Abstract
Purpose
Acanthamoeba keratitis is associated with keratocyte depletion in humans. We investigated how Acanthamoebae isolated from corneas affected by Acanthamoeba keratitis interacted with human corneal stromal cells in vitro.
Methods
Acanthamoebae were isolated from 6 patients with Acanthamoeba keratitis and genotyping was done. Whether the isolated Acanthamoebae could invade the corneal stroma was assessed with denuded corneal stroma ex vivo. The cytopathic effect of Acanthamoeba on cultured corneal fibroblasts from donor corneas was quantitatively evaluated by the MTT assay after culture under various conditions. Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) and Annexin V staining were employed to detect apoptotic cells among the corneal fibroblasts co-cultured with Acanthamoebae.
Results
All 6 Acanthamoebae isolated from the patients with Acanthamoeba keratitis were shown to have the T4 genotype by 18S rDNA sequence analysis. Acanthamoebae invaded the denuded corneal stroma in the ex vivo experiments and had a cytopathic effect on human corneal fibroblasts after direct adhesion, but not via chemical mediators. A cytopathic effect was detected with all 6 Acanthamoebae and corneal fibroblasts mainly died by apoptosis, as evidenced by Annexin V staining.
Conclusions
Acanthamoebae isolated from patients with Acanthamoeba keratitis had a cytopathic effect on human corneal fibroblasts, mainly via induction of apoptosis after direct adhesion. Our findings may provide some clues to the pathophysiology of corneal keratocyte depletion in patients with Acanthamoeba keratitis.
Introduction
Acanthamoebae are free-living cyst-forming protozoans that can cause painful keratitis with the potential loss of vision [1,2]. Acanthamoeba keratitis (AK) is closely associated with use of contact lenses, but can also occur in non-contact lens wearers after corneal trauma or exposure to contaminated water [3-5]. Acanthamoebae are classified into 15 genotypes (T1-T15), among which the T4 genotype has been identified as the major cause of AK [6,7].
The human corneal epithelium contains immunoprotective dendritic cells as the first line of defense against corneal infection [8]. Creation of corneal epithelial damage before the application of Acanthamoeba-infected contact lenses is essential for the development of AK in experimental models [9,10]. Because AK is rare in comparison with the very large number of contact lens wearers, the occurrence of corneal epithelial damage seems to be a precondition leading to AK. Therefore, the interaction between Acanthamoeba and keratocytes could provide clues to the mechanism underlying the development of AK. Acanthamoeba has a cytopathic effect on various cells [11-16], and keratocyte depletion by invading trophozoites has been detected by histological examination of human corneas with AK [17], but it is still unknown whether Acanthamoeba has a direct or indirect effect on human corneal fibroblasts.
In the present study, we isolated Acanthamoebae from the corneas of patients with AK and obtained activated keratocytes (i.e., corneal fibroblasts) from human donor corneas. Then we investigated whether Acanthamoeba had any effect on cultured corneal fibroblasts. Our findings suggested that Acanthamoeba has a direct adverse influence on the survival of corneal fibroblasts.
Methods
Isolation of Acanthamoebae
Acanthamoeba isolates were obtained from the corneal scrapings of 6 patients with AK at the Medical Center East of Tokyo Women’s Medical University. The isolates were grown on non-nutrient agar plates with heat-killed Escherichea coli as a source of nutrients and were designated as follows: E44, E46, E51, E52, E57, and E58. This research was done according to the tenets of the Declaration of Helsinki and was approved by the institutional review board of Tokyo Women’s Medical University. Written informed consent was obtained from the patients with AK.
Isolation and culture of human corneal fibroblasts
Human corneas for research were obtained from the Northwest Lions Eye Bank (Seattle, WA) and the tissue outside the sclerocorneal button was removed. The endothelial layer of the cornea with Descemet’s membrane was removed as a sheet, and then the corneal epithelium was removed mechanically. Denuded corneas were treated with collagenase (2 mg/ml in low glucose D-MEM; Wako, Osaka, Japan) at 37 °C until a single cell suspension of corneal keratocytes was obtained. Then human corneal fibroblasts were cultured from the corneal keratocytes in D-MEM with 10% fetal bovine serum (FBS) at 37 °C under 5% CO2 in 6-cm diameter dishes (Thermo Fisher Scientific, Roskilde, Denmark), glass-bottomed culture dishes (MatTek Corporation, Ashland, MA), and 24-well plates (Thermo Fisher Scientific). Cells were used for the present experiments after two to four passages.
