Abstract
Chemical investigation of two cultured cyanobacteria, Westiellopsis sp. (SAG strain number 20.93) and Fischerella muscicola (UTEX strain number LB1829) led to the isolation of three hapalindole-type alkaloids, namely hapalindole X (1), deschloro hapalindole I (2), and 13-hydroxy dechlorofontonamide (3), along with ten known indole alkaloids (hapalindoles A, C, G, H, I, J, and U, hapalonamide H, anhydrohapaloxindole A, and fischerindole L) and fischerellins A and B. The structures were determined by a combination of spectroscopic analyses mainly based on 1D and 2D NMR and HRESIMS data. Selected compounds were evaluated for cytotoxicity and exhibited weak to moderate cytotoxicity against HT-29, MCF-7, NCI-H460, SF268, and IMR90 cells. All compounds, except hapalindole C, were evaluated for 20S proteasome inhibition and displayed either weak or no inhibition at 25 μg/mL. Selected compounds were also evaluated for antimicrobial activity, and hapalindoles X (1) and A, and hapalonamide H showed potent activity against both M. tuberculosis and C. albicans with MIC values ranging from 0.6 to 2.5 μM.
1. Introduction
More than 4000 strains of cyanobacteria (blue-green algae) have been studied to date, with more than 1000 secondary metabolites described.1–4 This includes over 70 indole alkaloids from branched filamentous cyanobacteria belonging to the order Stigonematales.5–21 These alkaloids include hapalindoles,5–9 fischerindoles,10–12 welwitindolinones,11 ambiguines,13–17 hapalindolinones,18 hapaloxindoles,19–20 and fontonamides.19–20 All have polycyclic carbon skeletons derived from L-tryptophan and geraniol pyrophosphate and possess diverse biological activities, such as antibacterial, antifungal, and antialgal activities.5, 10, 21
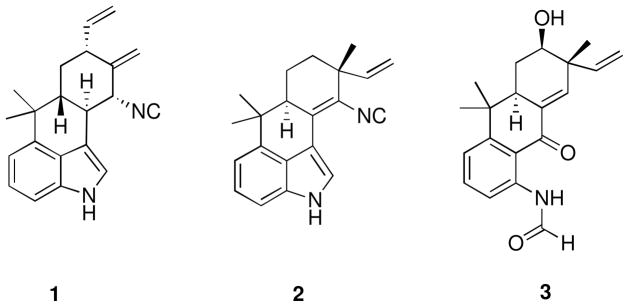
As part of our ongoing collaborative natural product drug discovery project,22 we evaluated organic extracts of cultured cyanobacteria for cytotoxicity using a set of human cancer cell lines designated HT-29 (colon), MCF-7 (breast), NCI-H460 (lung), and SF268 (CNS). The organic extract of Westiellopsis sp. (SAG 20.93) displayed cytotoxicity against HT-29 cells. Bioassay-guided fractionation led to isolation of hapalindole X (1) and deschloro hapalindole I (2) along with previously known hapalindoles A, C, G, H, I, J, and U, and hapalonamide H. The organic extract of Fischerella muscicola (UTEX LB1829) inhibited the growth of MCF-7, NCI-H460, and SF268 cells, and 13-hydroxy dechlorofontonamide (3) was isolated along with previously known hapalindoles A, H, I, and J, as well as fischerellins A and B. The three new hapalindole-type alkaloids were named hapalindole X (1), deschloro hapalindole I (2), and 13-hydroxy dechlorofontonamide (3) in line with the tradition used for previously reported hapalindole-type alkaloids from cyanobacteria (Figure 1). We herein present the isolation, structure elucidation, and bioactivity evaluation of these compounds.
Figure 1.
Structures of hapalindole X (1), deschloro hapalindole I (2), and 13-hydroxy dechlorofontonamide (3) isolated from Westiellopsis sp. (SAG 20.93) and Fischerella muscicola (UTEX LB1829)
*Absolute configurations shown are arbitrary as only relative configurations were determined in all three compounds.
