Abstract
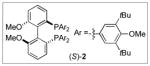
A 1:2 mixture of [(S)-2](AuCl)2 [(S)-2 = (S)-DTBM-MeOBIPHEP] and AgClO4 catalyzes the intramolecular enantioselective dehydrative amination of allylic alcohols with carbamates to form five- and six-membered aliphatic nitrogen heterocycles in high yield with up to 95% ee.
Keywords: asymmetric synthesis, alcohols, amination, Au, stereoselective catalysis
The transition metal-catalyzed enantioselective amination of allylic esters and carbonates represents one of the most well established routes to the synthesis of chiral, nonracemic allylic amines.[1] With the potential to condense synthetic sequences and reduce waste streams, there has been considerable interest in the dehydrative amination of allylic alcohols as a route to enantiomerically enriched allylic amines. However, while the stereospecific amination of chiral 2°-allylic alcohols has been demonstrated,[2–4] the enantioselective amination of allylic alcohols remains problematic.[5] Carreira has reported the Ir(I)-catalyzed enantioselective amination of 1-cyclohexylprop-2-en-ol with sulfamic acid in 70% ee.[6] Hartwig has reported the Ir(I)/BPh3-catalyzed enantioselective intermolecular amination of 1°-allylic alcohols with aromatic amines with up to 94% ee, but this method was restricted to cinnamyl alcohols in the absence of a stoichiometric Lewis acid promoter.[7] Yamamoto[8] and Kitamura[9] have independently reported the enantioselective intramolecular amination of allylic alcohols catalyzed by Hg(II) and Ru(II) complexes, respectively. However, these methods were restricted to sulfonamide nucleophiles and high enantioselectivity was realized only for the formation of arene-fused nitrogen heterocycles. Here we report a gold-catalyzed protocol for the intramolecular enantioselective amination of allylic alcohols with carbamates to form five- and six-membered aliphatic nitrogen heterocycles with up to 95% ee.
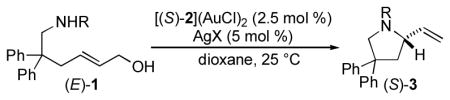
We recently reported the intramolecular dehydrative amination of allylic alcohols with alkylamines catalyzed by an achiral gold(I) phosphine complex.[4,10] Encouraged by the high efficiency and stereospecificity of this transformation and guided by both our previous work in the area of gold(I)-catalyzed enantioselective allene hydroamination[11,12] and Bandini’s recent demonstration of the gold(I)-catalyzed enantioselective arylation[13] and alkoxylation[14] of allylic alcohols,[15] we targeted axially chiral bis(gold) complexes as catalysts for the intramolecular enantioselective amination of the γ-benzylamino allylic alcohol (E)-1a. Unfortunately, optimization within this framework[16] proved largely unsuccessful and treatment of (E)-1a with a catalytic 1:2 mixture of [(S)-2](AuCl)2 [(S)-2 = (S)-DTBM-MeOBIPHEP; 2.5 mol %) and AgSbF6 (5 mol %) in dioxane at 25 °C for 5 h led to quantitative conversion to 2-vinylpyrrolidine 3a, but with only 29% ee (Table 1, entry 1).[17] We then focused our attention on the manipulation of the nitrogen nucleophile as a means to amplify stereoinduction (Table 1). These experiments proved fruitful and gold(I)-catalyzed cyclization of Fmoc-protected γ-amino allylic alcohol (E)-1g employing an optimized catalyst system comprised of [(S)-2](AuCl)2 (2.5 mol %) and AgClO4 (5 mol %) in dioxane at room temperature for 48 h led to isolation of (S)-3g in 95% yield with 91% ee (Table 1, entry 7).[16,18]
Table 1.
Effect of nucleophile on the gold(I)-catalyzed intramolecular amination of allylic alcohols (E)-1 under optimized conditions.[16,18]
| ||||||
|---|---|---|---|---|---|---|
| entry | R | X | time [h] | pyrrolidine | yield [%][a] | ee [%][b] |
| 1 | Bn | SbF6 | 5 | 3a | 100[c] | 29 |
| 2 | Cbz | ClO4 | 48 | 3b | 99 | 79 |
| 3 | Boc | ClO4 | 48 | 3c | 97 | 80 |
| 4[d] | Troc | ClO4 | 48 | 3d | 62 | 84 |
| 5 | CO2Me | ClO4 | 48 | 3e | 97 | 75 |
| 6 | Ts | ClO4 | 48 | 3f | 98 | 76 |
| 7 | Fmoc | ClO4 | 48 | 3g | 95 | 91 |
| ||||||
Isolated yield.