Isolation and sequencing of Acanthamoeba DNA
Acanthamoebae were grown in PYGC medium (10 g proteose peptone, 10 g yeast extract, 1 g glucose, 5 g NaCl and 1 g L-Cysteine in 1,000 ml of 5 mM phosphate buffer pH 7.0) [18] at 30 °C for 3–4 days in 25 cm2 culture flasks, and then were harvested and washed with phosphate-buffered saline (PBS). DNA was extracted using the QIAmp DNA mini kit® (Qiagen, Valencia, CA) according to the manufacturer’s instructions. To identify each Acanthamoeba strain, a fragment of the 18S rDNA gene was amplified using two Acanthamoeba-specific primers (JDP1 and JDP2) [19,20]. A 25 µl reaction mixture including 1 µl of extracted DNA was prepared and PCR was performed with a thermal cycler (GeneAmp PCR System; Applied Biosystems, Carlsbad, CA) using 45 cycles of 94 °C for 30 s, 60 °C for 45 s, and 72 °C for 30 s.
The amplified fragments of 18S rDNA were visualized by 1.5% agarose gel electrophoresis with ethidium bromide staining and compared to DNA size markers. Amplicons were analyzed with a 310 ABI PRISM automated sequencer (Applied Biosystems) and the sequences obtained were compared with those published in sequence databases (e.g., Genebank) by using the BLAST search program. The genotype of each isolate was identified by comparison with the previously reported reference sequences of each T type.
Ex vivo invasion of Acanthamoeba into corneal stroma
Denuded corneas without epithelial or endothelial cells were placed endothelial side up in the wells of a 24-well plate containing D-MEM. Acanthamoebae (1×105; >95% trophozoites) were added to the center of each cornea and incubated at 37 °C under serum-free conditions. Two days later, the corneas were fixed in 10% formalin and stained with hematoxylin and eosin (HE) solution for light microscopy. Besides, Acanthamoebae were checked not to transform into cyst form in 37 °C condition.
Cytopathic effect of Acanthamoeba on corneal fibroblasts
After human corneal fibroblasts were grown to form confluent monolayers, the culture dishes were washed three times with PBS to remove the medium containing FBS and then serum-free D-MEM was added. Next, Acanthamoebae (1×103 E44, >95% trophozoites) were carefully added to the center of each 6-cm diameter dish and incubated at 37 °C for 2 days.
To test whether the cytopathic effect of Acanthamoeba was due to direct adherence to corneal fibroblasts or to soluble factors such as chemical mediators, we used insert culture dishes with 0.4 µm pores (Transwell; Corning Life Sciences, Corning, NY), and corneal fibroblasts were cultured in the outer plate. Acanthamoebae could not pass through these pores under any culture conditions (data not shown). We used 6-well plates with insert culture dishes to investigate the morphological change of corneal fibroblasts. We prepared four times as many Acanthamoebae (4×104) as 24-well plates (1×104) based on our experiments, because the well size of 6-well plate is about four times as much as one of 24-well plate. The number of 1×104 Acanthamoeba used in 24-well plate was the highest number used in our experiments. Corneal fibroblasts were harvested by using 0.05% trypsin. The optical density of each well was read using a Cell Proliferation Kit I (MTT assay; Roche Molecular Biochemicals, Mannheim, Germany) at 655 nm in a microplate reader (Bio-Rad Laboratories, Hercules, CA) to quantify viable cells.
Next, Acanthamoebae (1.3×103 to 1×104) were added to 4 wells of a 24-well plate and incubated at 37 °C for 2 days. Dishes without Acanthamoebae were used as the negative control. After incubation, corneal fibroblasts were harvested by using 0.05% trypsin and the optical density was determined with the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) assay. This assay was done in the 6 different isolates of Acanthamoeba using quadricate samples (1×104 of E44, E46, E51, E52, E57, and E58).
TUNEL assay
Corneal fibroblasts were cultured in 5 ml flasks (Thermo Fisher Scientific) until confluence and 1×105 Acanthamoebae (E44) were added. After 4 days, cells were collected and the terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick-end labeling (TUNEL) assay was done to detect DNA fragmentation in apoptotic cells using an in situ Apoptosis Detection Kit (TaKaRa, Shiga, Japan). Corneal fibroblasts cultured without Acanthamoebae were used as the negative control, while corneal fibroblasts in dishes with Actinomysin D, Streptomyces sp. (MERCK, Darmstadt, Germany) were the positive control.