2. Results and discussion
2.1. Structure elucidation
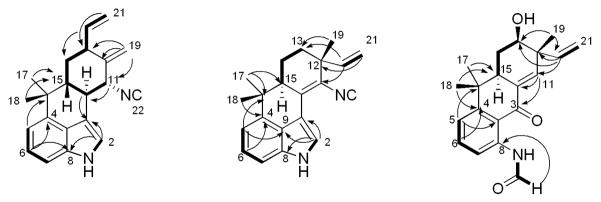
Hapalindole X (1) was obtained as a yellow amorphous powder. The HRESIMS pseudo-molecular [M+H]+ ion at m/z 303.1853 indicated a molecular formula of C21H22N2. The UV absorptions [λmax (log ε) 223 (6.48) 275 (7.32) nm] and the 1H NMR signals [δH 8.07 (H-1), 7.67 (H-2), 7.18 (H-6 and 7), and 7.02 (H-5)] indicated the presence of an indole moiety. Resonances at δH 5.94 (H-20), 5.19 (H-21E), and 5.14 (H-21Z) and at δH 1.48 (H3-17) and 1.08 (H3-18) showed the presence of a vinyl and two methyl groups, respectively. The two resonances at δH 5.45 (H-19a) and 5.06 (H-19b) were assigned to an exomethylene moiety. In addition, one methylene [δH 2.11 (H-14eq) and 1.37 (H-14ax)] and four methines [δH 4.24 (H-11), 3.08 (H-10), 2.64 (H-13), and 1.77 (H-15)] were observed. Correlations observed in the COSY spectrum connected these latter resonances and the vinyl group to form a 7-carbon spin system (Figure 2 and Table 1). The 13C NMR spectrum of 1 displayed all 21 carbon resonances required by the molecular formula. Of the thirteen resonances observed between 100 and 160 ppm, eight (δC 140.7, 133.7, 125.2, 123.3, 118.8, 113.4, 113.2, and 108.54) were assigned to the indole moiety and two (δC 139.1 and 116.3) were attributed to a vinyl group. The carbon resonances at δC 144.7 and 108.53 were assigned to an exomethylene moiety, and the final carbon resonance at δC 159.5 was assigned to an isonitrile moiety. These partial structures were connected by correlations observed in the HMBC spectrum (Figure 2 and Table 1). A correlation from H-5 to C-16 connected the indole moiety to the gem-dimethyl group. Correlations from both H3-17 and H3-18 to C-15 further connected this group to the 7-carbon H-11 to H2-21 fragment established in the COSY spectrum. The exomethylene moiety was placed at C-12 by a correlation from H-19a to C-12, and connected to C-11 and C-13 by correlations from both H-19a and H-19b to C-11 and C-13. The final connectivity from C-10 to C-3 was determined by a correlation from H-10 to C-3, and completed the planar structure of 1. Although a direct correlation from H-11 to C-22 was not observed, the 13C NMR chemical shift at δC 159.5 and a characteristic IR peak at 2142 cm−1 indicated the presence of an isonitrile. The position of the isonitrile at C-11 was determined based on the 13C NMR chemical shift of C-11 (δC 63.0). The relative configuration of 1 was determined by correlations observed from 2D NOESY spectrum (Table 1). Correlations observed between H-13 and H-15, H-11, and H-14eq indicated H-11, H-13, and H-15 all to be in the same plane. Correlations observed between H-14ax and H-10 indicated these to be in the opposite plane. Thus, the relative configuration of 1 was established as 10S*, 11R*, 13R*, and 15R*.
Figure 2.
Key COSY (▬) and HMBC (
 ) correlations of compounds 1–3
) correlations of compounds 1–3
Table 1.
NMR data of hapalindole X (1) in CDCl3
| Position | δC, mult.a | δH | mult. (J in Hz)b | COSYb | HMBCb | NOESYb |
|---|---|---|---|---|---|---|
| 1 | 8.07 | br s | 2 | 2 | ||
| 2 | 118.8, CH | 7.67 | t (1.7) | 1, 10c | 3, 8, 9 | 1, 10, 11 |
| 3 | 113.4, C | |||||
| 4 | 140.7, C | |||||
| 5 | 113.2, CH | 7.02 | d (6.8) | 6 | 7, 9, 16 | 17, 18 |
| 6 | 123.3, CH | 7.18 | t (6.8) | 5, 7 | 4, 8 | |
| 7 | 108.54, CH | 7.18 | d (6.8) | 5, 9 | ||
| 8 | 133.7, C | |||||
| 9 | 125.2, C | |||||
| 10 | 42.7, CH | 3.08 | t (11.1) | 11, 15 | 2, 3, 11, 15 | 11, 14ax, 15, 18 |
| 11 | 63.0, CH | 4.24 | d (11.1) | 10, 19a, 19b | 10, 12 | 10, 13, 15 |
| 12 | 144.7, C | |||||
| 13 | 45.9, CH | 2.64 | br t | 14eq, 14ax, 20 | 11, 14eq, 15, 20 | |
| 14eq | 33.1, CH2 | 2.11 | dt (12.6, 3.0) | 13, 14ax, 15 | 10, 12 | 13, 14ax, 15 |
| 14ax | 1.38 | ddd (12.6) | 13, 14eq, 15 | 10, 12, 13, 15, 20 | 10, 14eq, 18 | |
| 15 | 48.7, CH | 1.77 | ddd (12.6, 11.1, 3.0) | 10, 14eq, 14ax | 11, 13, 14eq | |
| 16 | 37.9, C | |||||
| 17 | 25.08, CH3 | 1.48 | s | 18 | 4, 15, 16, 18 | 5 |
| 18 | 25.05, CH3 | 1.08 | s | 17 | 4, 15, 16, 17 | 5, 10 |
| 19a | 108.53, CH2 | 5.45 | t (1.7) | 11 | 11, 12, 13 | 11, 19b |
| 19b | 5.06 | t (1.7) | 11 | 11, 13 | 19a, 20 | |
| 20 | 139.1, CH | 5.94 | ddd (17.6, 10.4, 7.8) | 13, 21E, 21Z | 13, 14 | 14ax, 21E, 21Z |
| 21E | 116.3, CH2 | 5.19 | dd (10.4, 1.7) | 20 | 13 | |
| 21Z | 5.14 | dt (17.6, 1.7) | 20 | 13, 20 | 13, 20 | |
| 22 | 159.5, C |
Recorded at 225 MHz.
