Abstract
Harmine is a β-carboline compound that targets glutamatergic, monoaminergic, and GABAergic pathways underlying drug addiction. We compared the efficacy of harmine against different psychoactive drugs using an invertebrate (planarian) assay designed to quantify ‘C-shape’ responses. Harmine itself (0.01 – 10 µM) did not produce C-shapes. However, when applied over the same concentration range, harmine significantly inhibited C-shapes elicited by cocaine, with a concentration of 0.1 µM producing almost 90% inhibition. Consistent with its putative actions, harmine produced a similar, though less efficacious, inhibition of C-shapes elicited by the substituted amphetamines methamphetamine and mephedrone (4-methylmethcathinone) but was much less effective against nicotine. When tested in the presence of the glutamate transporter inhibitor dihydrokainate (DHK) (0.1, 1 µM), harmine (0.1 µM) efficacy against cocaine-induced C-shapes was significantly reduced. Harmine also attenuated C-shapes elicited by N-methyl-D-aspartate (NMDA) and by glutamate itself. The present data suggest that harmine displays preferential efficacy against different addictive substances (cocaine > amphetamines > nicotine) and, at least for cocaine, is dependent on the glutamate system.
Keywords: harmine, β-carboline, mephedrone, cocaine, methamphetamine, glutamate, planaria, drug abuse, invertebrate
Introduction
Harmine is a member of the heterocyclic β-carboline family of indole alkaloid compounds (consisting of a pyridine ring fused to an indole skeleton). It produces multiple biological effects, including glutamate transporter subtype 1 (GLT-1) activation [20], monoamine oxidase A inhibition [17], 5-HT2A receptor activation [11], cyclin-dependent kinase inhibition [34], imidazoline site interactions, and inverse agonist actions at the benzodiazepine site of GABAA receptors [1,8,15,25]. Despite targeting biological systems that underlie drug addiction, harmine has not been extensively tested for its efficacy against different classes of abused drugs. Rat studies indicate that norharman, a related β-carboline compound, reduces cocaine intake in a U-shaped manner and that harmine reduces severity of the naloxone-precipitated morphine withdrawal syndrome and antagonize licking induced by apomorphine [2,5,8]. The present study used a simple invertebrate (planarian) assay to compare the efficacy of harmine against psychostimulants. Planarians possess a simple, yet centralized, nervous system and express mammalian-like neurotransmitter systems, including glutamate, dopamine, 5-HT, acetylcholine, and GABA [7,22–24,26,37]. Furthermore, similar to rats and mice [19], planarians display C-shape responses during exposure to cocaine, amphetamines, nicotine, and glutamate. C-shapes provide a reproducible, quantifiable, and common endpoint for comparing the efficacy of a test compound such as harmine [28,31,35]. Using the invertebrate assay, we now report that harmine displays particular efficacy (inhibition) against cocaine-induced C-shapes (nearly 90%) through a glutamate-based (dihydrokainate (DHK)-sensitive) mechanism.
Materials and Methods
Planarians (Dugesia dorotocephala) purchased from Carolina Biological Supply (Burlington, NC, USA) were acclimated to room temperature (21 °C) and tested within 3 days of receipt. Cocaine and methamphetamine were provided by the National Institute on Drug Abuse. (R,S)-mephedrone (4-methylmethcathinone or 4-MMC) was purchased from Fox Chase Chemical Diversity Center (Doylestown, PA, USA). Dihydrokainate (DHK), N-methyl-D-aspartate (NMDA), (−)-nicotine ditartrate, and L-Glutamic acid (glutamate) was purchased from Tocris Biosciences (St. Louis, MO, USA). Harmine (free base) was purchased from Sigma-Aldrich Corporation (St. Louis, MO, USA). Stock solutions of harmine (1 mM) were prepared in 0.1% dimethylsulfoxide (DMSO) and then diluted down to desired concentrations with distilled water containing Amquel® water conditioner. To control for any residual effect of the organic solvent, all experimental solutions (including controls that did not contain harmine) contained 0.1% DMSO. Group mean (± S.E.M.) comparisons were evaluated by two-way ANOVA followed by a Dunnett's analysis or one-way ANOVA followed by Tukey’s analysis. Values of p < 0.05 were considered statistically significant.