Determined by HPLC analysis on chiral support.
Conversion.
Reaction run at 40 °C.
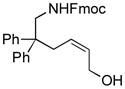
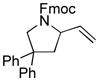
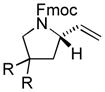
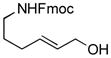
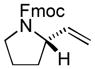
The scope of gold(I)-catalyzed enantioselective intramolecular amination was evaluated as a function of alkene geometry, substitution, and ring size (Table 2). The enantioselectivity of gold(I)-catalyzed amination was sensitive to alkene geometry and gold-catalyzed cyclization of (Z)-1g formed 3g in 99% yield with <5% ee (Table 2, entry 1). Although γ-amino allylic alcohols that possessed gem-dialkyl substitution at the homoallylic position cyclized with higher enantioselectivity than did an unsubstituted γ-amino allylic alcohol (Table 2, entries 2 – 4), homoallylic gem-disubstitution was not required for high enantioselectivity (Table 2, entries 5 and 6). For example, gold(I)-catalyzed cyclization of 4, which possessed a single phenyl group at the homoallylic position, led to isolation of pyrrolidine 5 in 87% yield as ~1:1 mixture of cis/trans diastereomers, both of which were formed with ≥90% ee, indicative of overriding catalyst control of stereoinduction. Gold-catalyzed enantioselective amination also tolerated gem-dialkyl substitution at the hydroxyl-bound carbon atom (Table 2, entry 7) and was applicable to the synthesis of six-membered nitrogen heterocycles, proving particularly effective for the synthesis of differentially protected 2-vinyl piperazines (Table 2, entries 8–11).
Table 2.
Substrate Scope of the Intramolecular Amination of Allylic Alcohols (0.6 M) Catalyzed by a 1:2 Mixture of [(S)-2](AuCl)2 (2.5 mol %) and AgClO4 (5 mol %) in Dioxane at 25 °C for 48 h.[18]
| entry | substrate | heterocycle | yield [%][a] | ee [%][b] |
|---|---|---|---|---|
| 1 |
 (Z)-1g |
 3g |
95 | Š5 |
|
|
|||
| 2 | (R = Me) | 94 | 90 | |
| 3 | [R-R = –(CH2)5–] | 95 | 94 | |
| 4 |
|
|
89 | 62 |
| 5 |
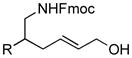 4 (R = Ph) |
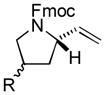 5 |
87[c] | 90/92 |
| 6 | (R = i-Pr) | 98[c] | 85/91 | |
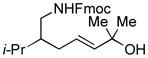
| 7 |
|
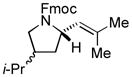
|
87[c] | 88/90 |
| 8 |
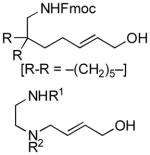
|
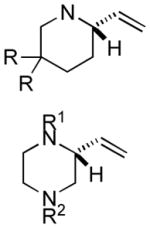
|
69 | 77 |
| 9[d] | (R1 = Fmoc, R2 = Boc) | 86 | 94 | |
| 10[d] | (R1 = Fmoc, R2 = Ts) | 99 | 92 | |
| 11[d] | (R1 = Boc, R2 = Fmoc) | 99 | 91 |
Isolated yield.
Determined by HPLC analysis on chiral support.
Diastereomeric ratio = ~1:1.
Reaction run at 50 °C.