Recorded at 600 MHz.
A four-bond correlation resulting from “W” configuration.
Deschloro hapalindole I (2) was obtained as a white amorphous powder. The HRESIMS pseudo-molecular [M+H]+ ion at m/z 303.1863 indicated a molecular formula of C21H22N2. The 1H NMR signals of an indole moiety [δH 8.32 (H-1), 7.89 (H-2), 7.206 (H-7), 7.202 (H-6), and 7.04 (H-5)], a vinyl group [δH 5.94 (H-20), 5.21 (H-21E), and 5.18 (H-21Z)], and three methyl singlets [δH 1.52 (H3-17), 1.36 (H3-19), and 1.04 (H3-18)] indicated 2 to be a hapalindole-type alkaloid. The UV absorptions [λmax (log ε) 223 (8.31) 247 (7.63) 280 (7.32) 324 (7.67)] were markedly similar as those reported for hapalindole I, structure of which contained a chlorine atom on position 13.6 Taken together, 2 was suggested to be the deschloro derivative of hapalindole I and this was explained by a comparison of the 1H NMR chemical shifts. The major differences between hapalindole I and 2 were observed at H-13 [δH 4.14 vs δH 1.76 (H-13eq) and 1.72 (H-13ax) in 2] and H-14 [H-14eq (δH 2.41 vs δH 1.97 in 2) and H-14ax (δH 2.23 vs δH 1.83 in 2)]. In addition, minor differences in the 1H NMR chemical shifts were observed around H-13 [H-21E (δH 5.46 vs δH 5.21 in 2), H-21Z (δH 5.36 vs δH 5.18 in 2), H-20 (δH 5.85 vs δH 5.94 in 2), and H3-19 (δH 1.47 vs δH 1.36 in 2)]. The planar structure was confirmed by correlations observed in the COSY and HMBC spectra (Figure 2 and Table 2). The correlation to an isonitrile moiety was not observed in the HMBC spectrum and amount of the compound was not enough to obtain a carbon spectrum. Howevr, the presence of the isonitrile moiety was confirmed by a characteristic IR peak at 2135 cm−1. The 2D NOESY spectrum was analyzed to determine the relative configuration of 2. Correlations observed between H-14ax and H3-18 and H3-19, and between H-15 and H-13ax, H-14eq, and H3-17 indicated H-15 and H3-19 to be in the opposite plane. Thus, the relative configuration of 2 was established as 12R* and 15S*, which is identical to that observed for hapalindole I.
Table 2.
NMR data of deschloro hapalindole I (2) in CDCl3
| Position | δC, mult.a | δH | mult. (J in Hz)b | COSYb | HMBCb | NOESYb |
|---|---|---|---|---|---|---|
| 1 | 8.32 | br s | 2 | |||
| 2 | 123.3, CH | 7.89 | d (2.4) | 1 | 3, 8, 9 | |
| 3 | 109.9, C | |||||
| 4 | 140.7, C | |||||
| 5 | 114.5, CH | 7.04 | m | 6 | 4, 7, 9, 10, 16 | 17, 18 |
| 6 | 124.4, CH | 7.202 | m | 7 | 4, 5, 8 | |
| 7 | 109.2, CH | 7.206 | m | 6 | 5, 9 | |
| 8 | 133.1, C | |||||
| 9 | 124.9, C | |||||
| 10 | 132.4, C | |||||
| 11 | 121.6, C | |||||
| 12 | 40.6, C | |||||
| 13eq | 35.8, CH2 | 1.76 | m | 14ax | 12, 19 | 19 |
| 13ax | 1.72 | m | 13eq | 15 | ||
| 14eq | 20.1, CH2 | 1.97 | m | 14ax, 15 | 13 | 15 |
| 14ax | 1.83 | m | 13eq, 14eq, 15 | 13 | 18, 19 | |
| 15 | 47.4, CH | 2.66 | dd (9.6, 6.6) | 14eq, 14ax | 10, 11, 16, 17 | 13ax, 14eq, 14ax, 17 |
| 16 | 38.9, C | |||||
| 17 | 24.6, CH3 | 1.52 | s | 18 | 4, 5, 16, 18 | |
| 18 | 26.1, CH3 | 1.04 | s | 17 | 4, 5, 16, 17 | 14ax |
| 19 | 23.6, CH3 | 1.36 | s | 11, 12, 13, 20 | ||
| 20 | 145.2, CH | 5.94 | dd (17.4, 10.8) | 21E, 21Z | 11, 12, 13, 20 | 13ax, 19 |
| 21E | 114.7, CH2 | 5.21 | d (10.8) | 20 | 12 | 19 |
| 21Z | 5.18 | d (17.4) | 20 | 12, 20 | 19 | |
| 22 | c |
Chemical shifts determined from gHSQC and gHMBC experiments recorded at 600 MHz.