A range of harmine concentrations was tested against C-shapes produced by cocaine, methamphetamine, mephedrone, nicotine, and NMDA. The nature of the C-shape responses has been described [26,31]. Approximately equi-effective concentrations of each substance were chosen based on prior work [23–24, 28,30–31]. Individual planarians were placed randomly into a petri dish (5.5 cm diameter) containing cocaine (3 mM) or a combination of cocaine (3 mM) and harmine (0.01, 0.1, 1, 10 µM) for 5 min. The number of C-shapes during the 5-min exposure interval was determined. Subsequent testing of harmine against methamphetamine (3 mM), mephedrone (1 mM), nicotine (3 mM), and NMDA (5 mM) used the identical experimental design. Harmine (0.01, 0.1, 1, 10, 100 µM) effects by itself were also investigated. The possibility that harmine acted through a glutamatergic mechanism to inhibit C-shapes elicited by cocaine was investigated by testing the following groups: cocaine (3 mM); harmine (0.1 µM)/cocaine (3 mM); DHK (0.01, 0.1, 1 µM)/harmine (0.1 µM)/cocaine (3 mM); and DHK (1 µM)/cocaine (3 mM).
Results
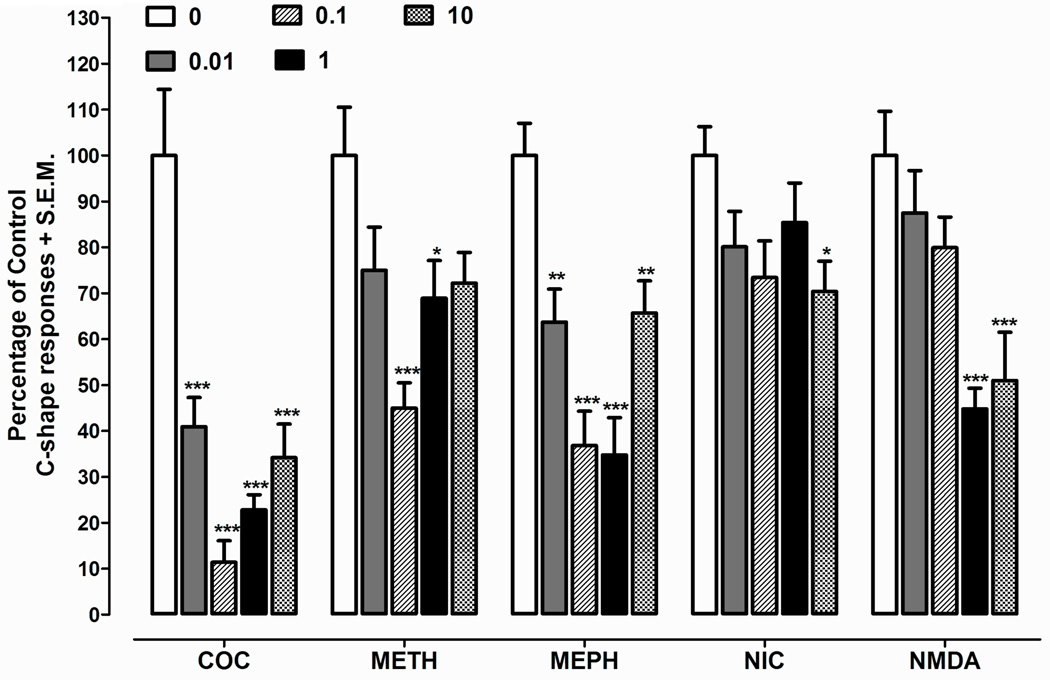
Effects of harmine on C-shapes elicited by different drugs of abuse are presented in Fig.1. Two-way ANOVA indicated a significant drug effect [F(4, 35) = 18.8, p < 0.0001], dose effect [F(4, 175) = 33.9, p < 0.0001], and interaction [F(16, 175) = 3.28, p < 0.0001]. Harmine (0.01, 0.1, 1, 10 µM) did not produce C-shapes or observable behaviors at the dose range tested here. For combination experiments presented in Fig. 1, C-shapes induced by cocaine were inhibited during co-exposure with harmine (µM harmine concentration, % inhibition): (0.01, 59.1); (0.1, 88.6); (1, 77.2) and (10, 65.8) (p < 0.001). C-shapes induced by mephedrone were also attenuated by harmine (µM harmine concentration, % inhibition): (0.01, 36.3) (p < 0.05); (0.1, 63.2) (p < 0.001); (1, 65.2) (p < 0.001); and (10, 34.3) (p < 0.05). For experiments with harmine and methamphetamine, C-shapes produced by methamphetamine were reduced by 55% during co-exposure with harmine (0.1 µM) (p < 0.001). Harmine was less effecting in reducing C-shapes produced by nicotine as only a concentration of 10 µM produced significant inhibition (29.6%) (p < 0.05). For harmine and NMDA experiments, C-shapes induced by NMDA were inhibited during co-exposure with harmine (µM harmine concentration, % inhibition): (1, 55) and (10, 49) (p < 0.001).