Quantification of apoptotic cells
Acanthamoebae (1×104) were added to human corneal fibroblasts and cultured at 37 °C for up to 5 days under serum-free conditions. On days 0, 1, 2, 3, 4, and 5 of incubation, the corneal fibroblasts were harvested using 0.05% trypsin-EDTA (Invitrogen, Tokyo, Japan) and were centrifuged at 200× g for 5 min at 4 °C. After removal of the supernatant, cells were labeled with Annexin V-FITC and propidium iodide (PI) by using an Annexin V-FITC apoptosis detection kit (Beckman Coulter, Brea, CA) according to the manufacturer’s instructions. Fluorescence from FITC and PI was detected under a fluorescence microscope (Axioplan 2 Imaging; Micro-optik, Deursen, Netherlands) at 518 nm and 620 nm, respectively. FITC/Annexin V-positive cells were counted in ten fields because these cells were regarded as being in the initial stage of apoptosis. FITC/Annexin V-negative and PI-negative (unstained) cells were defined as viable cells. While FITC/Annexin V-positive cells showing green fluorescence were regarded as early apoptotic cells, FITC/Annexin-positive and PI-positive cells showing red and green fluorescence were regarded as necrotic cells, because PI-positive cells include both necrotic cells and cells that have gone through apoptosis. The percentage of apoptotic cells among total cells was calculated.
Statistical analysis
Statistical comparisons between two groups were performed by the Mann–Whitney U-test. For multiple comparisons among groups, one-way ANOVA was used and then a post-hoc least significant difference test was performed. Statistical significance was set at p<0.05.
Results
Genotyping of Acanthamoebae isolates
The isolates were termed E44, E46, E51, E52, E57, and E58. Based on 18S rDNA sequence analysis, all of these Acanthamoebae isolates belonged to the T4 genotype, but their sequences varied. Table 1 summarizes the results of genotyping and the BLAST search findings. The sequences of the isolates showed 98~100% correspondence with those of clinical isolates reported previously.
Table 1. Results of genotyping of the isolates and BLAST search.
| Isolate | Sex | Age (y) | Strain [GeneBank] | Tissue source | Homology | Genotype |
|---|---|---|---|---|---|---|
| E44 |
F |
35 |
ATCC 50497 [U07410] |
cornea |
100% |
T4 |
| E46 |
M |
28 |
ATCC 30461 [AY026243] |
cornea |
99% |
T4 |
| E51 |
F |
34 |
AC 29 [AB554228] |
cornea |
99% |
T4 |
| E52 |
M |
59 |
CDC V390 [AY703004] |
cornea |
99% |
T4 |
| E57 |
F |
47 |
CDC V062 [AY702989] |
cornea |
100% |
T4 |
| E58 | M | 17 | CDC V029 [U07402] | cornea | 98% | T4 |
We summarized the results of each isolates. E44, 46, 51, 52, 57, and 58. Those isolates were analyzed based on 18S rDNA and compared with isolates reported previously. “Strain” shows isolates that had high homology(“Homology”) with isolates examined in our hospital. “Tissue source ” shows the organ where “Strain ” isolates were obtained. “Genotype” shows each isolates’ genotype in 18S rDNA classification. All isolates were T4 genotype. F; female, M; male, CDC; Centers for Disease Control and preventation, ATCC; American Type Culture Collection.
Effect of Acanthamoeba on denuded human corneal stroma
We used the E44 isolate throughout our experiments because it was the first Acanthamoeba isolated at our hospital that had the typical morphological and proliferative features of Acanthamoebae. To test whether E44 could invade human corneal stroma, trophozoites were added to denuded corneal stroma and incubated at 37 °C for 2 days. HE staining showed Acanthamoebae in the inside the corneal stroma (Figure 1), indicating that the E44 isolate of Acanthamoeba could attach to the corneal surface and invade the corneal stroma through fine collagen fibrils. Similar findings were detected in different three donor corneas.
Figure 1.
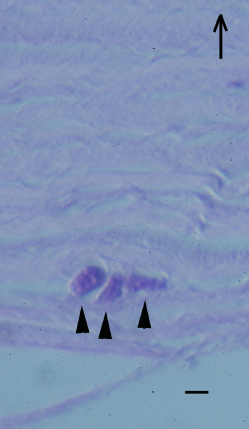
Ex vivo invasion of Acanthamoeba into corneal stroma. Acanthamoebae were added to denuded human corneal stroma. Acanthamoebae were placed on the denuded corneal stroma (endothelial side up) and incubated at 25 °C for 2 days. Hematoxylin and eosin staining shows Acanthamoebae (arrowheads) located in fine collagen fibrils. Arrow shows the direction of corneal epithelium. Scale bar=10 µm.