Recorded at 600 MHz.
Signal not observed.
13-hydroxy dechlorofontonamide (3) was obtained as a pale yellow amorphous powder. The HRESIMS pseudo-molecular [M-H]− ion at m/z 324.1630 indicated a molecular formula of C20H23NO3. The 1H NMR chemical shifts of 3 (Table 3) closely resembled those reported for dechlorofontonamide,20 with the major differences being observed at H-13 [δH 1.73 (H-13eq) and 1.64 (H-13ax) vs δH 4.05 in 3] and H-14 [H-14eq (δH 1.96 vs δH 2.32 in 3) and H-14ax (δH 1.69 vs δH 2.09 in 3)]. Some additional minor differences in the 1H NMR chemical shifts between dechlorofontonamide and 3 were observed at H-21E (δH 5.05 vs δH 5.20 in 3), H-21Z (δH 5.01 vs δH 5.18 in 3), H-20 (δH 5.85 vs δH 5.92 in 3), and H3-19 (δH 1.19 vs δH 1.35 in 3). Together, these findings indicated 3 to be the hydroxyl derivative of dechlorofontonamide, consistent with the molecular formula of C20H23NO3. The hydroxyl group was placed at C-13 by analysis of the COSY spectrum, which indicated a CH-CH2-CH spin system from C-15 to C-13, combined with the 13C NMR chemical shift of C-13 (δC 64.8) (Table 3). The relative configuration of 3 was determined by analysis of 2D NOESY spectrum. Correlations observed between H-13 and H-15 and H-14eq indicated H-13 and H-15 to be in the same plane. Correlations observed between H-14ax and H3-18 and H3-19 indicated H-15 and H3-17 to be in the opposite plane. Thus, the relative configuration of 3 was established as 12R*, 13S*, and 15S*, which is identical to those reported for both fontonamide and dechlorofontonamide.
Table 3.
NMR data of 13-hydroxy dechlorofontonamide (3) in CDCl3
| Position | δC, mult.a | δH | mult. (J in Hz)b | COSYb | HMBCb | NOESYb |
|---|---|---|---|---|---|---|
| 1 | 11.67 | s | 2 | 2 | ||
| 2 | 159.9, CH | 8.47 | s | 1 | 8 | 1 |
| 3 | 188.9, C | |||||
| 4 | 153.7, C | |||||
| 5 | 118.8, CH | 7.19 | d (8.2) | 6 | 7, 9, 16 | 17 |
| 6 | 135.3, CH | 7.51 | t (8.2) | 5, 7 | 4, 8 | 5 |
| 7 | 119.3, CH | 8.59 | d (8.2) | 6 | 5 | |
| 8 | 140.8, C | |||||
| 9 | 117.7, C | |||||
| 10 | 133.6, C | |||||
| 11 | 144.5, CH | 7.10 | d (2.4) | 15 | 3, 10, 13, 15, 20 | 19 |
| 12 | 44.0, C | |||||
| 13 | 64.8, CH | 4.05 | dd (12.8, 3.4) | 14eq, 14ax | 14, 15, 19, 20 | 14eq, 15, 20 |
| 13-OH | 1.54 | s | ||||
| 14eq | 30.8, CH2 | 2.32 | ddd (12.8, 6.0, 3.4) | 13, 14ax, 15 | 10, 12, 13 | 13, 15, 17 |
| 14ax | 2.09 | q (12.8) | 13, 14eq, 15 | 12, 13, 16 | 18, 19 | |
| 15 | 44.2, CH | 2.82 | ddd (12.8, 6.0, 2.4) | 14eq, 14ax | 11, 14, 16, 18 | 13, 17 |
| 16 | 37.8, C | |||||
| 17 | 24.2, CH3 | 1.44 | s | 4, 15, 16, 18 | 5 | |
| 18 | 25.4, CH3 | 1.04 | s | 4, 15, 16, 17 | ||
| 19 | 19.9, CH3 | 1.35 | s | 11, 12, 13, 20 | 11, 20, 21Z | |
| 20 | 142.9, CH | 5.92 | dd (17.4, 10.7) | 21E, 21Z | 11, 12, 13, 19 | 19 |
| 21E | 115.1, CH2 | 5.20 | d (10.7) | 20 | 12, 13 c | |
| 21Z | 5.18 | d (17.4) | 20 | 12, 20 | 19 |
Chemical shifts determined from gHSQC and gHMBC experiments recorded at 600 MHz.