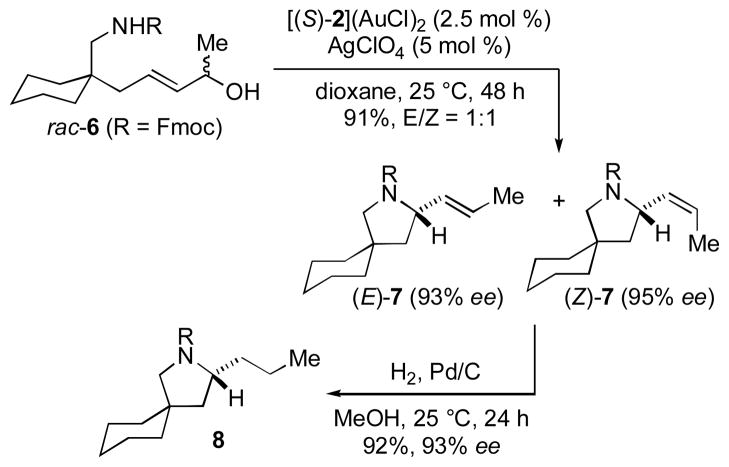
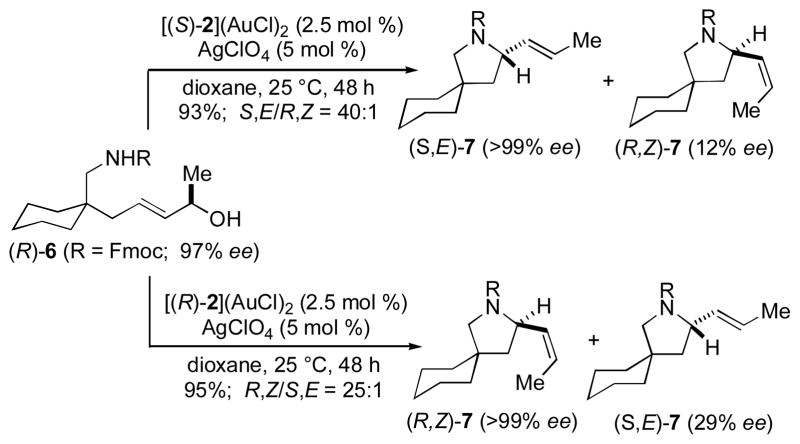
The effect of a chiral 2°-allylic alcohol moiety on the efficiency and stereoselectivity of gold-catalyzed allylic amination was evaluated employing γ-amino allylic alcohol 6. In one experiment, cyclization of rac-6 catalyzed by [(S)-2](AuCl)2/AgClO4 led to isolation of a 1:1 mixture of (E)-7 (93% ee) and (Z)-7 (95% ee) in 91% combined yield (Scheme 1). Hydrogenation of this mixture formed 2-propyl pyrrolidine 8 in 92% yield with 93% ee (Scheme 1), which established that (E)-7 and (Z)-7 possessed the same absolute configuration (S by analogy),[18] and HPLC analysis of the conversion of rac-6 to 7 revealed that both enantiomers of 6 reacted at similar rates (kS/kR = 1.06). In two additional experiments, cyclization of enantiomerically enriched (R)-6 (97% ee) catalyzed by [(S)-2](AuCl)2/AgClO4 led to isolation of a 40:1 mixture of (S,E)-7 (>99% ee) and (R,Z)-7 (12% ee) in 93% combined yield while cyclization of (R)-6 catalyzed by [(R)-2](AuCl)2/AgClO4 led to isolation of 25:1 mixture (R,Z)-7 (>99% ee) and (S,E)-7 (29% ee) in 95% combined yield (Scheme 2). Together, these results established that asymmetric induction is determined solely by catalyst configuration (S → S; R → R) and that E/Z selectivity is determined by the stereochemical relationship between the incipient N-bound stereocenter and the extant O-bound stereocenter (S/R → E; R/R → Z), consistent with the net syn displacement of the hydroxyl group by the attacking carbamate nucleophile.
Scheme 1.
Scheme 2.