Cytopathic effect on corneal fibroblasts
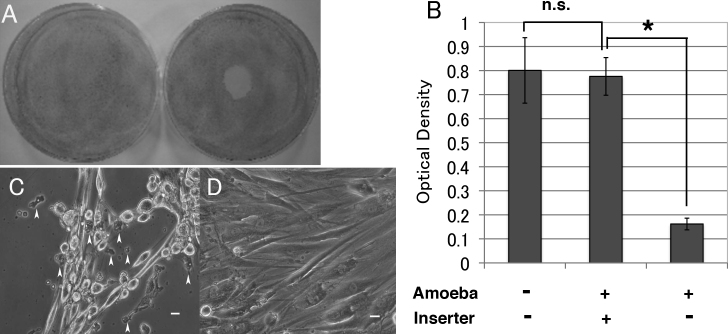
To investigate the effect of Acanthamoeba on corneal fibroblasts, isolates were added to the center of 6-cm dishes containing human corneal fibroblasts and were stained with Giemsa solution after culture. In this experiment, Acanthamoebae were placed carefully on the center of the dish not to spread to the periphery. It was found that fibroblasts had disappeared from the center of the dishes and this central area with no staining (Figure 2A, right) corresponded to the site where Acanthamoebae were placed. Although corneal fibroblasts on the peripheral area should have been exposed to soluble factors Acanthamoebae produced, there was no apparent change in the outside area of corneal fibroblasts at least 2 days after Acanthamoebae addition, suggesting that direct adhesion of Acanthamoebae to corneal fibroblasts is essential for the cytopathic effect of Acanthamoebae and soluble factors Acanthamoebae produced does not affect the fate of corneal fibroblasts.
Figure 2.
Direct and indirect cytopathic effects of Acanthamoeba on corneal fibroblasts. A: Acanthamoebae were placed on corneal fibroblasts at the center of 6-cm dishes and incubated at 25 °C for 2 days. The fibroblasts are uniformly stained with Giemsa solution in a control dish (left). The central area where Acanthamoebae were placed shows no staining (indicating loss of corneal fibroblasts) in a treated dish (right). B: MTT assay showed there was no significant difference of optical density value in the outer dishes with corneal fibroblasts with or without insert culture dishes bearing Acanthamoebae. Significant low optical density value is detected in Acanthamoebae direct adhesion group, compared with insert culture dishes bearing Acanthamoebae. (n=6) Amoeba; Acanthamoeba, Inserter; insert culture dish. C: Phase contrast microscopy shows many corneal fibroblasts are detached and Acanthamoebae adhere to corneal fibroblasts and the dish surface. Arrowheads show active Acanthamoebae co-cultured with corneal fibroblasts. D: Confluent human corneal fibroblasts are seen. Acanthamoebae in the insert culture dishes with 0.4 µm pores are not observed. Similar findings were obtained with repeated two sets of experiments. Representative data are shown. Scale bar=10 µm.
To test whether the cytopathic effect of Acanthamoeba was due to direct adherence to corneal fibroblasts or to soluble factors such as chemical mediators, we used insert culture dishes with 0.4 µm pores, and corneal fibroblasts were cultured in the outer plate. Acanthamoebae could not pass through these pores under any culture conditions. MTT assay showed there was no significant difference of optical density value in the outer dishes with corneal fibroblasts with or without insert culture dishes bearing Acanthamoebae. However, significant low optical density value was detected in Acanthamoebae direct adhesion group, compared with insert culture dishes bearing Acanthamoebae (Figure 2B). When Acanthamoebae were placed directly on the fibroblasts in the well without insert culture dishes, several fibroblasts were detached from dishes (Figure 2C). In contrast, corneal fibroblasts adhered surface of dishes and cell viability was maintained up to 7 days (Figure 2D) in case that insert culture dishes containing Acanthamoebae were placed in the wells, indicating that direct contact with Acanthamoebae, but not soluble factors, was essential for the cytopathic effect on corneal fibroblasts.
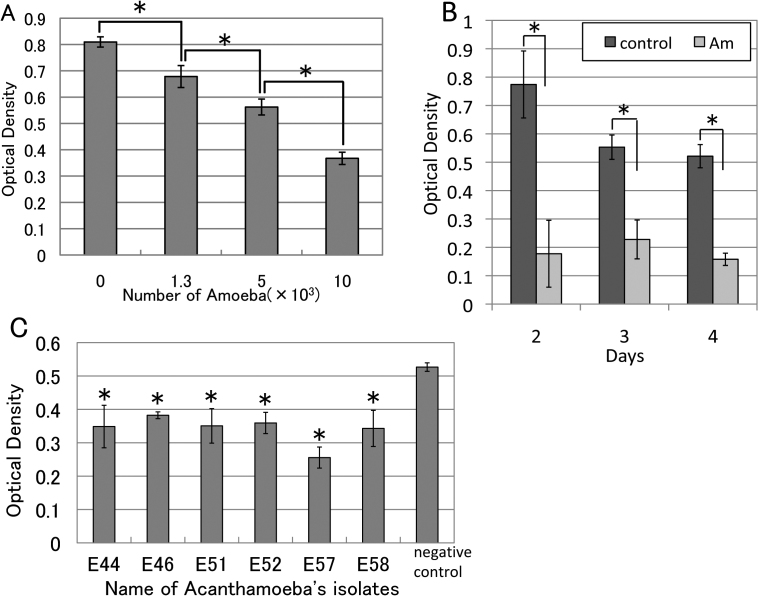
We also investigated whether an increase in the number of Acanthamoebae could affect the viability of corneal fibroblasts. We found that viable corneal fibroblasts decreased significantly in a dose-dependent manner after co-culture with Acanthamoebae (Figure 3A). We analyzed corneal fibroblast viability over time using 1×104 Acanthamoebae, because larger numbers of Acanthamoebae were too toxic for the fibroblasts. As shown in Figure 3B, the viability of corneal fibroblasts cultured with Acanthamoebae was significantly decreased from day 2 compared with the control cultures. Next, we evaluated the cytopathic effect of various T4 genotypes isolated from patients with AK. Significant decreases of corneal fibroblast viability were detected with all Acanthamoeba strains tested as compared to culture without Acanthamoebae (Figure 3C), suggesting that Acanthamoeba isolates from AK patients have a similar cytopathic effect on human corneal fibroblasts.