Recorded at 600 MHz.
A four-bond correlation resulting from “W” configuration.
2.2. Biological evaluation
Selected compounds were evaluated for cytotoxicity against HT-29 (colon), MCF-7 (breast), NCI-H460 (lung), and SF268 (CNS) cancer cells, and IMR90 (lung) cells (Table 4). Hapalindole X (1) showed moderate cytotoxicity against all four cancer cells and relatively low cytotoxicity against IMR90 cells. The most cytotoxic compound was hapalindole H with IC50 values ranging from 8.5 to 31.9 μM, M, while 13-hydroxy dechlorofontonamide (3) was inactive in all cell lines tested. Mo et al previously reported antimicrobial activities of hapalindole-related alkaloids,16,17 and selected compounds were further evaluated for antimicrobial activity against a panel of microorganisms including M. tuberculosis, M. smegmatis, C. albicans, E. coli, and A. baumanii as well as for Vero cell toxicity (Table 5). Hapalindole X (1) displayed antimicrobial activities against both M. tuberculosis and C. albicans with MIC values of 2.5 μM and moderate cytotoxicity in the Vero cell assay with an IC50 value of 35.2 μM. Hapalindole I showed inhibitory activity against M. tuberculosis with a MIC value of 2.0 μM and no detectable cytotoxicity against Vero cells. Hapalindole A was the most active compound against M. tuberculosis with a MIC value of less than 0.6 μM. Both deschloro hapalindole I (2) and 13-hydroxy dechlorofontonamide (3) showed no antimicrobial activity. None of the evaluated compounds inhibited the growth of A. baumanii. Only hapalindole A inhibited the growth of the gram-negative bacteria E. coli (MIC value of 8.0 μM).
Table 4.
Cytotoxic activity of selected compounds isolated from Westiellopsis sp. (SAG 20.93) and Fischerella muscicola (UTEX LB1829)
| IC50 (μM)
|
|||||
|---|---|---|---|---|---|
| HT-29 a | MCF-7 b | NCI-H460 c | SF268 d | IMR90 e | |
| Hapalindole X (1) | 24.8 | 35.4 ± 2.8 | 23.0 ± 4.6 | 23.5 ± 9.5 | 113.2 ± 13.2 |
| 13-hydroxy dechlorofontonamide (3) | NA | >100 | >100 | >100 | >100 |
| Hapalindole I | NA | >100 | 68.5 ± 11.0 | 93.1 ± 14.0 | >100 |
| Hapalindole J | 28.6 | 43.7 ± 10.0 | 12.0 ± 1.9 | 16.9 ± 3.4 | 39.1 ± 6.6 |
| Hapalindole A | 31.3 | 30.7 ± 7.1 | 17.0 ± 4.8 | 16.3 ± 7.1 | 39.1 ± 10.2 |
| Hapalindole U | 52.6 | >100 | >100 | >100 | >100 |
| Hapalindole C | 52.6 | >100 | 53.7 ± 15.5 | 88.6 ± 17.6 | >100 |
| Hapalindole H | 10.8 | 16.3 ± 3.3 | 8.5 ± 3.7 | 10.6 ± 4.5 | 31.9 ± 11.6 |
| Anhydrohapaloxindole A | NT | 56.7 ± 10.5 | 18.7 ± 6.2 | 26.0 ± 9.0 | 80.1 ± 24.5 |
| Fischerindole L | 48.2 | 28.3 ± 8.1 | 15.1 ± 2.6 | 17.4 ± 8.6 | 46.3 ± 13.7 |
| Camptothecin | NT | 0.08 ± 0.1 | 0.002 ± 0.0 | 0.07 ± 0.1 | 0.43 ± 0.5 |
Human colon adenocarcinoma.
Human breast carcinoma.
Human large cell lung carcinoma.
Human glioblastoma cancer cell.
Human lung fibroblast normal cell. IC50 values were obtained by averaging the results from three independent experiments. NT represents not tested. NA represents not active.
Table 5.
