preparation window was defined as −0.25 to −0.05 s prior to probe onset.
For the sensory epoch, initial analysis focused on parieto-occipital electrodes (PO3, PO4) which are at the center of the representation of the visual ERP components P1 and N1 (Di Russo et al., 2003; Di Russo et al., 2002). For the other epochs, the statistical comparisons for alpha was done on the average power of a parieto-occipital region of interest (ROI) (Pz, P3, P1, P2, P4, POz, PO4, PO3) and for beta we used a left-central ROI (C3, C1, Cz). These ROIs were chosen based on previous studies. For beta, the largest modulations of beta activity in tasks involving motor preparation for movement of the contralateral hand have been found in this left central ROI (Alegre et al., 2006; Pfurtscheller, 1981; Ritter et al., 2009). For alpha, preparatory modulation following cues in attention tasks have been observed mainly in the parieto-occipital ROI (Thut et al., 2006).
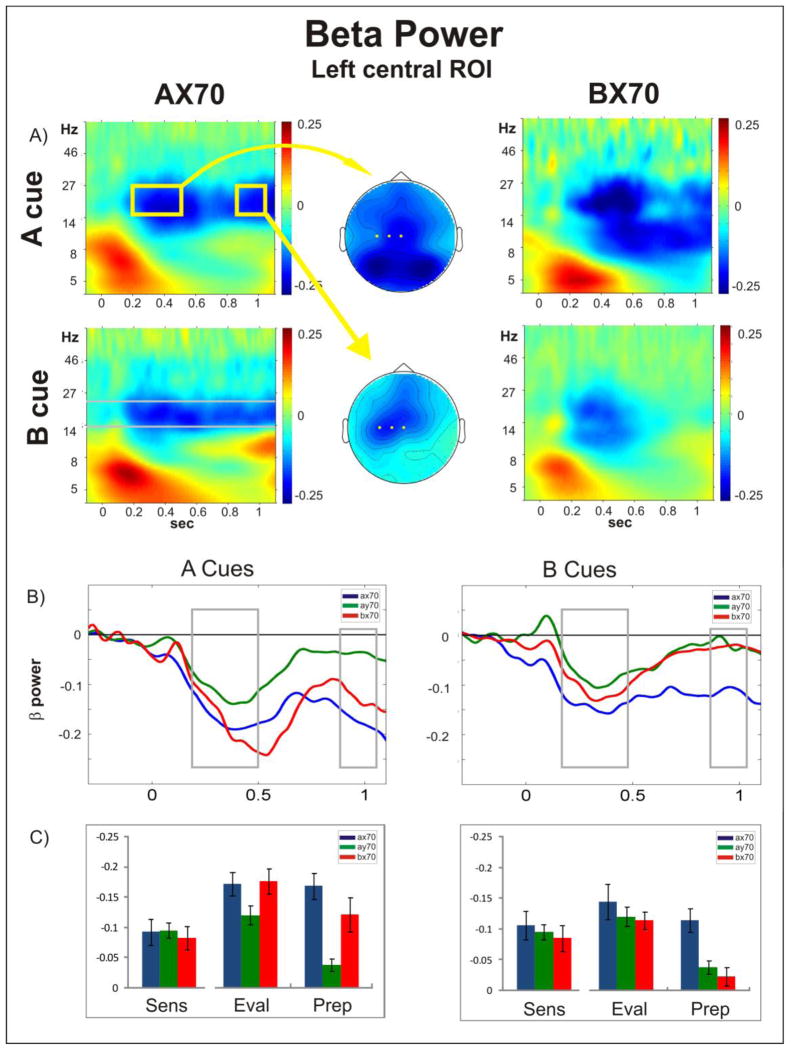
For gamma and theta, since we did not have an a priori hypothesis of possible effects, we computed the comparisons across all three regions (sensory, central, parieto-occipital). In addition, as can be seen in the headplot Figure 3A, there was activity in the beta range in parietal regions, during the evaluation window. This was unexpected, and a preliminary analysis of this activity, using the parieto-occipital ROI, was performed.
Figure 3. Beta Power.
A) Time frequency plots of oscillatory power following A (top) and B (bottom) cue onset (time = 0), for task AX70 (left) and BX70 (right) averaged across the channels over left somato-motor cortex that were chosen for statistical analysis (C3, C1, Cz). The middle column shows the top view of the spatial distribution of the beta power in the evaluation (top) and preparation (bottom) window following A cues in task AX70, as highlighted by the yellow boxes, with the electrodes used on the ROI statistical analysis highlighted in yellow; B) Comparison of the temporal evolution of beta power (frequencies within the grey bars in A) in all tasks for cues A (left) and B (right). Time zero refers to cue onset. Following an initial dip of beta power in the evaluation period in all tasks, beta remains suppressed in tasks with high response probability (A cues in AX70 and BX70) or high need for control (B cues in AX70); C) Histograms showing mean power (+/− standard error) in the beta range for the three periods analyzed (Sens: sensory; Eval: evaluation; Prep: preparatory), for the three task variants.
For the analysis of CNV amplitude we computed the mean amplitude of a fronto-central ROI (FC1-4, FCZ, CZ, C1, C2) across the whole preparation time window. The average activity in each frequency band individually (theta, alpha, beta, gamma) for their defined ROI, across time window (sensory, evaluation, preparation), was subjected to repeated measures ANOVA using Greenhouse-Geisser adjustments.