Figure 3.
Cytopathic effect of Acanthamoeba on corneal fibroblasts in various conditions. A: Acanthamoebae (0 to 10×103) were added to corneal fibroblasts in each well and incubated at 25 °C for 2 days. Acanthamoebae (1×104) significantly decreased the viability of corneal fibroblasts compared with no Acanthamoebae (n=4). B: A significant decrease of corneal fibroblast viability was detected from day 2 (n=4). C: Cytopathic effect on corneal fibroblasts for 6 Acanthamoebae isolates from our AK patients. A significant decrease of optical density (indicating a cytopathic effect on corneal fibroblasts) was detected with all tested Acanthamoebae compared to control cultures with no Acanthamoebae (n=4). Similar findings were obtained with repeated two experiments. Representative data are shown. *p<0.05.
Detection of apoptotic corneal fibroblasts
To test whether or not apoptosis of corneal fibroblasts was induced by Acanthamoeba infection, co-culture of Acanthamoeba with human corneal fibroblasts was done and TUNEL staining was performed to detect DNA fragmentation. As a result, DAB-positive apoptotic corneal fibroblasts were found in the positive control cultures and the cultures with Acanthamoeba, but not in the negative control cultures (Figure 4A-C). Next, to evaluate the extent of apoptosis when corneal fibroblasts were cultured with Acanthamoebae, we calculated the percentage of cells stained with Annexin V, which identifies early apoptosis (Figure 4D). Annexin V-positive cells increased significantly compared with necrotic cells on days 2 to 5. More than 50% of cells were Annexin V-positive on days 4 and 5, indicating that Acanthamoeba mainly killed corneal fibroblasts by apoptosis.
Figure 4.
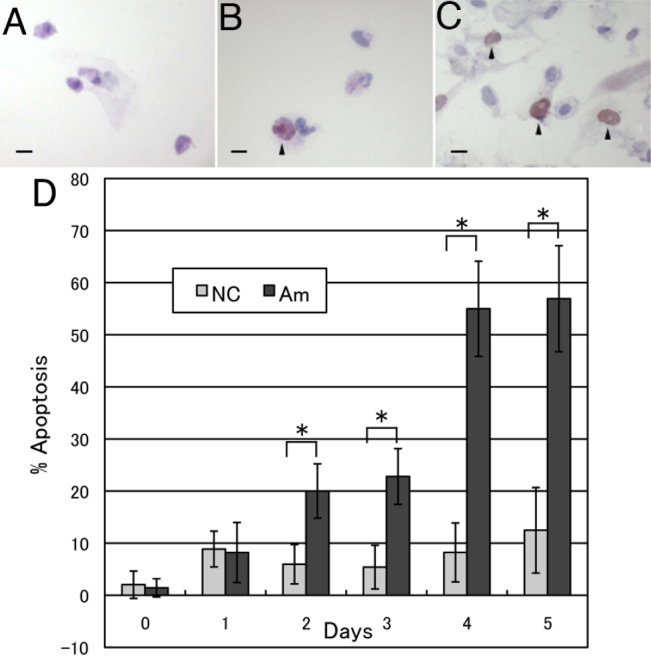
Detection of apoptotic human corneal fibroblasts. TUNEL staining was performed after human corneal fibroblasts were co-cultured with Acanthamoebae. TUNEL-positive cells are not detected in cultures of fibroblasts without Acanthamoebae (A). Arrowheads show TUNEL-positive apoptotic corneal fibroblasts cultured with Acanthamoebae (B) and with Actinomysin D (C). Scale bar=100 µm. D: Percentage of Annexin V-positive cells. A significant increase of Annexin V-positive corneal fibroblasts was detected on day 2 or later of culture with Acanthamoebae. More than 50% of corneal fibroblasts were Annexin V-positive on days 4 and 5 (n=4–5). Similar results were obtained with repeated two experiments. NC; Negative control, corneal fibroblasts without Acanthamoebae, Am; Corneal fibroblasts co-cultured with Acanthamoebae. *p<0.05.
