Presentation of Case
A 27-year-old male farmer presented himself to an integrated health centre in the Bankim health district of the Adamaoua region of Cameroon with two ulcerative lesions with undermined edges on the upper chest and neck as well as enlarged and indurated lymph nodes of the neck (see Figure 1). He had not sought any traditional treatment and indicated that the condition had been ongoing for one month. Given the known high prevalence of Mycobacterium ulcerans (M. ulcerans) disease (Buruli ulcer) in the Bankim health district [1], ulcer exudates were examined for acid-fast bacilli by Ziehl-Neelsen (ZN) staining at the local health centre and tested positive. Based on the positive ZN stain, a diagnosis of Buruli ulcer (BU) was made and the treatment recommended for BU by the World Health Organization (WHO) [2], daily rifampicin (600 mg p.o.) and streptomycin (1 g i.m.) for 56 days, was administered to the patient. Additional swabs of ulcer exudates were analyzed using the M. ulcerans–specific IS2404 quantitative polymerase chain reaction (qPCR) assay [3]. All four swabs obtained from the patient tested negative. Exudate swabs were also used for the initiation of a culture on Löwensein-Jensen medium after decontamination with 2.5% oxalic acid for 30 minutes at room temperature. After 8.5 weeks of incubation at 30°C, the optimal growth temperature of M. ulcerans, mycobacterial growth was observed. The cultured mycobacteria were ZN positive, but negative in the M. ulcerans–specific IS2404 qPCR. PCR amplification [4] and DNA sequencing of the rifampicin resistance determining region (RRDR) of the rpoB gene identified the strain as belonging to the M. tuberculosis complex; no rifampicin resistance conferring mutation in the RRDR was found. A qPCR identifying a single-nucleotide (A to C) change at position 2′154′724 further characterized the cultivated strain as belonging to Lineage 4 (Euro-American Linage) of M. tuberculosis [5]. Spoligotyping and analysis using the SITVITWEB database revealed that the strain belonged to the “T-family” [6] of M. tuberculosis, a spoligotype of Lineage 4 which encompasses all strains that are difficult to classify into other spoligotype families. While the strain therefore does not belong to the “Cameroon Family” of TB, the obtained spoligo type has previously been reported to occur in Cameroon [6].
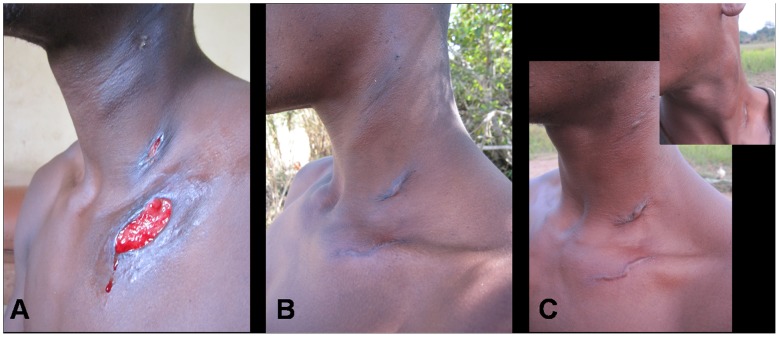
Figure 1. Clinical evolution of TB patient discovered in BU-endemic area.
A 27-year-old male presented to an integrated health centre in a BU-endemic area in Cameroon with multiple lesions on the neck and upper chest. After acid-fast bacilli were observed in the wound exudates, the patient was diagnosed with BU and treated according to WHO guidelines. Figure 1A shows the patient on day 17 of BU treatment. The lesions healed following eight weeks of rifampicin/streptomycin combination therapy. Figure 1B and C show the patient 86 and 178 days after completion of BU treatment, respectively. Further laboratory analysis on the original wound exudates showed that the patient was suffering from TB as opposed to BU, and the appropriate long-term TB treatment was administered.