Two further analyses were done to investigate the spatial distribution of the main effects: source localization and a cluster-based permutation test. Source localization was performed using BESA (Brain Electric Source Analysis, MEGIS Software GmbH, www.besa.de) and the results are shown in Supplementary Figure 4. The cluster based test was used as implemented in the Fieldtrip toolbox and the results are shown in Supplementary Figure 3 (Maris and Oostenveld, 2007). This approach controls for multiple comparison testing when computing statistics across multiple channels, and/or frequency and time points. In the first step of the cluster test we computed the statistics of choice, e.g. dependent samples t-test, for each sample point. Significant values (alpha = 0.05) were clustered based on their adjacency in time, space, and frequency, and the t-values for all points in this cluster were summed. In the following Monte-Carlo permutation, responses were randomly reassigned to different tasks and the previous steps were repeated yielding a sum t-value for a cluster spanning the same data points as in the initial cluster. The permutation was repeated 5000 times to obtain the distribution of cluster sum t-values of the null hypothesis. Subsequently, the summed t-values of the initial cluster of interest were compared to the obtained null distribution to get significance levels of the cluster. A cluster level alpha of 0.05 was used. Since the most commonly used version of AX-CPT is the version here called AX70, we focused on effects in this task.
Reaction times from trials that survived artifact rejection were analyzed and repeated measures ANOVA with subsequent paired t-tests were used to assess significance. Linear relationships were assessed using Pearson’s correlation coefficient, with task as a co-variate. The commercial software programs SPSS and Matlab were used to compute statistical tests.
3. Results
3.1. Behaviour
Behavioural results from this dataset were previously discussed in more detail (Dias et al., 2003). Reaction times from trials that survived artifact rejection are reported because this measure was used for correlation analysis with the neurophysiological data (beta ERD, CNV amplitude). As in the previous study, there was a significant effect of task on reaction times (F(1.89,18.89) = 54.72, p < 0.01) (Figure 4). Paired t-tests showed that reaction times were slowest in task AY70 (mean= 517 ms; standard error (SEM) = 78 ms) compared to the other variations (AX70: mean = 336 ms, SEM = 76; BX70: mean = 361 ms, SEM = 58 ms): AY70 vs. BX70: df = 10, t = 8.029, p < 0.01 and AX70 vs. AY70: df = 10, t = −11, p < 0.01. P. There was no significant difference between tasks BX70 and AX70.
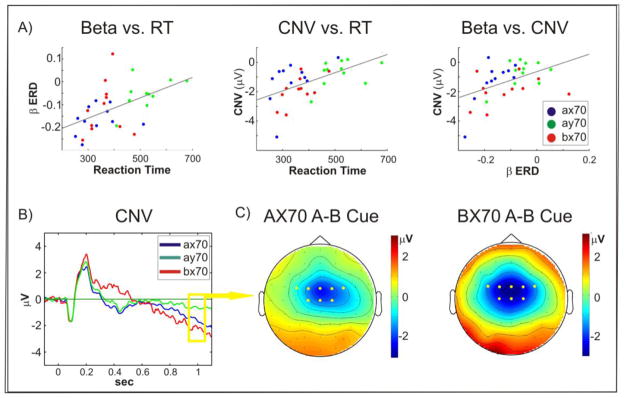
Figure 4. Correlations.
A) Beta ERD (left panel), as well as CNV amplitudes (middle panel), were significantly correlated with reaction time across all tasks. In addition, Beta ERD correlated with CNV amplitude (right panel); B) Grand average of evoked potentials recorded at FCZ to A cues in the three tasks. The CNV was more negative in AX70 and BX70 than in AY-70. C) Spatial distribution of the voltage difference between A and B cues for tasks AX70 and BX70.
3.2. EEG
The average number of correct trials that passed the criteria for artefact rejection varied for each task variation, because of the different percentages of trials in each variation. Thus, for the A cues, the mean number of trials per subject was 249in task variation AX70, 40 in BX70 and 253 in AY70. For B cues, the mean number was 77 for AX70, 293 for BX70 and 76 for AY70.
3.2.1. Sensory Window (0 – 0.2 s)
The main effects in the sensory window consisted of typical power increases in frequency bands similar to frequency components present in the ERP (Figure 1). To test for effects across stimuli and tasks we computed a repeated measures ANOVA for the mean increase in power for each frequency band individually (theta, alpha, beta, and gamma) with within-subject factors task and stimulus. We did not observe any significant stimulus or task effects and no stimulus versus task interactions for any of the frequency bands, and thus did not further pursue analysis of this epoch (Figures 2, 3 and Supplementary Figure 1).
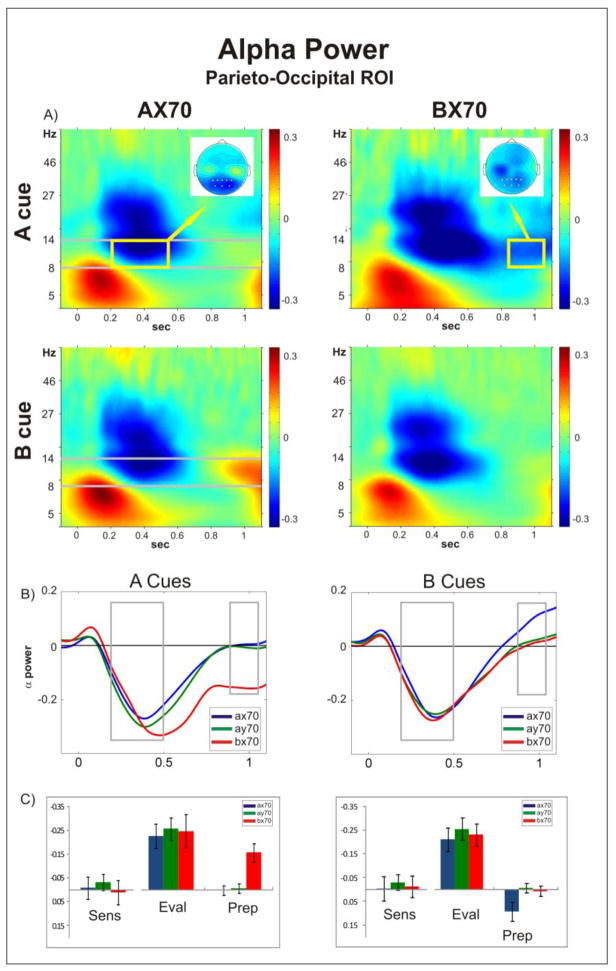
Figure 2. Alpha Power.