MIC and IC50 values for selected compounds isolated from and Westiellopsis sp. (SAG 20.93) and Fischerella muscicola (UTEX LB1829)
| MIC (μM)
|
IC50 (μM)
|
||||||
|---|---|---|---|---|---|---|---|
| M. tuberculosis a | M. smegmatis b | C. albicans c | S. aureus d | E. coli e | A. baumanii f | Vero | |
| Hapalindole X (1) | 2.5 | 78.8 | 2.5 | 9.1 | > 100 | > 100 | 35.2 |
| Deschloro hapalindole I (2) | > 100 | > 100 | > 100 | > 100 | > 100 | > 100 | > 100 |
| 13-hydroxy dechlorofontonamide (3) | > 100 | > 100 | > 100 | > 100 | > 100 | > 100 | > 100 |
| Hapalindole I | 2.0 | > 100 | > 100 | > 100 | > 100 | > 100 | > 100 |
| Hapalindole J | 4.3 | 39.0 | 0.7 | 8.4 | > 100 | > 100 | 31.9 |
| Hapalindole A | < 0.6 | 18.2 | 1.2 | 3.9 | 8.0 | > 100 | 25.6 |
| Anhydrohapaloxindole A | 16.2 | >100 | 1.9 | 27.3 | > 100 | > 100 | 79.9 |
| Hapalonamide H | 1.2 | 34.3 | < 0.6 | > 100 | > 100 | > 100 | 13.6 |
| Fischerindole L | 22.0 | 63.0 | 1.2 | 6.4 | > 100 | > 100 | < 9.2 |
| Fischerellin A | 43.1 | >100 | > 100 | > 100 | > 100 | > 100 | 94.8 |
| Fischerellin B | 23.0 | >100 | > 100 | > 100 | > 100 | > 100 | > 100 |
| Rifampin | 0.24 | ||||||
| Streptomycin | 0.41 | ||||||
| Amphotericin B | 0.12 | ||||||
| Ampicillin | 1.54 | ||||||
| Gentamycin | 2.3 | ||||||
| Doxycycline | 0.35 | ||||||
Mycobacterium tuberculosis.
Mycobacterium smegmatis.
Candida albicans.
Staphylococcus aureus.
Escherichia coli.
Acinetobacter baumannii.
3. Conclusions
The bioassay-guided investigation of two cultured cyanobacteria, Westiellopsis sp. and Fischerella muscicola, both belonging to the order Stigonematales, led to the isolation of three new hapalindole-type alkaloids. Hapalindole X (1) represents the first report of a tetracyclic hapalindole with an exocyclic methylene moiety. This moiety was found at C-12, a position substituted with a vinyl group and a methyl group in all previously reported tetracyclic hapalindoles. In hapalindole X (1), the vinyl group was located at C-13, suggesting the presence of a new biosynthetic pathway in this organism. Most hapalindoles have been reported in both chlorinated and deschlorinated forms, and we herein report the isolation of deschlorinated hapalindole I. Hapalonamides are considered oxidation products of the hapalindoles,19 and it can be assumed that 13-hydroxy dechlorofontonamide (3) is the oxidation product of hapalindole A.
4. Experimental
4.1. General experimental procedures
Optical rotations were measured with a Perkin-Elmer 241 polarimeter. UV spectra were recorded on a Varian Cary 50 Bio spectrophotometer. IR spectra were obtained on a FTIR-410 Fourier transform infrared spectrometer. 1D and 2D NMR spectra were obtained at room temperature on a Bruker Avance DRX 600 MHz spectrometer with a 5 mm CPTXI Z-gradient probe. 13C NMR spectrum was obtained on a Bruker AV 900 MHz NMR spectrometer with a 5 mm ATM CPTCI Z-gradient probe. 1H and 13C chemical shifts were referenced to a residual proton, 7.24 and 77.0 ppm in CDCl3. HRESIMS were obtained using a Shimadzu IT-TOF spectrometer.
4.2. Biological material
Westiellopsis sp. and Fischerella muscicola were initially acquired from the culture collections of Algae at the University of Göttingen (SAG strain number 20.93) and at the University of Texas at Austin (UTEX strain number LB1829), respectively. Each cyanobacterium was grown in a 2.8 L Fernbach flask containing 2 L of inorganic media (Z media for SAG 20.93 and BG-12 media for UTEX LB1829) under aeration.23 Cultures were illuminated with fluorescent lamps at 1.93 klx with an 18/6 hours light/dark cycle at 22 °C. After eight weeks, the biomass of each cyanobacterium was harvested by centrifugation, and lyophilized.