Fig. 1.
Harmine (0.01 – 100 µM) effects on C-shapes elicited by cocaine (COC, 3 mM), methamphetamine (METH, 3 mM), mephedrone (MEPH, 1 mM), glutamate (3 mM), nicotine (NIC, 3 mM), and NMDA (5 mM) during a 5-min exposure. Mean number of C-shapes (± S.E.M.) for the respective control groups (i.e., 0 µM harmine) were: COC (18.6 ± 2.9); METH (22.5 ± 2.5); MEPH (25.5 ± 1.9); NIC (33.4 ± 2.3); and NMDA (36.0 ± 3.7). Data are presented as percentage of control C-shapes + S.E.M. in respective harmine-naïve group. N = 8 planarians/ group. ***p < 0.001, **p < 0.01, *p < 0.05 compared to control group (0 µM harmine).
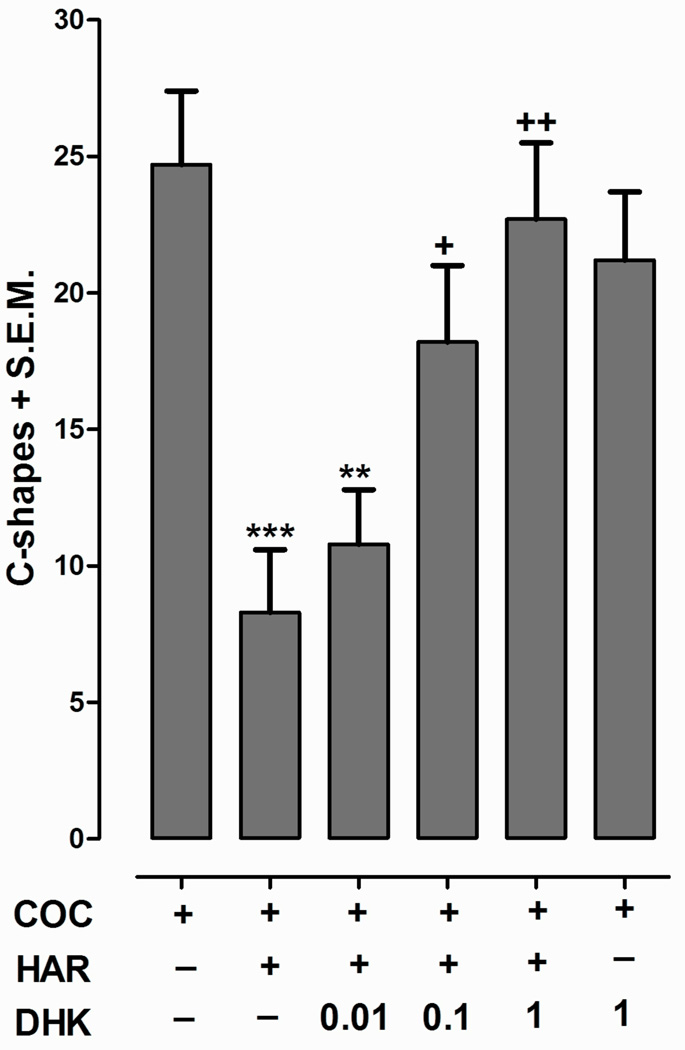
Harmine (0.1 µM) efficacy against C-shapes elicited by cocaine (3 mM) was investigated in the presence of the glutamate uptake inhibitor DHK (Fig. 2). One-way ANOVA indicated a significant main effect [F(5, 54) = 6.93, p < 0.0001]. Harmine inhibited C-shapes elicited by cocaine (p < 0.001), but its efficacy was significantly reduced by co-administration with 0.1 µM (p < 0.05) and 1 µM (p < 0.01) DHK. DHK (1 µM) did not significantly affect C-shapes produced by cocaine (p > 0.05) or cause C-shapes by itself (not shown). A role for glutamate in the effects of harmine was further investigated by testing a range of harmine concentrations (0, 0.01, 0.1, 1, 10 µM) against C-shapes induced by glutamate (3 mM) [31]. One-way ANOVA indicated a significant main effect [F(4, 35) = 16.9, p < 0.0001]. The mean number of C-shapes produced by glutamate (3 mM) was 21.9 ± 2.3. For combination experiments, harmine attenuated C-shapes induced by glutamate (3 mM) (µM harmine concentration, % inhibition): (0.01, 54.9 ± 4.8); (0.1, 42.3 ± 4.9); (1, 36.6 ± 2.7) and (10, 52.0 ± 5.3) (p < 0.001).