A) Time-frequency plots of oscillatory power following A (top) and B (bottom) cue onset (time = 0), for task AX70 (left) and BX70 (right) averaged across the channels over parieto-occipital area that were used for statistical analysis (Pz, P3, P1, P2, P4, POz, PO4, PO3, marked in yellow on the top view insets of the spatial distribution of the alpha activity); B) Comparison of the temporal evolution of alpha power (delimited in grey in A) in all tasks for cues A (left) and B (right). The initial alpha ERD returned to baseline in most tasks, but remained suppressed following the A cue in BX70 (left panel). In addition, following the B cue ERS was observed in task AX70 preceding onset of the target (right panel). C) Histograms showing mean power (+/− standard error) in the alpha range for the three periods analyzed (Sens: sensory; Eval: evaluation; Prep: preparatory), for the three task variants.
3.2.2. Evaluation Window (0.2 – 0.5 s)
Significant main effects in the alpha and beta frequency bands were observed in the induced power during the evaluation period (Figures 2, 3), and are detailed below. We did not observe significant modulatory effects of the gamma power due to expectancy in this period (Supplementary Figure 1). Theta power showed a significant stimulus versus task interaction (F(1.2,12.0)=10.85, p<0.01), but this was not pursued further since the effects likely reflect previously described modulations of evoked potentials (Dias et al., 2003).
3.2.2.1. Alpha
Following cue presentation, an initial reduction of alpha power was observed, starting at around 0.2 s (Figure 2B). This initial alpha ERD was invariably induced, independent of task and stimulus. Repeated measures ANOVA for this period showed no main effect of task (F(1.41,14.11)=0.63, p=0.41) or stimulus (F(1,10)=4, p=0.07), and no task versus stimulus interaction (F(1.8,18)=0.58, p=0.55). The parieto-occipital distribution of this effect (inset in Figure 2A, left panel) is in line with the well established stimulus induced alpha ERD (Neuper and Pfurtscheller, 2001; Pfurtscheller and Lopes da Silva, 1999). Thus, while reliably induced by both cue types and in all tasks, alpha ERD in the evaluation period was not modulated by either local or global context. In addition, there was no variation of baseline alpha activity dependent on the task variation (Supplementary Figure 2).
3.2.2.2. Beta
Similar to the alpha, power in the beta frequency band showed a marked decrease in the evaluation period. However, as opposed to the alpha ERD, beta suppression in the central-left hemispheric ROI in this time interval showed a significant stimulus effect (F(1,10)=20.33, p<0.01), a task effect (F(1.69,16.93)=9.69, p<0.01), and a stimulus versus task interaction (F(1.84,18.37)=5.4, p=0.016). These effects were driven by an increased beta suppression following A cues (contrast of stimulus type: F(1,10)=20.33, p<0.01), and this effect was most pronounced in task BX70, which was the only task with a significant difference in a post-hoc paired t-tests between A cue and B cue (t=−5.37, df=10, p<0.01, AX70 p=0.09, AY70 p=0.22).
However, since these results were computed on baseline corrected data it was important to control for possible task effects in the baseline (Supplementary Figure 2). For the absolute beta power in this ROI there was a task effect (F(1.79,17.93)=21.95, p<0.01), but no main effect of stimulus, though there was a task versus stimulus effect (F(1.94,19.35)=6.04, p=0.01),. Between-factor contrasts showed increased absolute beta power in the baseline in task AX70 compared to BX70 (p<0.01), AX70 compared to AY70 (p<0.01), and BX70 compared to AY70 (p=0.03). There was no statistically significant difference between the baselines of cue A and cue B in task AX70 (p=0.23) or AY70 (0.93) and a statistical trend for a higher baseline in A cues in BX70 (p=0.06). In line with the baseline corrected data, paired t-tests of absolute power in the evaluation epoch showed that power was significantly reduced following the A cue compared to B cue in the BX70 task (t =−2.92, df =10, p = 0.02). However, in this case, power following A cues was also significantly lower compared to B cues in the other two tasks (AX70: t = −3.51, df = 10, p < 0.01; AY70: t = −3.37, df = 10, p<0.01). In summary, in the non-baseline corrected data the beta ERD was more pronounced following A cues compared to B cues, and, relative to baseline, this ERD difference between cues was most prominent in the BX70 task.
The spatial distribution of the beta ERD in the evaluation window spanned not only the expected fronto-central electrodes, but extended into bilateral parieto-occipital areas (Figure 3A, middle column). A preliminary investigation of this activity, using ANOVA, showed a main effect of cue (F(1,10)=6.902, p=0.025), with activity to cue A showing larger beta ERD compared to cue B, but no main effect of task and no task x cue interaction. Since these were not part of the hypotheses for this study, they will be further investigated in upcoming studies.
3.2.3. Preparation Window (0.85–1.05 s)
No significant modulatory effect on gamma power was observed in this period (Supplementary Figure 1). Again theta power showed a significant stimulus versus task interaction (F(1.27,12.72)=4.712, p=0.04), but was not further pursued due to reasons mentioned above. Significant main effects in the alpha and beta frequency bands were observed during the preparation period (Figures 2, 3), and are detailed below.
3.2.3.1. Alpha
The repeated measures ANOVA for the preparation window showed a significant effect of task (F(1.67,16.66) = 9.37, p<0.01), stimulus (F(1,10) = 23.99, p < 0.01), and a significant task versus stimulus interaction (F(1.69,16.93) = 4.62, p = 0.03). This was due to two effects.