Discussion
All 6 Acanthamoeba isolates from the corneas of our AK patients had the T4 genotype according to 18S rDNA sequence analysis, as shown in Table 1. Our present findings clearly demonstrated that all of the Acanthamoeba isolated from AK patients had a cytopathic effect on human corneal fibroblasts and that this cytopathic effect was due to direct adhesion to corneal fibroblasts rather than soluble factors, suggesting that the keratocyte depletion demonstrated by histological examination of human corneas with AK [17] is induced by direct adhesion of Acanthamoeba to activated corneal keratocytes (i.e., corneal fibroblasts). DNA fragmentation was detected in corneal fibroblasts cultured with Acanthamoebae and the fibroblasts were mainly depleted by apoptosis, as evidenced by Annexin V staining to detect early apoptosis. In vitro observation showed active Acanthamoeba trophozoites phagocytosing fragments of corneal fibroblasts (unpublished observation 2010). These findings may reproduce those occurring in AK induced by pathogenic Acanthamoeba.
Our in vitro study suggested that activated corneal fibroblasts may be extremely vulnerable to Acanthamoeba infection in vivo. However, AK is localized to the central area of the cornea and does not expand to the periphery or the conjunctiva. This may be because all layers of the peripheral corneal stroma contain monocytes [21] and the substantia propria of the conjunctiva has several leukocytes (mainly macrophages) [19]. Moreover, corneal fibroblasts have the potential to produce abundant chemokines that attract macrophages and neutrophils [21-23]. Clinically, vascular invasion of AK lesions dramatically suppresses disease activity, implying a critical role of leukocytes from the blood vessels in combating AK. Thus, differences in the immunoprotective microenvironment of the ocular surface may confine AK lesions to the central area of the avascular cornea, but severe visual impairment due to corneal scarring is a common problem. These findings suggest that local application of host leukocytes and use of chemokines to attract neutrophils and macrophages may be treatment options for AK that is uncontrolled by current therapies.
Sequencing of nuclear 18S rDNA [6,7] is a useful method of classifying Acanthamoebae accurately. Fifteen types of Acanthamoebae (T1 to T15) have been identified, but isolates obtained from AK are mainly of the T4 genotype. All 6 isolates obtained at our hospital were of the T4 genotype and all isolates had a cytopathic effect on corneal fibroblasts (Figure 3C). However, the extent of the cytopathic effect varied, suggesting pathophysiological diversity of Acanthamoebae with the T4 genotype. Therefore, we retrospectively examined the correlation between the cytopathic effect in vitro and clinical severity of AK caused by each isolate, but no correlation was detected in our series (unpublished observation). This may be because clinical severity of AK depends on various factors, such as the stage at the first visit to hospital, previous treatment, and use of topical corticosteroids due to misdiagnosis. However, detailed classification of Acanthamoeba by DNA typing may contribute to prediction of the prognosis and selection of appropriate treatment for each isolate in the near future.
The following limitations of our study should be noted. Cultured human corneal fibroblasts are not the same as keratocytes in the normal corneal stroma, because corneal fibroblasts are activated in response to inflammation [24,25]. Considering that AK causes inflammation of the cornea, corneal fibroblasts rather than corneal keratocytes may be more suitable for analysis of interactions between corneal stromal cells and Acanthamoebae. In this study, Annexin V expression was used to detect early apoptosis and PI-positive cells were regarded as necrotic cells. Therefore, we could not determine the actual proportion of necrotic cells, because PI-positive cells resulting from apoptosis were not completely excluded. However, we at least did not overestimate the number of apoptotic cells. Further studies need to be performed to determine the exact percentage of apoptotic cells. Acanthamoebae express a trans-membrane protein with the characteristics of a cell surface receptor, which is called mannose-binding protein (MBP) and mediates adhesion to the surface of the cornea. Following MBP-mediated adhesion to host cells, the amoebae produce a contact-dependent metalloproteinase and several contact-independent serine proteinases [26]. Kinnear [27] reported indirect cytopathic effects of Acanthamoeba on corneal fibroblasts using insert culture dishes, suggestive of contact-independent serine proteinases. This may be because approximately 400× higher number of Acanthamoebae were employed in the study than our experimental setting. Considering that Acanthamoebae used in our experiment was enough number to kill directly activated corneal fibroblasts and Acanthamoebae are sparse in the corneal stroma of patients with confocal microscopic observation [28], indirect cytopathic effects of Acanthamoebae on corneal fibroblasts can be at least ignorable in an actual clinical setting.