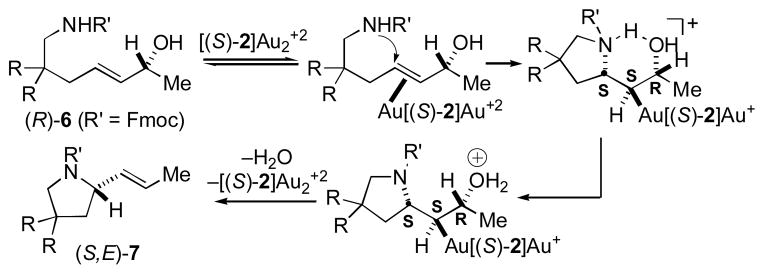
The net syn displacement of the hydroxyl group by the nitrogen nucleophile, which was also documented for the amination of allylic alcohols catalyzed by achiral mono(gold) complexes,[3,4] is consistent with a mechanism involving π-complexation of gold to the C=C bond followed by anti-addition of the nucleophile and anti-elimination of the hydroxyl group, perhaps facilitated by an intramolecular N–H–O hydrogen bond (Scheme 3).[19] Alternatively, syn-substitution is also consistent with a mechanism involving σ-activation of the hydroxyl group followed by concerted SN2′ displacement[20] and Toste has recently demonstrated that bis(gold) phosphine complexes are sufficiently Lewis acidic to acidify the hydroxyl proton of an alcohol.[21] However, the failure of either triflic acid or BF3·OEt2 (10 mol %, 25 °C, 48 h) to catalyze the cyclization of (E)-1g argues against a σ-activation pathway for allylic amination.
Scheme 3.
In summary, we have developed a gold(I)-catalyzed protocol for the intramolecular enantioselective amination of allylic alcohols with carbamates to form five- and six-membered nitrogen heterocycles with up to 95% ee. Cyclization of chiral γ-amino allylic alcohols that possessed a stereogenic homoallylic or hydroxyl-bound carbon atom occurred with overriding catalyst control of asymmetric induction. Stereochemical analysis of the cyclization of (R)-6, which possessed a 2°-allylic alcohol moiety, established the net syn-displacement of the hydroxyl group by the carbamate nucleophile.
Acknowledgments
We thank the NIH (GM-080422) for support of this research. PM thanks Duke University for support provided through the Burroughs Welcome and C. R. Hauser Fellowships.
Footnotes
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
Contributor Information
Paramita Mukherjee, French Family Science Center, Duke University, Durham, NC 27708–0346 (USA), Fax: (+1) 919-660-1605.
Prof. Ross A. Widenhoefer, Email: rwidenho@chem.duke.edu, French Family Science Center, Duke University, Durham, NC 27708–0346 (USA), Fax: (+1) 919-660-1605
References
- 1.a) Trost BM, Zhang T, Sieber JD. Chem Sci. 2010;1:427–440. [Google Scholar]; b) Lu Z, Ma S. Angew Chem. 2008;120:264–303. [Google Scholar]; Angew Chem Int Ed. 2008;47:258–297. doi: 10.1002/anie.200605113. [DOI] [PubMed] [Google Scholar]; c) Trost BM, Van Vranken DL. Chem Rev. 1996;96:395–422. doi: 10.1021/cr9409804. [DOI] [PubMed] [Google Scholar]; d) Trost BM, Crawley ML. Chem Rev. 2003;103:2921–2943. doi: 10.1021/cr020027w. [DOI] [PubMed] [Google Scholar]
- 2.a) Roggen M, Carreira EM. J Am Chem Soc. 2010;132:11917–11919. doi: 10.1021/ja105271z. [DOI] [PubMed] [Google Scholar]; b) Hande SM, Kawai N, Uenishi J. J Org Chem. 2009;74:244–253. doi: 10.1021/jo801926g. [DOI] [PubMed] [Google Scholar]; c) Kawai N, Abe R, Uenishi J. Tetrahedron Lett. 2009;50:6580–6583. [Google Scholar]; d) Yokoyama H, Hirai Y. Heterocycles. 2008;75:2133–2153. [Google Scholar]; e) Ozawa F, Okamoto H, Kawagishi S, Yamamoto S, Minami T, Yoshifuji M. J Am Chem Soc. 2002;124:10968–10969. doi: 10.1021/ja0274406. [DOI] [PubMed] [Google Scholar]
- 3.Mukherjee P, Widenhoefer RA. Org Lett. 2010;12:1184–1187. doi: 10.1021/ol902923e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mukherjee P, Widenhoefer RA. Org Lett. 2011;13:1334–1337. doi: 10.1021/ol103175w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.For a recent highlight of the enantioselective dehydrative functionalization of allylic alcohols see: Bandini M. Angew Chem. 2011;123:1026–1027.Angew Chem Int Ed. 2011;50:994–995. doi: 10.1002/anie.201006522.