First, following the sensory induced parieto-occipital alpha ERD in the evaluation period, alpha activity returned to baseline levels following both A and B cues in AY-70, cue A in AX70 and cue B in AX-70 (Figure 2B). However, alpha ERD following A cues was significantly larger compared to B cues in task BX70 (df = 10, t =−3.98, p < 0.01), as well as compared to the A cues in the other tasks (A cue AY70 vs. BX70: df = 10, t =3.5, p < 0.01; AX70 vs. BX70: df = 10, t =3.94, p < 0.01). This increased ERD following A cues in task BX70 is probably analogous to the previously reported suppression of alpha power that was shown to be related to anticipatory visual attention (Foxe et al., 1998). In addition, in task variation AX70 there was an increase in alpha power (ERS) following B cues, compared to A cues (df = 10, t =−2.99, p = 0.01).
The spatial distribution of both effects, ERS in AX70 and ERD in BX70, were overlapping as revealed by cluster analysis (Supplementary Figure 3A). The comparison of B vs. A in AX70 yielded one extensive significant positive cluster that included right anterior frontal, left central and bilateral parietal regions and in BX70 resulted in one significant negative cluster that covered approximately the same areas.
3.2.3.2. Beta
Following the parieto-occipital and fronto-central distribution of the beta ERD in the evaluation window, beta ERD in the preparation window was observed mainly in left central electrodes (Figure 3A, middle column).
In the left central ROI (represented by the yellow dots in figure 3A), we observed significant effects of task (F(2,19.9) = 20.94, p < 0.01), stimulus (F(1,10) = 7.85, p = 0.019), and a statistical trend in the task versus stimulus interaction (F(1.47,14.72) = 3.8, p < 0.057) (Figure 3A,B). Post-hoc t tests showed significantly less power following A cues compared to B cues in task AX70 (df = 10, t = −3.96) and BX70 (df = 10, t = −2.59, p = 0.027), but no significant difference in task AY70. Thus, beta power was decreased in the context of high response probabilities. While both high response probability tasks AX70 and BX70 showed significant increased suppression following A cues compared to AY70 (AX70 vs. AY70: df = 10, t = −8.79, p < 0.01, AY70 vs. BX70: df = 10, t = 2.94, p =0.03), there was no statistical difference between BX70 and AX70 (Figure 3B, C). Significant negative clusters including the electrodes in the left central ROI were found in tasks AX70 and BX70 when comparing the decrease in beta power following A cues compared to B cues (Supplementary Figure 3B). Interestingly, beta power was also suppressed following the B cue in task AX70. There was a significant difference for activity following B cues between AX70 and both BX70 (df = 10, t = 10.92, p < 0.01) and AY70 (df = 10, t = 3.87, p < 0.01) (Figure 3C).
3.2.3.3. CNV vs. Beta
As previously reported for this dataset, an increasing negative potential developed in the preparation phase following instructive cues, reflecting the CNV potential (Dias et al., 2003; Walter et al., 1964). The CNV showed a symmetric fronto-central distribution across all tasks (Figure 4C). As previously mentioned the spatial distribution of the beta ERD was also central, however, it was lateralized to the contralateral hemisphere of the right hand with which all subjects were instructed to respond to correct targets. Both beta desynchronization and CNV have been implicated in motor preparation or expectancy of a response. Supporting this relation, the average values in the preparation window for both measures correlated with reaction time in AX trials, which were the only ones that required a motor response (beta vs. reaction time: r=0.51, p<0.001, CNV vs. reaction time: r=0.59, p<0.001) (Figure 4A). Furthermore, there was a significant correlation between CNV amplitude and beta desynchronization (r=0.53, p<0.001). All of these correlations remained significant when controlled for task (beta vs. reaction time: r=0.51, p=0.002, CNV vs. reaction time: r=0.60, p<0.001, CNV vs beta (r=0.56, p<0.01). Repeated measures ANOVA for CNV amplitude showed a significant effect of task (F(1.36,13.64) = 7.14, p < 0.01) and cue stimulus (F(1,10) = 40.68, p < 0.01), and a significant task versus cue interaction (F(1.55,15.52) =21.27, p < 0.01). Although no significant differences were observed between tasks AX70 and BX70, CNV amplitudes tended to be larger in task BX70 (mean = −2.0 μV) compared to AX70 (mean = −1.42 μV), while beta ERD tended to be more pronounced in AX70.
4. Discussion
The AX-CPT is widely used to evaluate cognitive control, working memory, and aspects of attention. Several recent reports investigated neural processes involved in AX-CPT performance using fMRI and ERPs in the EEG (Braver et al., 2009; Dias et al., 2003; Dias et al., 2006), but results regarding the possible role of neural oscillations for task performance were still missing. Here we report two main findings, one in the alpha frequency band and one in the beta frequency band. Activity in the gamma frequency band was not modulated by this task in the areas tested, and activity in the theta band was strongly related to the evoked potential, which may have obscured any possible induced activity, and has already been reported (Dias et al., 2003). Thus the discussion will focus on the alpha and beta findings.
In the alpha band we found a broad parieto-occipital ERD starting within 0.1 s following stimulation and persisting throughout the cue evaluation period. As an ERD (as opposed to an increase in synchrony), it had no obvious correlate in the average ERP waveform and so represents a process that is missed in traditional ERP analyses of CPT data. Although the onset of the ERD was similar for all task variations and stimuli, the extent and duration was modulated by task demands, suggesting both “bottom-up” and “top-down” modulations. In particular, the ERD was larger if a prepotency to not respond had to be overcome following an instructive cue signalling high probability of response (cue A in BX70). In contrast, the ERD was reduced and alpha power was eventually increased if a prepotency to respond had to be overcome following a cue signalling that response had to be withheld (cue B in AX70). We hypothesize that this alpha modulation could reflect not only a disinhibition of visual associative brain regions to support high level decoding of cue information, but also an anticipatory attentional mechanism to gate visuomotor processing.