4.3. Extraction and isolation
The lyophilized biomass (1.00 g) of Westiellopsis sp., from total of 4 L culture, was extracted by maceration with CH2Cl2/MeOH (1:1 v/v) at room temperature and the solvent was evaporated in vacuo. The extract (330.7 mg) was redissolved in CH2Cl2/MeOH (1:1 v/v) and mixed with Diaion® HP20SS resin. The mixture was dried in vacuo and the dried mixture was fractionated on a Diaion® column using a step gradient with increasing amount of 2-propanol in water to afford eight fractions. Fractions 5–7, eluting with 70%, 80%, and 90% 2-propanol, respectively, displayed cytotoxicity against HT-29 cells. Fractions 5 and 6 were subjected to HPLC-ESIMS analysis (Varian Microsob C8, 2.0 × 250 mm, flow rate 0.2 mL/min) with 30–100% MeOH gradient over 35 min containing 0.1% formic acid. The analysis of MS spectra indicated the presence of potentially new indole alkaloids. Fractions 5 and 6 were combined and further subjected to reversed-phase HPLC (column Alltima C8 Dynamax, 10 × 250 mm, flow rate 3 mL/min) with 70–85% MeOH/H2O gradient over 40 min and yielded 1 (1.05 mg, tR 40.0 min) and 2 (0.62 mg, tR 42.8 min) along with hapalonamide H (1.02 mg, tR 16.0 min), hapalindoles U (0.66 mg, tR 26.2 min), C (0.41 mg, tR 28.5 min), G (0.29 mg, tR 29.3 min), J (2.92 mg, tR 30.5 min), A (5.15 mg, tR 32.4 min), H (4.18 mg, tR 37.8 min), and I (0.64 mg, tR 43.5 min). The extract (327.0 mg) of Fischerella muscicola was obtained from the lyophilized biomass (6.26 g from 13 L culture) as described above. Eight fractions were obtained using the Diaion fractionation protocol described above. The combined fractions 5–7 (70%, 80%, and 90% 2-propanol) were subjected to reversed-phase HPLC (column Alltima C8, 10 × 250 mm, flow rate 3.0 mL/min) with 80% MeOH isocratic to yield 3 (0.94 mg, tR 15.2 min) along with fischerindole L (1.09 mg, tR 13.3 min), hapalindoles J (1.14 mg, tR 14.1 min), A (3.86 mg, tR 15.9 min), and I (0.49 mg, tR 17.6 min), fischerellins A (1.06 mg, tR 18.8 min) and B (1.27 mg, tR 21.7 min). Fraction 6 was subjected to reversed-phase HPLC (Alltima C8, 10 × 250 mm, flow rate 3.0 mL/min) with 70–85% MeOH/H2O gradient over 50 min and yielded anhydrohapaloxindole A (1.13 mg, tR 26.8 min).
4.3.1. Hapalindole X (1)
Yellow amorphous powder; [ ]25D +138.0° (CHCl3, c 0.14); UV (MeOH) max (log ε) 223 (6.48) 275 (7.32); IR (neat) max 3410, 2969, 2142, 1630, 1437, 1374, 1093, 920, 825, 776, 749 cm−1; 1H and 13C NMR, see Table 1; HRESIMS m/z 303.1853 [M+H]+ (calcd for C21H23N2, 303.1861).
4.3.2. Deschloro hapalindole I (2)
White amorphous powder; [ ]25 D +11.0° (CHCl3, c 0.05); UV (MeOH) max (log ε) 223 (8.31) 247 (7.63) 280 (7.32) 324 (7.67); IR (neat) max 3478, 2924, 2135, 1683, 1601, 1457, 1377, 1207, 1138, 1056, 843, 749, 724 cm−1; 1H and 13C NMR, see Table 1; HRESIMS m/z 303.1863 [M+H]+ (calcd for C21H23N2, 303.1861).
4.3.3. 13-hydroxy dechlorofontonamide (3)
Pale yellow amorphous powder; [ ]25D −45.6° (CHCl3, c 0.05); UV (MeOH) max (log ε) 207 (4.99) 238 (5.40) 287 (5.27) 343 (4.83); IR (neat) max 3750, 3200, 1698, 1654, 1599, 1507, 1252, 902, 813 cm−1; 1H and 13C NMR, see Table 1; HRESIMS m/z 324.1630 [M-H]− (calcd for C20H22NO3, 324.1600).
4.4. Cytotoxicity assay
Cytotoxicity against NCI-H460, MCF-7, SF268, and IMR90 cells was measured as described previously.24 Cytotoxicity against HT-29 cells was measured using a commercially available kit according to the manufacturer’s instructions (CellTiter 96® Aqueous One Solution Cell Proliferation Assay, Promega Corp, Madison, WI, USA).