Fig. 2.
Dihydrokainate (DHK, 0.01 – 10 µM) effects on C-shapes elicited by cocaine (COC, 3 mM) in the absence and presence of harmine (HAR, 0.1 µM) during a 5-min exposure. Data are presented as C-shapes + S.E.M. N = 8 planarians/ group. ***p < 0.001, **p < 0.01 compared to COC group and +p < 0.05, ++p < 0.05 compared to COC + HAR group.
Discussion
Harmine displayed preferential efficacy against different psychoactive compounds. Effects of harmine were most robust against cocaine and substituted amphetamines with lesser efficacy detected against nicotine. Inhibition of cocaine-evoked C-shapes was notably pronounced, with a concentration of 0.1 µM producing about 90% inhibition. The U-shaped concentration curve for harmine against cocaine, methamphetamine, and mephedrone is consistent with the U-shaped inhibition of cocaine intake by norharman in rats and indicative of more than one mechanism of action [1,5,8,15,25]. A recently documented effect of harmine is enhancement of cellular glutamate uptake [20]. In vivo studies indicate that harmine displays efficacy in a mouse model of amyotrophic lateral sclerosis and increases both glutamate uptake activity and GLT-1 protein expression [20]. In the present experiments, cocaine responses were most effectively inhibited by harmine concentrations (0.1 –10 µM) that also induce GLT-1 gene expression in mouse and human astrocytes (3 µM) and elicit promoter activation in cultured cells (10 µM). Our results also revealed that harmine failed to inhibit cocaine-induced C-shapes in the presence of a glutamate transporter inhibitor (DHK), providing pharmacological evidence that the efficacy of harmine is dependent on enhancement of glutamate uptake. Specific glutamate transporter genes and proteins have not yet been identified in planarians, although elements of a functionally active glutamate system, including endogenous glutamate and ionotropic glutamate receptors, are present [6,37]. A more established glutamate uptake activator, ceftriaxone, displays efficacy against cocaine responses across different species [32]. Vertebrate studies indicate that ceftriaxone reduces reinforcing and drug-seeking properties of cocaine in self-administration assays [18,38]. Planarian studies reveal that ceftriaxone blocks C-shapes produced by acute cocaine exposure and withdrawal responses following discontinuation of cocaine exposure [30–31]. A comparison of ceftriaxone and harmine efficacies against cocaine-induced C-shapes indicates that ceftriaxone was less efficacious (about a 50% inhibition) than harmine, perhaps due to the latter compound acting through at non-glutamate substrates such as 5-HT receptors, imidazoline sites, or cyclin-dependent kinases [15].
The substituted amphetamines, methamphetamine and mephedrone, elicited C-shapes that were also attenuated by harmine. Compared to its effects against cocaine, harmine displayed lesser efficacy against mephedrone and methamphetamine, a finding possibly related to amphetamines acting through a broader range of mechanisms (i.e., monoamine release, monoamine transporter uptake block, monoamine oxidase inhibition, etc.). The underlying mechanism of action of harmine is unclear, but GLT-1 transporter activation is again a possibility because ceftriaxone inhibits acute and sensitized amphetamine-induced responses in rats [29]. Harmine inhibits monoamine oxidases [10], but this mechanism is unlikely to have contributed significantly to results presented here because amphetamines produce directionally similar effects on monoamine oxidase activity [12]. Furthermore, monoamine oxidase inhibition prevents dopamine catabolism leading to increased dopamine concentrations, and dopamine and dopamine agonists elicit behavioral responses in planarians [36]. Other possible mechanisms of action for harmine observed here include 5-HT2A receptor activation, cyclin-dependent kinase inhibition, imidazoline site interactions, and inverse agonist actions at the benzodiazepine site of GABAA receptors [1, 8, 11, 15, 25, 34].