In the beta frequency band, we found modulation of beta power in fronto-central regions persisting throughout the cue/probe interval, putatively reflecting motor preparation. There was beta ERD over left central regions, consistent with localization of the motor region controlling right hand response. Beta power in this region was modulated by expectancy of response and was negatively correlated with reaction time. In other words, beta ERD following instructive cues was most pronounced if the cue signalled a high probability that the subject would have to respond to the subsequent probe. There was also prominent symmetrically distributed beta ERD in the posterior parietal regions during the evaluation period, which returned to baseline in the preparation period, and was not affected by task.
4.1. Alpha
Parieto-occipital alpha oscillations in the human have been traditionally viewed as representing a sensory ‘idling’ process. However, several recent studies have suggested a more dynamic role, in which increases in alpha are associated with active suppression of to-be-ignored information while, suppression of ongoing alpha is seen when attention and/or preparation is required (Bastiaansen and Brunia, 2001; Foxe et al., 1998; Kelly et al., 2006; Sauseng et al., 2005; Worden et al., 2000). Furthermore, this effect may be modulated by both frontal and parietal areas (Capotosto et al., 2009; Rajagovindan and Ding,; Sauseng et al., 2005).
It is important to distinguish between modulation of ongoing alpha oscillations and externally induced alpha ERD (Kelly et al., 2006; Pfurtscheller and Lopes da Silva, 1999). As seen in the evaluation period in this study, external stimulation invariably causes a suppression of alpha power (Pfurtscheller and Aranibar, 1977). In the present study, stimulus-driven ERD showed no modulation by either type of cue or task. In keeping with the evolving concept of ongoing alpha activity as a mechanism for suppression of response to potentially distracting information, the early onset of stimulus-induced ERD and lack of task or stimulus modulation suggests a rapid, bottom-up system in the brain for removing suppression from visual object processing regions, once a stimulus is presented.
In addition to this early, presumed bottom-up process, a late phase was observed that was sensitive to both stimulus and task, suggesting top-down modulation. Alpha suppression during this response preparation window appears to be similar to that shown in previous studies of anticipatory attention in humans (Bastiaansen and Brunia, 2001; Foxe et al., 1998; Kelly et al., 2006; Sauseng et al., 2005; Worden et al., 2000; Yamagishi et al., 2005). However, all previous studies of alpha modulation used either spatial or cross-modal attention paradigms, thus this is the first study to investigate role of alpha ERD to different instructional stimuli within the visual modality itself.
The alpha ERD to the A cue during the preparatory interval was significant only in the BX-70 task variations, and was absent in the other task variations. The task variation BX70 is unique in that the information from the A cue must be maintained and used to override a prepotent tendency to not respond to an “X” probe when it is presented, as X is usually preceded by a B cue in this task. In addition, both X and Y probes are equiprobable after presentation of the A cue, putting greatest stress on the subsequent discrimination. Thus, the sustained alpha ERD in this task variant may keep visual object recognition regions maximally “on line” throughout the response preparation interval.
By contrast, following B cues in the AX-70 task variant, there was increased alpha power throughout the preparatory interval. In the AX-70, but not other tasks, the B cue must be used to override a prepotent response to respond to an X when it appears. Furthermore, following the B cue, the identity of the subsequent probe stimulus (i.e. X vs. Y) becomes irrelevant since both necessitate withholding of response. Thus, the increased alpha may be used to suppress processing of the probe stimulus under this task. Some increase in alpha in the preparatory period was also observed for B vs. A cues in the other tasks, suggesting that alpha generation may be used to dynamically modulate regional brain activation even within sensory channels.
4.2. Beta
Similar to parieto-occipital areas, the somato-motor cortex shows a dominant rhythm in the alpha frequency range, called the rolandic alpha rhythm (Pineda, 2005). Generators of this rhythm are located primarily within post-central somato-sensory cortex, but may span across the central sulcus to include pre-central cortex as well (Salmelin et al., 1995) (see also Supplementary figure 4). In contrast, a second dominant rhythm, the rolandic beta rhythm (Hari, 2006), is associated primarily with the pre-central primary motor cortex. Indeed, a study using transcranial magnetic stimulation suggested that beta is the characteristic frequency range of the motor cortex (Rosanova et al., 2009). Similar to the parieto-occipital alpha, power of pre-central beta and post-central alpha are inversely correlated with BOLD signal in motor fMRI studies (Ritter et al., 2009). Thus, decreased power in these rhythms has been equalized with cortical activation.
Further extensive evidence from studies in human and non-human primates links the pre-central beta rhythm to motor control. For example, in self paced movement tasks, beta power decreases prior to movement onset and rebounds following movement termination and remains increased during steady contraction (Crone et al., 1998; Donner et al., 2009; Farmer, 1998; Pfurtscheller, 1981). During isometric contraction pre-central beta is coherent with simultaneously recorded contra-lateral EMG (Baker, 2007; Rubino et al., 2006; Schoffelen et al., 2008). Furthermore, the motor system is more effective in executing corrective movements during periods of increased pre-central beta, and also shows a bias to maintain an existing motor state at the expense of initiating new movements (Androulidakis et al., 2006; Engel and Fries,; Gilbertson et al., 2005). Thus, it could be hypothesized that during periods of low beta power the motor cortex is in a receiving state for new information or commands from other cortical areas.
In our study, all stimuli elicited beta ERD in the evaluation period, suggesting visuo-motor interaction or motor planning independent of the information conveyed by the instructive cue. In the subsequent preparation window, beta ERD was most pronounced following A cues in tasks AX70 and BX70, concordant with the biggest probability of response, while following no-go B cues, the beta power returned to baseline in most cases. This is in line with a study in which monkeys were trained to release a button to go, but not to no-go cues (Zhang et al., 2008). Both stimuli induced an initial dip in beta activity before recovery following the no-go cues and maintenance of suppression following the go cues.