- 6.Defieber C, Ariger MA, Moriel P, Carreira EM. Angew Chem. 2007;119:3200–3204. doi: 10.1002/anie.200700159. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2007;46:3139–3143. doi: 10.1002/anie.200700159. [DOI] [PubMed] [Google Scholar]
- 7.Yamashita Y, Gopalarathnam A, Hartwig JF. J Am Chem Soc. 2007;129:7508–7509. doi: 10.1021/ja0730718. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto H, Ho E, Namba K, Imagawa H, Nishizawa M. Chem Eur J. 2010;16:11271–11274. doi: 10.1002/chem.201001656. [DOI] [PubMed] [Google Scholar]
- 9.Miyata K, Kutsuna H, Kawakami S, Kitamura M. Angew Chem. 2011;123:4745–4749. doi: 10.1002/anie.201100772. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2011;50:4649–4653. doi: 10.1002/anie.201100772. [DOI] [PubMed] [Google Scholar]
- 10.For a recent review of gold-catalyzed dehydrative functionalization of allylic alcohols see: Biannic B, Aponick A. Eur J Org Chem. doi: 10.1002/ejoc.201100858. In press.
- 11.a) Zhang Z, Bender CF, Widenhoefer RA. J Am Chem Soc. 2007;129:14148–14149. doi: 10.1021/ja0760731. [DOI] [PubMed] [Google Scholar]; b) Zhang Z, Bender CF, Widenhoefer RA. Org Lett. 2007;9:2887–2889. doi: 10.1021/ol071108n. [DOI] [PubMed] [Google Scholar]
- 12.For a recent review of enantioselective gold(I) catalysis see: Pradal A, Toullec PY, Michelet V. Synthesis. 2011:1501–1514.
- 13.Bandini M, Eichholzer A. Angew Chem. 2009;121:9697–9701. [Google Scholar]; Angew Chem Int Ed. 2009;48:9533–9537. doi: 10.1002/anie.200904388. [DOI] [PubMed] [Google Scholar]
- 14.Bandini M, Monari M, Romaniello A, Tragni M. Chem Eur J. 2010;16:14272–14277. doi: 10.1002/chem.201002606. [DOI] [PubMed] [Google Scholar]
- 15.For additional examples of the catalytic enantioselective addition of carbon and oxygen nucleophiles to underivatized allylic alcohols see reference 9 and also: Roggen M, Carreira EM. Angew Chem. 2011;123:5683–5686.Angew Chem Int Ed. 2011;50:5568–5571. doi: 10.1002/anie.201007716.Tanaka S, Seki T, Kitamura M. Angew Chem. 2009;121:9110–9113. doi: 10.1002/anie.200904671.Angew Chem Int Ed. 2009;48:8948–58951.Rueping M, Nachtsheim BJ, Moreth SA, Bolte M. Angew Chem. 2008;120:603–606. doi: 10.1002/anie.200703668.Angew Chem Int Ed. 2008;47:593–596. doi: 10.1002/anie.200703668.Trost BM, Quancard J. J Am Chem Soc. 2006;128:6314–6315. doi: 10.1021/ja0608139.
- 16.A full description of optimization studies is provided in the Supporting Information.
- 17.Employment of a 1:1 mixture of [(S)-2](AuCl)2 and AgSbF6 led to significant decrease in both rate and enantioselectivity.[16]
- 18.The absolute configuration of (S)-3b was determined by comparison to the HPLC trace of (R)-3b.[11a] The S configuration of the heterocycles depicted in Table 2, entries 4 and 10 was established by chemical correlation. The absolute configurations of all other heterocycles were assigned by analogy.
- 19.Paton RS, Maseras F. Org Lett. 2009;11:2237–2240. doi: 10.1021/ol9004646. [DOI] [PubMed] [Google Scholar]
- 20.a) Magid RM. Tetrahedron. 1980;36:1901–1930. [Google Scholar]; b) Paquette LA, Stirling CJM. Tetrahedron. 1992;48:7383–7423. [Google Scholar]
- 21.Cheon CH, Kanno O, Toste FD. J Am Chem Soc. 2011;133:13248–13251. doi: 10.1021/ja204331w. [DOI] [PMC free article] [PubMed] [Google Scholar]