Harmine effects on mephedrone are especially interesting because mephedrone is a principal ingredient of psychoactive bath salts, a dangerous street drug linked to fatalities and non-fatal overdoses [39]. The American Association of Poison Control reported that mephedrone exposures increased at least 10-fold from 2010 to 2011, and several factors associated with mephedrone, such as its relative purity, ease of synthesis from available precursors, widespread internet marketing, and perceived quality of high, are attractive to end users of the drug and elements of organized crime [40]. Limited preclinical evidence suggests a psychostimulant profile for mephedrone, including enhancement of extracellular monoamine levels in the limbic system, augmentation of locomotor activity, reinforcing properties in self-administration assays, and serotonergic deficits following repeated exposure [3,13–14,16,21]. The psychostimulant-like effects of mephedrone also extend to planarians, where it elicits dopamine-sensitive C-shapes following acute exposure, withdrawal responses during drug absence, and place conditioning effects [28]. The present results suggest mephedrone displays an intermediate efficacy relative to more established psychostimulants, producing C-shapes that are greater than those produced by methamphetamine but less than those elicited by cocaine. Furthermore, the present findings suggest that mephedrone-induced C-shapes are not only sensitive to dopamine receptor activation [28], but also to the actions of harmine.
Harmine inhibited NMDA-evoked C-shapes by approximately 55%. Prior work indicates that planarians display C-shapes following exposure to NMDA that are antagonized by topiramate and carbamazepine [26–27]. Although NMDA can activate its receptors independent of glutamate release, in vivo microdialysis experiments indicate that it does increase extracellular glutamate levels in the rat brain [41]. Thus, NMDA may have produced C-shapes in planarians through overlapping mechanisms that include direct receptor activation and enhanced glutamate release. In this case, it is possible that harmine, by increasing glutamate uptake activity, inhibited the component of NMDA-evoked C-shapes that is dependent on enhanced glutamate release. The interpretation is supported by our finding that harmine also inhibits C-shapes induced by administration of glutamate itself. Future behavioral and neurochemical studies using rats are planned to test this hypothesis.
Although pharmacological evidence presented here suggests a glutamate-based mechanism underlies the efficacy of harmine against cocaine, pharmacokinetic factors and alternative mechanisms of action may have contributed. One consideration regarding planarian assays is that the entire organism is exposed to the drug(s). Thus, in the present study, one could speculate that the efficacy of harmine was related to occlusion of substances across the planarian tegument. However, in the case in which a glutamate transporter inhibitor (DHK) was added to a solution containing cocaine and harmine, the cocaine-induced response was completely restored even in the presence of harmine. This finding indicates that simple occlusion of diffusion across the tegument by harmine could not have been responsible for its efficacy against cocaine. It should also be noted that in order for compounds to display efficacy in planarian assays, higher concentrations are often required relative to those needed for rodent brain studies. The rate of uptake of compounds into planarians has not been determined, but at least two possibilities may account for the concentration differences between planarians and rodents that we and others have reported [26–28, 30–31], neither of which alter the conclusions of the present results. The first possibility is that there is not ‘free diffusion’ in planarians and the diffusion barrier is greater than that in rodents’ brain, which seems unlikely but would not affect the results with harmine. A second possibility is that the affinity of compounds for mammalian targets of drugs (receptors, uptake sites, etc.), or second messenger transduction efficacy of compounds, is lower in planarians than in higher-order animals, thus requiring higher concentrations to achieve the same level of receptor/uptake site occupancy or signal transduction (level of effect). Finally, the presence of glutamatergic and cholinergic receptors on muscles and the body surface of flatworms raises the possibility that C-shapes elicited by stimulants were muscle contractions [33]. While conducting contraction assays is beyond our capability, any effect on contraction is unlikely to be phasic in nature and simultaneously caused by all six of the substances tested viz., cocaine, methamphetamine, mephedrone, nicotine, NMDA, and glutamate.
In conclusion, harmine displayed selective efficacy against drugs of abuse that was dependent on the class of compound (cocaine > substituted amphetamines > nicotine). The experiments utilized an invertebrate assay that quantifies C-shape responses [23–24,26,27]. With the information obtained in the present study, future studies will investigate more detailed aspects of the mechanisms of action of β-carboline compounds in mammals [20].
Research Highlights.
Harmine displays selective efficacy against different drugs of abuse in vivo.