Interestingly, while beta power following B cues recovered after the evaluation period in tasks AY70 and BX70, it remained low in task AX70, although less than when following A cues. A possible reason for this could be that during this task most cue target pairs are go trials and a certain automatism was established that prepared premotor areas rhythmically for a response. An interesting question is if this “incorrect” preparatory activity following B cues in the AX70 task is related to the increase in BX-errors observed in clinical populations such as in patients with schizophrenia (Barch et al., 2001; Cohen et al., 1999; Javitt et al., 2000). Unfortunately, due to too few BX error trials in this dataset, we could not compare activity in correct and error BX trials.
Although task-related suppression of beta activity was most prominent over motor cortex in this study, we also observed modulation of parietal beta activity. As compared to the beta modulation over motor cortex, which began during the evaluation period and was maintained throughout the preparation period, parietal beta modulation was restricted to the evaluation period. Furthermore, during the evaluation period, the temporal profile of activity was similar across parietal and central regions. It has been argued that beta synchrony between parietal and central as well as -frontal regions may represent a “spectral fingerprint” of sensorimotor integration, which would be consistent with results of the present study (Engel and Fries, 2010; Siegel et al., 2012).
4.3. CNV
Given that both CNV and beta modulations persisted during the cue-probe interval and both showed correlations with reaction time, a final series of analyses evaluated the relationship between these two measures. Despite similar timing and correlations, the two measures appear to reflect distinct processes based upon both spatial localization and task-related variation.
In terms of localization the main beta desynchronization during the motor preparation period was observed on the sensorimotor cortex contralateral to the response hand (Supplementary Figure 4), suggestive of generators within underlying sensorimotor cortex (Pfurtscheller and Lopes da Silva, 1999).
In contrast, the CNV was non-lateralized and maximal at the vertex, consistent with prior studies that have localized CNV generators not only to primary motor cortex, but also to anterior cingulate, and supplementary motor cortices (Dias et al., 2003; Liu et al., 1996).
In terms of task-related modulation, we observed most pronounced beta modulation to A cues in the AX70, and smallest modulation to A cues in the AY70 task. Thus, the beta modulation appears to track primarily the probability that a valid cue will be followed by a correct target, and therefore that a motor response will be required. Consistent with this, beta modulation was also absent to B cues in both the BX-70 and AY-70 conditions. Interestingly, in the AX-70 condition, a sustained beta ERD was observed to the B as well as A cues, suggesting that in this condition, in which prepotent tendency to response is strongest, there may be a sustained active inhibition of the motor response, rather than a passive no-response set.
In contrast, CNV tended to be larger to A cues in the BX70 vs. AX70 task, suggesting that it may track not only the probability of response, but also the need to overcome a prepotent no-response set (Dias et al., 2011; Dias et al., 2003). As for the beta ERD, no significant CNV was observed to B cues in any task.
To date, most studies of oscillatory activity related to motor response have used self paced or motor imagery tasks, or paradigms with simpler warning and target cues. Here we show the feasibility of analyzing motor beta in widely used cognitive tasks as well, such as the AX-CPT, and demonstrate dissociability of oscillatory modulations related to more traditional time domain motor-response measures.
5. Conclusion
The current study investigated the oscillatory signature of cortical cognitive operations during AX-CPT performance using frequency domain analysis across a range of previously described, informative parametric task variations. The most salient effects consisted of a decrease of power in the alpha frequency band during stimulus evaluation, modulated during the preparation period, and in the beta frequency band relating to response preparation and maintenance. Importantly, these effects have no direct correlate in time-domain ERP and cannot be temporally resolved in fMRI studies with this paradigm, and thus complement existing neurocognitive measures. Deficits in AX-CPT performance have been demonstrated across a variety of neuropsychiatric disorders, most notably schizophrenia and ADHD. In addition to delineating critical neurocognitive processes, the present study thus also provides a basis for dissociating patterns of dysfunction in individuals with discrete neuropsychiatric disorders.
Supplementary Material
Acknowledgments
Funding:
This work was supported by National Institute of Heath (R37MH49334, P50MH086385 to DCJ).
The authors would like to thank Beth Higgins and Erica Saccente for technical assistance.
Footnotes
All authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
S. Bickel, Email: Bick35@gmail.com.
E.C. Dias, Email: Dias@nki.rfmh.org.
M.L. Epstein, Email: Mepstein@nki.rfmh.org.
D.C. Javitt, Email: Javitt@nki.rfmh.org.