Based on this laboratory diagnosis of an M. tuberculosis infection, the patient was re-examined 186 days after completion of the BU treatment. At this point, the ulcers had fully scarred (see Figure 1). However, the lymph nodes of the neck remained enlarged and indurated. A chest X-ray provided no evidence for pulmonary tuberculosis (TB), and the patient tested negative for human immunodeficiency virus (HIV) infection. Given the laboratory results and the clinical presentation, the patient was retrospectively diagnosed as a case of cutaneous TB [7]. Given the insufficiency of the BU treatment to cure TB, the patient was started on the full regimen of the standard TB treatment recommended by the Cameroon National TB Control Program: two months of isoniazid, rifampicin, ethambutol, and pyrazinamide followed by four months of isoniazid and rifampicin.
Case Discussion
Ethical approval (clearance N° 041/CNE/DNM/09, 19/06/2009) to analyze patient specimens was obtained from the National Ethics Committee of Cameroon, registered under the N° IRB00001954. Written informed consent from the patient was obtained before specimens were used for reconfirmation of clinical diagnosis and detailed laboratory analysis.
BU disease presents with a variety of clinical manifestations including nonulcerative forms such as movable subcutaneous nodules, plaques, and oedema, which may eventually progress to ulcerative lesions with characteristic undermined edges. Without treatment, ulcers may enlarge considerably and involve entire limbs or large areas of the trunk [8]. It is believed that mycolactone, the macrolide toxin produced by M. ulcerans, largely contributes to the pathogenesis of BU disease [9]. The diversity in clinical presentation renders clinical diagnosis difficult. Of the four currently available methods for laboratory reconfirmation of BU [8], only one, ZN microscopy, is suitable as a point-of-care diagnostic test in the African endemic areas, which are usually remote and rural. However, its sensitivity is limited [10], [11]. Cultivation of the slow-growing mycobacteria, histopathology, and PCR-based detection of M. ulcerans DNA can only be performed in central reference laboratories.
Given its wide range of clinical presentations, the differential diagnosis of cutaneous tuberculosis, which makes up 1%–2% of all TB cases worldwide [7], [12], is also difficult. Both infectious and noninfectious diseases of the skin need to be considered when examining a potential case of cutaneous TB [13]. For a definite diagnosis, histological examination, PCR, or optimally isolation of M. tuberculosis is required. All of these diagnostic methods again require sophisticated reference laboratories [7], [12]. Once diagnosed, patients can be treated with the regiment that is also used to treat pulmonary TB [7]. It is remarkable that a clinical manifestation very similar to that of BU disease was observed in the case presented here, although M. tuberculosis does not produce a potent macrolide toxin.
For patients from BU-endemic regions, ZN microscopy is usually accepted as reconfirmation of the clinical diagnosis and, to reduce costs, it has been suggested that only ZN negative swabs should be sent to a reference laboratory for analysis by PCR [11]. While the vast majority of ZN positive samples are also PCR positive, sensitivity of PCR is much higher than microscopy and many ZN negative samples still turn out PCR positive [11]. In BU-endemic areas as remote as the Bankim health district, clinical diagnosis by local medical staff is often considered sufficient for treatment decision. It has been shown that if clinical diagnosis is performed by highly trained and experienced staff, more than 90% of suspected cases can be reconfirmed by PCR [14]. However in regions where health care staff does not regularly encounter BU cases, a large proportion of suspected BU cases cannot be confirmed by laboratory tests [15]. Clinical overdiagnosis on one side, and true BU cases missed by relying only on ZN microscopy on the other side, can lead to over- or undertreatment of patients, respectively. It is therefore recommended that all BU cases should be laboratory confirmed [15]. If the decision is made to send only ZN negative swabs for confirmation to a reference laboratory, training of health care staff in BU differential diagnosis becomes even more important. Furthermore, such training should include the clinical presentation of other skin diseases, including other mycobacterial diseases. As demonstrated by the case presented here, lymphadenopathy—not a typical sign of BU—should lead to a more detailed clinical examination.
Overall, the case of a misdiagnosed patient with cutaneous TB in a BU-endemic area presented here further underscores the need for a simple, highly sensitive, and specific point-of-care diagnostic test for BU.
Learning Points
Clinical diagnosis of Buruli ulcer should be supplemented with laboratory examinations.
Microscopy, the only point-of-care laboratory test currently available, does not differentiate between Buruli ulcer and infections by other mycobacteria.
Clinical presentation of other mycobacterial diseases should be included when training staff on the differential diagnosis of Buruli ulcer.