Harmine preferentially antagonizes cocaine-induced stereotyped responses in planarians.
Harmine efficacy against cocaine is reduced by a glutamate transporter subtype 1 (GLT-1) inhibitor.
Harmine displays efficacy against methamphetamine.
Harmine displays efficacy against the psychoactive bath salt compound mephedrone.
Acknowledgements
This study was supported by NIDA grants P30 DA013429 and DA030676. The authors thank Timothy Shickley, Ph.D., for suggesting Planaria as an in vivo test model.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allen MS, LaLoggia AJ, Dorn LJ, Martin MJ, Costantino G, Hagen TJ, Koehler KF, Skolnick P, Cook JM. Predictive binding of beta-carboline inverse agonists and antagonists via the CoMFA/GOLPE approach. J. Med. Chem. 1992;35:4001–4010. doi: 10.1021/jm00100a004. [DOI] [PubMed] [Google Scholar]
- 2.Aricioglu-Kartal F, Kayir H, Tayfun Uzbay I. Effects of harman and harmine on naloxone-precipitated withdrawal syndrome in morphine-dependent rats. Life Sci. 2003;73:2363–2371. doi: 10.1016/s0024-3205(03)00647-7. [DOI] [PubMed] [Google Scholar]
- 3.Baumann MH, Ayestas MA, Jr, Partilla JS, Sink JR, Shulgin AT, Daley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV. The Designer Methcathinone Analogs, Mephedrone and Methylone, are Substrates for Monoamine Transporters in Brain Tissue. Neuropsychopharmacology. 2012;37:1192–1203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cappendijk SL, Fekkes D, Dzoljic MR. The inhibitory effect of norharman on morphine withdrawal syndrome in rats: comparison with ibogaine. Behav. Brain Res. 1994;65:117–119. doi: 10.1016/0166-4328(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 5.Cappendijk SL, Fekkes D, Van Dalen A, Pepplinkhuizen L. The acute effects of norharman on cocaine self-administration and sensorimotor function in male Wistar rats. Eur. Neuropsychopharmacol. 2001;11:233–239. doi: 10.1016/s0924-977x(01)00090-6. [DOI] [PubMed] [Google Scholar]
- 6.Cebria F, Kudome T, Nakazawa M, Mineta K, Ikeo K, Gojobori T, Agata K. The expression of neural-specific genes reveals the structural and molecular complexity of the planarian central nervous system. Mech. Dev. 2002;116:199–204. doi: 10.1016/s0925-4773(02)00134-x. [DOI] [PubMed] [Google Scholar]
- 7.Eriksson KS, Panula P. gamma-Aminobutyric acid in the nervous system of a planarian. J. Comp. Neurol. 1994;345:528–536. doi: 10.1002/cne.903450405. [DOI] [PubMed] [Google Scholar]
- 8.Farzin D, Haghparast A, Motaman S, Barya F, Mansouri N. Effects of harmane and other β-carbolines on apomorphine-induced licking behavior in rat. Pharmacol. Biochem. Behav. 2011;98:215–219. doi: 10.1016/j.pbb.2011.01.001. Epub (2011) [DOI] [PubMed] [Google Scholar]
- 9.Farzin D, Mansouri N. Antidepressant-like effect of harmane and other beta-carbolines in the mouse forced swim test. Eur. Neuropsychopharmacol. 2006;16:324–328. doi: 10.1016/j.euroneuro.2005.08.005. Epub (2005) [DOI] [PubMed] [Google Scholar]
- 10.Ginovart N, Meyer JH, Boovariwala A, Hussey D, Rabiner EA, Houle S, Wilson AA. Positron emission tomography quantification of [11C]-harmine binding to monoamine oxidase-A in the human brain. J. Cereb. Blood Flow Metab. 2006;26:330–344. doi: 10.1038/sj.jcbfm.9600197. [DOI] [PubMed] [Google Scholar]
- 11.Glennon RA, Dukat M, Grella B, Hong S, Costantino L, Teitler M, Smith C, Egan C, Davis K, Mattson MV. Binding of beta-carbolines and related agents at serotonin (5-HT(2) and 5-HT(1A)), dopamine (D(2)) and benzodiazepine receptors. Drug Alcohol Depend. 2000;60:121–132. doi: 10.1016/s0376-8716(99)00148-9. [DOI] [PubMed] [Google Scholar]