References
- Alegre M, Imirizaldu L, Valencia M, Iriarte J, Arcocha J, Artieda J. Alpha and beta changes in cortical oscillatory activity in a go/no go randomly-delayed-response choice reaction time paradigm. Clin Neurophysiol. 2006;117:16–25. doi: 10.1016/j.clinph.2005.08.030. [DOI] [PubMed] [Google Scholar]
- Androulidakis AG, Doyle LM, Gilbertson TP, Brown P. Corrective movements in response to displacements in visual feedback are more effective during periods of 13–35 Hz oscillatory synchrony in the human corticospinal system. Eur J Neurosci. 2006;24:3299–3304. doi: 10.1111/j.1460-9568.2006.05201.x. [DOI] [PubMed] [Google Scholar]
- Baker SN. Oscillatory interactions between sensorimotor cortex and the periphery. Curr Opin Neurobiol. 2007;17:649–655. doi: 10.1016/j.conb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banaschewski T, Yordanova J, Kolev V, Heinrich H, Albrecht B, Rothenberger A. Stimulus context and motor preparation in attention-deficit/hyperactivity disorder. Biol Psychol. 2008;77:53–62. doi: 10.1016/j.biopsycho.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Barch DM, Carter CS, Braver TS, Sabb FW, MacDonald A, 3rd, Noll DC, Cohen JD. Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Arch Gen Psychiatry. 2001;58:280–288. doi: 10.1001/archpsyc.58.3.280. [DOI] [PubMed] [Google Scholar]
- Bastiaansen MC, Brunia CH. Anticipatory attention: an event-related desynchronization approach. Int J Psychophysiol. 2001;43:91–107. doi: 10.1016/s0167-8760(01)00181-7. [DOI] [PubMed] [Google Scholar]
- Braver TS, Paxton JL, Locke HS, Barch DM. Flexible neural mechanisms of cognitive control within human prefrontal cortex. Proc Natl Acad Sci U S A. 2009;106:7351–7356. doi: 10.1073/pnas.0808187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capotosto P, Babiloni C, Romani GL, Corbetta M. Frontoparietal cortex controls spatial attention through modulation of anticipatory alpha rhythms. J Neurosci. 2009;29:5863–5872. doi: 10.1523/JNEUROSCI.0539-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Barch DM, Carter C, Servan-Schreiber D. Context-processing deficits in schizophrenia: converging evidence from three theoretically motivated cognitive tasks. J Abnorm Psychol. 1999;108:120–133. doi: 10.1037//0021-843x.108.1.120. [DOI] [PubMed] [Google Scholar]
- Crone NE, Miglioretti DL, Gordon B, Sieracki JM, Wilson MT, Uematsu S, Lesser RP. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. I. Alpha and beta event-related desynchronization. Brain. 1998;121 ( Pt 12):2271–2299. doi: 10.1093/brain/121.12.2271. [DOI] [PubMed] [Google Scholar]
- Di Russo F, Martinez A, Hillyard SA. Source analysis of event-related cortical activity during visuo-spatial attention. Cereb Cortex. 2003;13:486–499. doi: 10.1093/cercor/13.5.486. [DOI] [PubMed] [Google Scholar]
- Di Russo F, Martinez A, Sereno MI, Pitzalis S, Hillyard SA. Cortical sources of the early components of the visual evoked potential. Hum Brain Mapp. 2002;15:95–111. doi: 10.1002/hbm.10010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias EC, Butler PD, Hoptman MJ, Javitt DC. Early sensory contributions to contextual encoding deficits in schizophrenia. Arch Gen Psychiatry. 2011;68:654–664. doi: 10.1001/archgenpsychiatry.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias EC, Foxe JJ, Javitt DC. Changing plans: a high density electrical mapping study of cortical control. Cereb Cortex. 2003;13:701–715. doi: 10.1093/cercor/13.7.701. [DOI] [PubMed] [Google Scholar]
- Dias EC, McGinnis T, Smiley JF, Foxe JJ, Schroeder CE, Javitt DC. Changing plans: neural correlates of executive control in monkey and human frontal cortex. Exp Brain Res. 2006;174:279–291. doi: 10.1007/s00221-006-0444-4. [DOI] [PubMed] [Google Scholar]
- Donner TH, Siegel M, Fries P, Engel AK. Buildup of choice-predictive activity in human motor cortex during perceptual decision making. Curr Biol. 2009;19:1581–1585. doi: 10.1016/j.cub.2009.07.066. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P. Beta-band oscillations--signalling the status quo? Curr Opin Neurobiol. 2010;20:156–165. doi: 10.1016/j.conb.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Farmer SF. Rhythmicity, synchronization and binding in human and primate motor systems. J Physiol. 1998;509 ( Pt 1):3–14. doi: 10.1111/j.1469-7793.1998.003bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxe JJ, Simpson GV, Ahlfors SP. Parieto-occipital approximately 10 Hz activity reflects anticipatory state of visual attention mechanisms. Neuroreport. 1998;9:3929–3933. doi: 10.1097/00001756-199812010-00030. [DOI] [PubMed] [Google Scholar]
- Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291:1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- Gilbertson T, Lalo E, Doyle L, Di Lazzaro V, Cioni B, Brown P. Existing motor state is favored at the expense of new movement during 13–35 Hz oscillatory synchrony in the human corticospinal system. J Neurosci. 2005;25:7771–7779. doi: 10.1523/JNEUROSCI.1762-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray CM, Konig P, Engel AK, Singer W. Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature. 1989;338:334–337. doi: 10.1038/338334a0. [DOI] [PubMed] [Google Scholar]
- Hari R. Action-perception connection and the cortical mu rhythm. Prog Brain Res. 2006;159:253–260. doi: 10.1016/S0079-6123(06)59017-X. [DOI] [PubMed] [Google Scholar]
- Holmes AJ, MacDonald A, III, Carter CS, Barch DM, Andrew SV, Cohen JD. Prefrontal functioning during context processing in schizophrenia and major depression: an event-related fMRI study. Schizophr Res. 2005;76:199–206. doi: 10.1016/j.schres.2005.01.021. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Shelley AM, Silipo G, Lieberman JA. Deficits in auditory and visual context-dependent processing in schizophrenia: defining the pattern. Arch Gen Psychiatry. 2000;57:1131–1137. doi: 10.1001/archpsyc.57.12.1131. [DOI] [PubMed] [Google Scholar]
- Kelly SP, Gomez-Ramirez M, Foxe JJ. The strength of anticipatory spatial biasing predicts target discrimination at attended locations: a high-density EEG study. Eur J Neurosci. 2009;30:2224–2234. doi: 10.1111/j.1460-9568.2009.06980.x. [DOI] [PubMed] [Google Scholar]