There is a pressing need for a sensitive and specific point-of-care laboratory test for Buruli ulcer.
Acknowledgments
We would like to thank Fidèle Gaetan Wantong, Jacques Christian Minyem, and all the Bankim health care staff for their great support in the field. Thank you also to Marie-Thérèse Ruf and Katharina Röltgen for their valuable help in the laboratory.
Funding Statement
This work was supported by the Medicor Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Marion E, Landier J, Boisier P, Marsollier L, Fontanet A, et al. (2011) Geographic expansion of Buruli ulcer disease, Cameroon. Emerging Infect Dis 17: 551–553 doi:10.3201/eid1703.091859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization (2004) Provisional guidance on the role of specific antibiotics in the management of Mycobacterium ulcerans disease (Buruli ulcer). [Google Scholar]
- 3. Fyfe JAM, Lavender CJ, Johnson PDR, Globan M, Sievers A, et al. (2007) Development and application of two multiplex real-time PCR assays for the detection of Mycobacterium ulcerans in clinical and environmental samples. Appl Environ Microbiol 73: 4733–4740 doi:10.1128/AEM.02971-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim BJ, Lee SH, Lyu MA, Kim SJ, Bai GH, et al. (1999) Identification of mycobacterial species by comparative sequence analysis of the RNA polymerase gene (rpoB). J Clin Microbiol 37: 1714–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gagneux S, DeRiemer K, Van T, Kato-Maeda M, de Jong BC, et al. (2006) Variable host-pathogen compatibility in Mycobacterium tuberculosis. Proc Natl Acad Sci USA 103: 2869–2873 doi:10.1073/pnas.0511240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Demay C, Liens B, Burguière T, Hill V, Couvin D, et al. (2012) SITVITWEB - A publicly available international multimarker database for studying Mycobacterium tuberculosis genetic diversity and molecular epidemiology. Infect Genet Evol 12: 755–766 doi:10.1016/j.meegid.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 7. Bravo FG, Gotuzzo E (2007) Cutaneous tuberculosis. Clin Dermatol 25: 173–180 doi:10.1016/j.clindermatol.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 8. Asiedu K, Scherpbier R, Raviglione M (2000) Buruli ulcer. World Health Organization, Global Buruli Ulcer Initiative [Google Scholar]
- 9. George KM, Chatterjee D, Gunawardana G, Welty D, Hayman J, et al. (1999) Mycolactone: a polyketide toxin from Mycobacterium ulcerans required for virulence. Science 283: 854–857. [DOI] [PubMed] [Google Scholar]
- 10. Bretzel G, Siegmund V, Nitschke J, Herbinger KH, Thompson W, et al. (2007) A stepwise approach to the laboratory diagnosis of Buruli ulcer disease. Trop Med Int Health 12: 89–96 doi:10.1111/j.1365-3156.2006.01761.x. [DOI] [PubMed] [Google Scholar]
- 11. Yeboah-Manu D, Asante-Poku A, Asan-Ampah K, Ampadu EDE, Pluschke G (2011) Combining PCR with microscopy to reduce costs of laboratory diagnosis of buruli ulcer. Am J Trop Med Hyg 85: 900–904 doi:10.4269/ajtmh.2011.11-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.James WD (1994) Military Dermatology. Dept. of the Army. 617 p. [Google Scholar]
- 13. Frankel A, Penrose C, Emer J (2009) Cutaneous tuberculosis: a practical case report and review for the dermatologist. J Clin Aesthet Dermatol 2: 19–27. [PMC free article] [PubMed] [Google Scholar]
- 14. Mensah-Quainoo E, Yeboah-Manu D, Asebi C, Patafuor F, Ofori-Adjei D, et al. (2008) Diagnosis of Mycobacterium ulcerans infection (Buruli ulcer) at a treatment centre in Ghana: a retrospective analysis of laboratory results of clinically diagnosed cases. Trop Med Int Health 13: 191–198 doi:10.1111/j.1365-3156.2007.01990.x. [DOI] [PubMed] [Google Scholar]
- 15. Portaels F, Silva MT, Meyers WM (2009) Buruli ulcer. Clin Dermatol 27: 291–305 doi:10.1016/j.clindermatol.2008.09.021. [DOI] [PubMed] [Google Scholar]