4.5. 20S proteasome assay
Evaluation of inhibition of the 20S proteasome was performed as previously described.25
4.6. Mycobacterium tuberculosis, other organisms and vero cell cytotoxicity
Supplementary Material
Acknowledgments
This research was supported by the National Cancer Institute (PO1CA125066). We thank the research Resources Center (RRC) at UIC for high-resolution mass spectrometry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Gademann K, Portmann C. Curr Org Chem. 2008;12:326–341. [Google Scholar]
- 2.Sielaff H, Christiansen G, Schwecke T. IDrugs. 2006;9:119–127. [PubMed] [Google Scholar]
- 3.Harvey AL. Drug Discov Today. 2008;13:894–901. doi: 10.1016/j.drudis.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Chlipala G, Mo S, Orjala J. Curr Drug Targets. 2011;11:1654–1673. doi: 10.2174/138945011798109455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore RE, Cheuk C, Patterson GML. J Am Chem Soc. 1984;106:6456–6457. [Google Scholar]
- 6.Moore RE, Cheuk C, Yang GX, Patterson GML. J Org Chem. 1987;52:1036–1043. [Google Scholar]
- 7.Klein D, Daloze D, Braekman JC. J Nat Prod. 1995;58:1781–1785. [Google Scholar]
- 8.Asthana RK, Srivastava A, Singh AP, Deepali, Singh SP. J Appl Phycol. 2006;18:33–39. [Google Scholar]
- 9.Becher PF, Keller S, Jung G, Sussmuth RD, Juttner F. Phytochemistry. 2007;68:2493–2497. doi: 10.1016/j.phytochem.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 10.Park A, Moore RE, Patterson GML. Tetrahedron Lett. 1992;33:3257–3260. [Google Scholar]
- 11.Stratmann K, Moore RE, Bonjouklian R, Deeter JB, Patterson GML, Shaffer S, Smith CD, Smitka TA. J Am Chem Soc. 1994;116:9935–9942. [Google Scholar]
- 12.Kim H, Krunic A, Lantvit D, Shen Q, Kroll JD, Swanson MS, Orjala J. Tetrahedron. 2012 doi: 10.1016/j.tet.2012.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smitka TA, Bonjouklian R, Doolin L, Jones ND, Deeter JB. J Org Chem. 1992;57:857–861. [Google Scholar]
- 14.Huber U, Moore RE, Patterson GML. J Nat Prod. 1998;61:1304–1306. doi: 10.1021/np9801561. [DOI] [PubMed] [Google Scholar]
- 15.Raveh A, Carmeli S. J Nat Prod. 2007;70:196–201. doi: 10.1021/np060495r. [DOI] [PubMed] [Google Scholar]
- 16.Mo S, Krunic A, Chlipala G, Orjala J. J Nat Prod. 2009;72:894–899. doi: 10.1021/np800751j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mo S, Krunic A, Santarsiero BD, Franzblau SG, Orjala J. Phytochemistry. 2010;71:2116–2123. doi: 10.1016/j.phytochem.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masuyama Y, Kinugawa N, Kurusu Y. J Org Chem. 1987;52:3704–3706. [Google Scholar]
- 19.Moore RE, Yang XG, Patterson GML. J Org Chem. 1987;52:3773–3777. [Google Scholar]
- 20.Moore RE, Yang XG, Patterson GML, Bonjouklian R, Smitka TA. Phytochemistry. 1989;28:1565–1567. [Google Scholar]
- 21.Richter JM, Ishihara Y, Masuda T, Whitefield BW, Llamas T, Pohjakallio A, Baran PS. J Am Chem Soc. 2008;130:17938–17954. doi: 10.1021/ja806981k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kinghorn AD, Carcache-Blanco EJ, Chai H-B, Orjala J, Farnsworth NR, Soejarto DD, Oberlies NH, Wani MC, Kroll DJ, Pearce CJ, Swanson SM, Kramer RA, Rose WC, Fairchild CR, Vite GD, Emanuel S, Jajoura D, Cope FO. Pure Appl Chem. 2009;81:1051–1063. doi: 10.1351/PAC-CON-08-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson RA, Berges JA, Harrison RJ, Watanabe MM. In Algal Culturing Techniques. Elsevier Academic Press; Burlington, MA: 2005. [Google Scholar]
- 24.Alali FQ, El-Elimat T, Li C, Qandil A, Alkofahi A, Tawaha K, Burgess JP, Nakanishi Y, Kroll DJ, Navarro HA, Falkinhan JO, Wani MC, Oberlies NH. J Nat Prod. 2005;68:173–178. doi: 10.1021/np0496587. [DOI] [PubMed] [Google Scholar]
- 25.Chlipala G, Mo S, De Blanco EJC, Ito A, Bazarek S, Orjala J. J Pharm Biol. 2009;47:53–60. doi: 10.1080/13880200802415483. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.