In summary, we showed that Acanthamoebae from AK patients had a cytopathic effect on human corneal fibroblasts by direct adhesion rather than soluble mediators. This cytopathic effect on corneal fibroblasts was mainly due to apoptosis. Our findings provide some clues to the pathophysiology of corneal keratocyte loss in human AK.
Acknowledgments
The authors thank Dr. Yuko Kamei for harvest of Acanthamoeba and helpful advice. This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1.Illingworth CD, Cook SD. Acanthamoeba keratitis. Surv Ophthalmol. 1998;42:493–508. doi: 10.1016/s0039-6257(98)00004-6. [DOI] [PubMed] [Google Scholar]
- 2.Dart JK, Saw VP, Kilvington S. Acanthamoeba keratitis: diagnosis and treatment update 2009. Am J Ophthalmol. 2009;148:487–99. doi: 10.1016/j.ajo.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Stehr-Green JK, Bailey TM, Visvesvara GS. The epidemiology of Acanthamoeba keratitis in the United States. Am J Ophthalmol. 1989;107:331–6. doi: 10.1016/0002-9394(89)90654-5. [DOI] [PubMed] [Google Scholar]
- 4.Sharma S, Garg P, Rao GN. Patient characteristics, diagnosis, and treatment of non-contact lens related Acanthamoeba keratitis. Br J Ophthalmol. 2000;84:1103–8. doi: 10.1136/bjo.84.10.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dart JK, Saw VP, Kilvington S. Acanthamoeba keratitis: diagnosis and treatment update 2009. Am J Ophthalmol. 2009;148:487–99. doi: 10.1016/j.ajo.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 6.Schroeder JM, Booton GC, Hay J, Niszl IA, Seal DV, Markus MB, Fuerst PA, Byers TJ. Use of subgenic 18S ribosomal DNA PCR and sequencing for genus and genotype identification of acanthamoebae from humans with keratitis and from sewage sludge. J Clin Microbiol. 2001;39:1903–11. doi: 10.1128/JCM.39.5.1903-1911.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yera H, Zamfir O, Bourcier T, Viscogliosi E, Noël C, Dupouy-Camet J, Chaumeil C. The genotypic characterisation of Acanthamoeba isolates from human ocular samples. Br J Ophthalmol. 2008;92:1139–41. doi: 10.1136/bjo.2007.132266. [DOI] [PubMed] [Google Scholar]
- 8.Yamagami S, Yokoo S, Usui T, Yamagami H, Amano S, Ebihara N. Distinct populations of dendritic cells in the normal human donor corneal epithelium. Invest Ophthalmol Vis Sci. 2005;46:4489–94. doi: 10.1167/iovs.05-0054. [DOI] [PubMed] [Google Scholar]
- 9.He YG, McCulley JP, Alizadeh H, Pidherney M, Mellon J, Ubelaker JE, Stewart GL, Silvany RE, Niederkorn JY. A pig model of Acanthamoeba keratitis: transmission via contaminated contact lenses. Invest Ophthalmol Vis Sci. 1992;33:126–33. [PubMed] [Google Scholar]
- 10.van Klink F, Alizadeh H, He Y, Mellon JA, Silvany RE, McCulley JP, Niederkorn JY. The role of contact lenses, trauma, and Langerhans cells in a Chinese hamster model of Acanthamoeba keratitis. Invest Ophthalmol Vis Sci. 1993;34:1937–44. [PubMed] [Google Scholar]
- 11.Stopak SS, Roar MI, Nauheim RC, Turgeon PW, Sossi G, Kowalski RP, Thoft RA. Growth of Aconthomoeba on human corneal epithelial cells and keratocytes in vitro. Invest Ophthalmol Vis Sci. 1991;32:354–9. [PubMed] [Google Scholar]
- 12.Niederkorn JY, Alizadeh H, Leher H, McCulley JP. The pathogenesis of Acanthamoeba keratitis. Microbes Infect. 1999;1:437–43. doi: 10.1016/s1286-4579(99)80047-1. [DOI] [PubMed] [Google Scholar]
- 13.Pidherney MS, Alizadeh H, Stewart GL, McCulley JP, Niederkorn JY. In vitro and in vivo tumoricidal properties of a pathogenic/free-living amoeba. Cancer Lett. 1993;72:91–8. doi: 10.1016/0304-3835(93)90016-3. [DOI] [PubMed] [Google Scholar]