- 12.Green AL. The human brain. Inhibition of rat and mouse brain monamine oxidases by (+)-amphetamine. Biochem J. 1971;121:37P–38P. doi: 10.1042/bj1210037p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hadlock GC, Webb KM, McFadden LM, Chu PW, Ellis JD, Allen SC, Andrenyak DM, Vieira-Brock PL, German CL, Conrad KM, Hoonakker AJ, Gibb JW, Wilkins DG, Hanson GR, Fleckenstein AE. 4-Methylmethcathinone (mephedrone): neuropharmacological effects of a designer stimulant of abuse. J. Pharmacol. Exp. Ther. 2011;339:530–536. doi: 10.1124/jpet.111.184119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang PK, Aarde SM, Angrish D, Houseknecht KL, Dickerson TJ, Taffe MA. Contrasting effects of d-methamphetamine, 3,4-methylenedioxymethamphetamine, 3,4-methylenedioxypyrovalerone, and 4-methylmethcathinone on wheel activity in rats. Drug Alcohol Depend. 2012 doi: 10.1016/j.drugalcdep.2012.05.011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Husbands SM, Glennon RA, Gorgerat S, Gough R, Tyacke R, Crosby J, Nutt DJ, Lewis JW, Hudson AL. Beta-carboline binding to imidazoline receptors. Drug Alcohol Depend. 2001;64:203–208. doi: 10.1016/s0376-8716(01)00123-5. [DOI] [PubMed] [Google Scholar]
- 16.Kehr J, Ichinose F, Yoshitake S, Goiny M, Sievertsson T, Nyberg F, Yoshitake T. Mephedrone, compared to MDMA (ecstasy) and amphetamine, rapidly increases both dopamine and serotonin levels in nucleus accumbens of awake rats. Br. J. Pharmacol. 2011;164:1949–1958. doi: 10.1111/j.1476-5381.2011.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim H, Sablin SO, Ramsay RR. Inhibition of monoamine oxidase A by beta-carboline derivatives. Arch. Biochem. Biophys. 1997;337:137–142. doi: 10.1006/abbi.1996.9771. [DOI] [PubMed] [Google Scholar]
- 18.Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol. Psychiatry. 2010;67:81–84. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koob GF. Neural mechanisms of drug reinforcement. Ann. NY Acad .Sci. 1992;654:171–191. doi: 10.1111/j.1749-6632.1992.tb25966.x. Review. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Sattler R, Yang EJ, Nunes A, Ayukawa Y, Akhtar S, Ji G, Zhang PW, Rothstein JD. Harmine, a natural beta-carboline alkaloid, upregulates astroglial glutamate transporter expression. Neuropharmacology. 2011;60:1168–1175. doi: 10.1016/j.neuropharm.2010.10.016. Epub (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez-Clemente J, Escubedo E, Pubill D, Camarasa J. Interaction of mephedrone with dopamine and serotonin targets in rats. Eur. Neuropsychopharmacol. 2011;22:231–236. doi: 10.1016/j.euroneuro.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Nishimura K, Kitamura Y, Taniguchi T, Agata K. Analysis of motor function modulated by cholinergic neurons in planarian Dugesia japonica. Neuroscience. 2010;168:18–30. doi: 10.1016/j.neuroscience.2010.03.038. [DOI] [PubMed] [Google Scholar]
- 23.Pagán OR, Rowlands AL, Azam M, Urban KR, Bidja AH, Roy DM, Feeney RB, Afshari LK. Reversal of cocaine-induced planarian behavior by parthenolide and related sesquiterpene lactones. Pharmacol. Biochem. Behav. 2008;89:160–170. doi: 10.1016/j.pbb.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Pagán OR, Rowlands AL, Fattore AL, Coudron T, Urban KR, Bidja AH, Eterović VA. A cembranoid from tobacco prevents the expression of nicotine-induced withdrawal behavior in planarian worms. Eur. J. Pharmacol. 2009;615:118–124. doi: 10.1016/j.ejphar.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parker CA, Anderson NJ, Robinson ES, Price R, Tyacke RJ, Husbands SM, Dillon MP, Eglan RM, Hudson AL, Nutt DJ, Crump MP, Crosby J. Harmane and harmalan are bioactive components of classical clonidine-displacing substance. Biochemistry. 2004;43:16385–16392. doi: 10.1021/bi048584v. [DOI] [PubMed] [Google Scholar]
- 26.Raffa RB, Rawls SM. Landes Bioscience. Austin, TX: 2010. A model for drug action and abuse. [Google Scholar]