- Kelly SP, Lalor EC, Reilly RB, Foxe JJ. Increases in alpha oscillatory power reflect an active retinotopic mechanism for distracter suppression during sustained visuospatial attention. J Neurophysiol. 2006;95:3844–3851. doi: 10.1152/jn.01234.2005. [DOI] [PubMed] [Google Scholar]
- Lakatos P, Karmos G, Mehta AD, Ulbert I, Schroeder CE. Entrainment of neuronal oscillations as a mechanism of attentional selection. Science. 2008;320:110–113. doi: 10.1126/science.1154735. [DOI] [PubMed] [Google Scholar]
- Liu MJ, Fenwick PB, Lumsden J, Lever C, Stephan KM, Ioannides AA. Averaged and single-trial analysis of cortical activation sequences in movement preparation, initiation, and inhibition. Hum Brain Mapp. 1996;4:254–264. doi: 10.1002/(SICI)1097-0193(1996)4:4<254::AID-HBM3>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007;164:177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Neuper C, Pfurtscheller G. Event-related dynamics of cortical rhythms: frequency-specific features and functional correlates. Int J Psychophysiol. 2001;43:41–58. doi: 10.1016/s0167-8760(01)00178-7. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G. Central beta rhythm during sensorimotor activities in man. Electroencephalogr Clin Neurophysiol. 1981;51:253–264. doi: 10.1016/0013-4694(81)90139-5. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Aranibar A. Event-related cortical desynchronization detected by power measurements of scalp EEG. Electroencephalogr Clin Neurophysiol. 1977;42:817–826. doi: 10.1016/0013-4694(77)90235-8. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 1999;110:1842–1857. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- Pineda JA. The functional significance of mu rhythms: translating “seeing” and “hearing” into “doing”. Brain Res Brain Res Rev. 2005;50:57–68. doi: 10.1016/j.brainresrev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Rajagovindan R, Ding M. From Prestimulus Alpha Oscillation to Visual-evoked Response: An Inverted-U Function and Its Attentional Modulation. J Cogn Neurosci. 2010 doi: 10.1162/jocn.2010.21478. [DOI] [PubMed] [Google Scholar]
- Rihs TA, Michel CM, Thut G. Mechanisms of selective inhibition in visual spatial attention are indexed by alpha-band EEG synchronization. Eur J Neurosci. 2007;25:603–610. doi: 10.1111/j.1460-9568.2007.05278.x. [DOI] [PubMed] [Google Scholar]
- Ritter P, Moosmann M, Villringer A. Rolandic alpha and beta EEG rhythms’ strengths are inversely related to fMRI-BOLD signal in primary somatosensory and motor cortex. Hum Brain Mapp. 2009;30:1168–1187. doi: 10.1002/hbm.20585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosanova M, Casali A, Bellina V, Resta F, Mariotti M, Massimini M. Natural frequencies of human corticothalamic circuits. J Neurosci. 2009;29:7679–7685. doi: 10.1523/JNEUROSCI.0445-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosvold HE, Mirsky AF, Sarason I, Bransome ED, Jr, Beck LH. A continuous performance test of brain damage. J Consult Psychol. 1956;20:343–350. doi: 10.1037/h0043220. [DOI] [PubMed] [Google Scholar]
- Rubino D, Robbins KA, Hatsopoulos NG. Propagating waves mediate information transfer in the motor cortex. Nat Neurosci. 2006;9:1549–1557. doi: 10.1038/nn1802. [DOI] [PubMed] [Google Scholar]
- Salmelin R, Hamalainen M, Kajola M, Hari R. Functional segregation of movement-related rhythmic activity in the human brain. Neuroimage. 1995;2:237–243. doi: 10.1006/nimg.1995.1031. [DOI] [PubMed] [Google Scholar]
- Sarnthein J, Petsche H, Rappelsberger P, Shaw GL, von SA. Synchronization between prefrontal and posterior association cortex during human working memory. Proc Natl Acad Sci USA. 1998;95:7092–7096. doi: 10.1073/pnas.95.12.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Stadler W, Schabus M, Doppelmayr M, Hanslmayr S, Gruber WR, Birbaumer N. A shift of visual spatial attention is selectively associated with human EEG alpha activity. Eur J Neurosci. 2005;22:2917–2926. doi: 10.1111/j.1460-9568.2005.04482.x. [DOI] [PubMed] [Google Scholar]
- Schoffelen JM, Oostenveld R, Fries P. Neuronal coherence as a mechanism of effective corticospinal interaction. Science. 2005;308:111–113. doi: 10.1126/science.1107027. [DOI] [PubMed] [Google Scholar]
- Schoffelen JM, Oostenveld R, Fries P. Imaging the human motor system’s beta-band synchronization during isometric contraction. Neuroimage. 2008;41:437–447. doi: 10.1016/j.neuroimage.2008.01.045. [DOI] [PubMed] [Google Scholar]
- Servan-Schreiber D, Cohen JD, Steingard S. Schizophrenic deficits in the processing of context. A test of a theoretical model. Arch Gen Psychiatry. 1996;53:1105–1112. doi: 10.1001/archpsyc.1996.01830120037008. [DOI] [PubMed] [Google Scholar]
- Siegel M, Donner TH, Engel AK. Spectral fingerprints of large-scale neuronal interactions. Nat Rev Neurosci. 2012;13:121–134. doi: 10.1038/nrn3137. [DOI] [PubMed] [Google Scholar]
- Thut G, Nietzel A, Brandt SA, Pascual-Leone A. Alpha-band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. J Neurosci. 2006;26:9494–9502. doi: 10.1523/JNEUROSCI.0875-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter WG, Cooper R, Aldridge VJ, McCallum WC, Winter AL. Contingent Negative Variation: An Electric Sign of Sensorimotor Association and Expectancy in the Human Brain. Nature. 1964;203:380–384. doi: 10.1038/203380a0. [DOI] [PubMed] [Google Scholar]
- Worden MS, Foxe JJ, Wang N, Simpson GV. Anticipatory biasing of visuospatial attention indexed by retinotopically specific alpha-band electroencephalography increases over occipital cortex. J Neurosci. 2000;20:RC63. doi: 10.1523/JNEUROSCI.20-06-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi N, Goda N, Callan DE, Anderson SJ, Kawato M. Attentional shifts towards an expected visual target alter the level of alpha-band oscillatory activity in the human calcarine cortex. Brain Res Cogn Brain Res. 2005;25:799–809. doi: 10.1016/j.cogbrainres.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen Y, Bressler SL, Ding M. Response preparation and inhibition: the role of the cortical sensorimotor beta rhythm. Neuroscience. 2008;156:238–246. doi: 10.1016/j.neuroscience.2008.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.