- 14.Alizadeh H, Pidherney MS, McCulley JP, Niederkorn JY. Apoptosis as a mechanism of cytolysis of tumor cells by a pathogenic free-living amoeba. Infect Immun. 1994;62:1298–303. doi: 10.1128/iai.62.4.1298-1303.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mattana A, Tozzi MG, Costa M, Delogu G, Fiori PL, Cappuccinelli P. By releasing ADP, Acanthamoeba castellanii causes an increase in the cytosolic free calcium concentration and apoptosis in wish cells. Infect Immun. 2001;69:4134–40. doi: 10.1128/IAI.69.6.4134-4140.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattana A, Bennardini F, Usai S, Fiori PL, Franconi F, Cappuccinelli P. Acanthamoeba castellanii metabolites increase the intracellular calcium level and cause cytotoxicity in wish cells. Microb Pathog. 1997;23:85–93. doi: 10.1006/mpat.1997.0138. [DOI] [PubMed] [Google Scholar]
- 17.Garner A. Pathogenesis of acanthamoebic keratitis: hypothesis based on a histological analysis of 30 cases. Br J Ophthalmol. 1993;77:366–70. doi: 10.1136/bjo.77.6.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson AG, Cann RL, Carr SM, George M, Gyllensten UB, Helm-Bychowski KM, Higuchi RG, Parumbi SR, Prager EM, Sage RD, Stoneking M. Mitochondrial DNA and two perspectives on evolutionary genetics. Biol J Linn Soc Lond. 1985;26:375–400. [Google Scholar]
- 19.Booton GC, Visvesvara GS, Byers TJ, Kelly DJ, Fuerst PA. Identification and distribution of Acanthamoeba species genotypes associated with nonkeratitis infections. J Clin Microbiol. 2005;43:1689–93. doi: 10.1128/JCM.43.4.1689-1693.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schroeder JM, Booton GC, Hay J, Niszl IA, Seal DV, Markus MB, Fuerst PA, Byers TJ. Use of subgenic 18S ribosomal DNA PCR and sequencing for genus and genotype identification of acahthamoebae from humans with keratitis and from sewage sludge. J Clin Microbiol. 2001;39:1903–11. doi: 10.1128/JCM.39.5.1903-1911.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamagami S, Ebihara N, Usui T, Yokoo S, Amano S. Bone marrow-derived cells in normal human corneal stroma. Arch Ophthalmol. 2006;124:62–9. doi: 10.1001/archopht.124.1.62. [DOI] [PubMed] [Google Scholar]
- 22.Yamagami S, Yokoo S, Amano S, Ebihara N. Characterization of bone marrow derived cells in the substantia propria of the human conjunctiva. Invest Ophthalmol Vis Sci. 2007;48:4476–81. doi: 10.1167/iovs.06-1543. [DOI] [PubMed] [Google Scholar]
- 23.Spandau UH, Toksoy A, Verhaart S, Gillitzer R, Kruse FE. High expression of chemokines Gro-alpha (CXCL-1), IL-8 (CXCL-8), and MCP-1 (CCL-2) in inflamed human corneas in vivo. Arch Ophthalmol. 2003;121:825–31. doi: 10.1001/archopht.121.6.825. [DOI] [PubMed] [Google Scholar]
- 24.Hong JW, Liu JJ, Lee JS, Mohan RR, Mohan RR, Woods DJ, He YG, Wilson SE. Proinflammatory chemokine induction in keratocytes and inflammatory cell infiltration into the cornea. Invest Ophthalmol Vis Sci. 2001;42:2795–803. [PubMed] [Google Scholar]
- 25.Kumagai N, Fukuda K, Fujitsu Y, Lu Y, Chikamoto N, Nishida T. Lipopolysaccharide-induced expression of intercellular adhesion molecule-1 and chemokines in cultured human corneal fibroblasts. Invest Ophthalmol Vis Sci. 2005;46:114–20. doi: 10.1167/iovs.04-0922. [DOI] [PubMed] [Google Scholar]
- 26.Panjwani N. Pathogenesis of acanthamoeba keratitis. Ocul Surf. 2010;8:70–9. doi: 10.1016/s1542-0124(12)70071-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kinnear FB. Cytopathogenicity of acanthamoeba, vahlkampfia and hartmannella: quantative & qualitative in vitro studies on keratocytes. J Infect. 2003;46:228–37. doi: 10.1053/jinf.2002.1116. [DOI] [PubMed] [Google Scholar]
- 28.Vaddavalli PK, Garg P, Sharma S, Sangwan VS, Rao GN, Thomas R. Role of confocal microscopy in the diagnosis of fungal and acanthamoeba keratitis. Ophthalmology. 2011;118:29–35. doi: 10.1016/j.ophtha.2010.05.018. [DOI] [PubMed] [Google Scholar]