- 27.Ramakrishnan L, Desaer C. Carbamazepine inhibits distinct chemoconvulsant-induced seizure-like activity in Dugesia tigrina. Pharmacol. Biochem. Behav. 2011;99:665–670. doi: 10.1016/j.pbb.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Ramoz L, Lodi S, Bhatt P, Reitz AB, Tallarida C, Tallarida RJ, Raffa RB, Rawls SM. Mephedrone ("bath salt") pharmacology: insights from invertebrates. Neuroscience. 2012;208:79–84. doi: 10.1016/j.neuroscience.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rasmussen B, Unterwald EM, Rawls SM. Glutamate transporter subtype 1 (GLT-1) activator ceftriaxone attenuates amphetamine-induced hyperactivity and behavioral sensitization in rats. Drug Alcohol Depend. 2011;118:484–488. doi: 10.1016/j.drugalcdep.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rawls SM, Cavallo F, Capasso A, Ding Z, Raffa RB. The beta-lactam antibiotic ceftriaxone inhibits physical dependence and abstinence-induced withdrawal from cocaine, amphetamine, methamphetamine, and clorazepate in planarians. Eur. J. Pharmacol. 2008;584:278–284. doi: 10.1016/j.ejphar.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 31.Rawls SM, Karaca F, Madhani I, Bhojani V, Martinez RL, Abou-Gharbia M, Raffa RB. β-lactamase inhibitors display anti-seizure properties in an invertebrate assay. Neuroscience. 2010;169:1800–1804. doi: 10.1016/j.neuroscience.2010.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes Hoberg M, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su ZZ, Gupta P, Fisher PB. β-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- 33.Ribeiro P, El-Shehabi F F, Patocka N. Classical transmitters and their receptors in flatworms. Parasitology. 2005;131(Suppl):S19–S40. doi: 10.1017/S0031182005008565. [DOI] [PubMed] [Google Scholar]
- 34.Song Y, Kesuma D, Wang J, Deng Y, Duan J, Wang JH, Qi RZ. Specific inhibition of cyclin-dependent kinases and cell proliferation by harmine. Biochem. Biophys. Res. Commun. 2004;317:128–132. doi: 10.1016/j.bbrc.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 35.Tallarida C, Song K, Raffa RB, Rawls SM. Glutamate carboxypeptidase II (GCPII) inhibitor displays anti-glutamate and anti-cocaine effects in an invertebrate assay. Amino Acids. 2011 doi: 10.1007/s00726-011-1052-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Venturini G, Stocchi F, Margotta V, Ruggieri S, Bravi D, Bellantuono P, Palladini G. A pharmacological study of dopaminergic receptors in planaria. Neuropharmacology. 1989;28:1377–1382. doi: 10.1016/0028-3908(89)90013-0. [DOI] [PubMed] [Google Scholar]
- 37.Vyas CA, Rawls SM, Raffa RB, Shackman JG. Glutamate and aspartate measurements in individual planaria by rapid capillary electrophoresis. J. Pharmacol. Toxicol. Methods. 2010;63:119–122. doi: 10.1016/j.vascn.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 38.Ward SJ, Rasmussen BA, Corley G, Henry C, Kim JK, Walker EA, Rawls SM. β-lactam antibiotic decreases acquisition of and motivation to respond for cocaine, but not sweet food, in C57Bl/6 mice. Behav. Pharmacol. 2011;22:370–373. doi: 10.1097/FBP.0b013e3283473c10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Winstock AR, Mitcheson LR, Deluca P, Davey Z, Corazza O, Schifano F. Mephedrone, new kid for the chop? Addiction. 2011;106:154–161. doi: 10.1111/j.1360-0443.2010.03130.x. [DOI] [PubMed] [Google Scholar]
- 40.Wood DM, Davies S, Greene SL, Button J, Holt DW, Ramsey J, Dargan PI. Case series of individuals with analytically confirmed acute mephedrone toxicity. Clin. Toxicol. 2010;48:924–927. doi: 10.3109/15563650.2010.531021. [DOI] [PubMed] [Google Scholar]
- 41.Young AM, Bradford HF. N-methyl-D-aspartate releases excitatory amino acids in rat corpus striatum in vivo. J. Neurochem. 1991;56:1677–1683. doi: 10.1111/j.1471-4159.1991.tb02067.x. [DOI] [PubMed] [Google Scholar]