Abstract
Anti-phospholipid syndrome (APS) is an autoimmune disorder characterized by the presence of autoantibody (AAb) to phospholipid (PL)-binding proteins, such as β2-glycoprotein I (β2GPI), and clinical manifestations including thrombosis and/or recurrent pregnancy loss. β2GPI-reactive T cells are clearly implicated in the generation of these AAb, but the mechanism responsible for their activation remains unclear. We hypothesized that immunization of mice with human β2GPI, in the context of a potent innate immune activator lipopolysaccharide (LPS), would generate not only high titers of anti-PL AAb, but also a strong β2GPI-specific T cell response. Healthy, nonautoimmune C57BL/6 mice were immunized repeatedly with human β2GPI in the presence of LPS. High titers of anti-PL to β2GPI appeared after the second immunization, with T cell reactivity to β2GPI detectable only after the fourth immunization. Splenic T cells from these mice proliferated in response to native β2GPI, alone or bound to anionic PL. These T cells produced IL-2 and IFN-γ, but not IL-4 or IL-10, indicating a Th1 bias of the β2GPI-specific response. These findings suggest that T cells responsive to β2GPI may become activated in APS patients by exposure to their cognate Ag in the context of innate immune activation and a pro-inflammatory environment.
Keywords: β2-Glycoprotein I, TC, anti-phospholipid syndrome, systemic lupus erythematosus (SLE), rodent, autoimmunity
Introduction
AAb to β2GPI have been studied extensively in patients with APS and systemic lupus erythematosus (SLE), but the T cell response to this protein has received little attention. T cells isolated from APS patients and healthy controls can recognize β2GPI, but mainly in non-native forms (e.g. reduced or recombinant), or when bound to anionic PL [1–3]. These findings suggest that β2GPI-reactive T cells recognize an epitope that is derived from altered, rather than native, β2GPI. However, the in vivo stimulus responsible for initiating this autoreactive T cell response remains unknown.
We hypothesized that immunization of mice with human β2GPI, in the context of a potent innate immune activator that results in systemic inflammation, would generate a strong β2GPI-specific T cell response. To induce a strong and persistent T cell response to β2GPI, we selected LPS, a prototypic activator of innate immunity and a mediator of inflammation known to cause up-regulation of CD80 and CD86 [4]. LPS, which signals via TLR4, can also have additional immunogenic effects, such as enhanced Ag presentation by dendritic cells (DC) and enhanced survival of memory T cells [5]. We have previously shown that immunization of C57BL/6 or BALB/c mice with human β2GPI, an apoptotic (apo) cell-binding protein, induces a long-lived, potent antibody (Ab) response not only to β2GPI, but also to multiple SLE autoantigen (AAg), presumably via epitope spread to apo cell-derived Ag. Autoimmunity culminated in overt glomerulonephritis closely resembling that in SLE [6].
We show here that T cells from healthy, non-autoimmune C57BL/6 mice immunized with β2GPI in the presence of LPS respond to both native and PL-bound β2GPI with a Th1-biased cytokine response. These data suggest that T cells responsive to native β2GPI may become activated by repeated exposure to this protein in the context of innate immune activation and systemic inflammation.
Materials and methods
Human β2GPI was from Crystal Chem (Downers, IL, USA), LPS (Escherichia coli-derived, serotype 0111:B4) from List Biological (Campbell, CA, USA), and phospholipid (PL) from Avanti Polar Lipids, Inc. (Alabaster, AL, USA).
Female C57BL/6 mice (10–12 week-old; Harlan Sprague Dawley, Inc., Indianapolis, IN, USA) were maintained under specific pathogen free (SPF) conditions, and immunized i.v. with either HEPES buffer (10, 150 mM NaCl, pH 7.4) or human β2GPI (20 μg for first, and 10 μg for subsequent immunizations), followed 24 h later by i.v. injection of LPS (10 μg) [6]. Mice received a total of two or four immunizations at 2-week intervals, and were bled 7–12 days post-immunization. Anti-β2GPI and anti-cardiolipin antibodies (ACL) were measured as previously described [6].
Splenic T cells, isolated using EasySep Mouse T cell Enrichment kits (StemCell Technologies, Vancouver, BC, Canada), were plated (1 × 105 cells/well) in RPMI 1640 supplemented with 10% β2GPI-depleted fetal bovine serum (FBS), L-glutamine, HEPES, non-essential amino acids, penicillin/streptomycin, and β-mercaptoethanol (βME). Antigen-presenting cells (APC) (mitomycin C-treated naïve C57BL/6 splenocytes) were added (4 × 105 cells/well), followed by antigen (Ag) (native β2GPI [15 μg/ml]), alone or preincubated with PL vesicles [0.15 μM]; human serum albumin (HSA) [30 μg/ml]; or concanavalin A (Con A) [1 or 5 μg/ml]. Cell proliferation was assessed by BrdU incorporation (Indianapolis, IN, USA). T cell (1 × 106 cells/well) were co-cultured with APC (4 × 106 cells/well) in the presence of Ag for 48 h. Secreted cytokines were measured using ELISA kits (BD Biosciences, San Jose, CA, USA).
Statistical significance was determined by a two-tailed unpaired t test with Welch correction using InStat 3.0 (GraphPad Software, El Camino Real, CA, USA).
Results and discussion
Immunization with β2GPI in the presence of LPS, a potent innate immune activator, induces a T cell response to native β2GPI
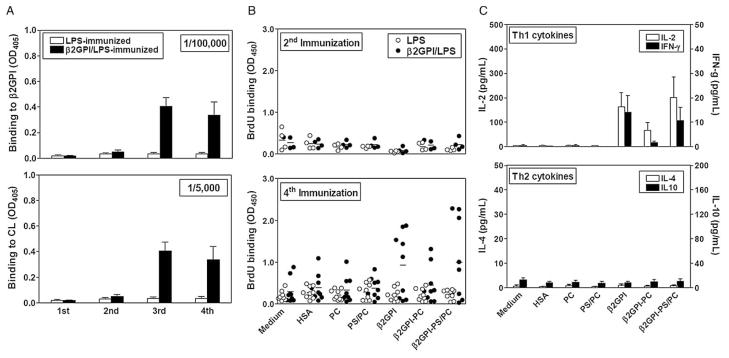
C57BL/6 mice immunized with β2GPI and LPS (β2GPI/LPS), but not LPS alone, developed high titers of anti-β2GPI and ACL IgG Ab (>1/100,000 and >1/5000, respectively) following the 2nd immunization, and 4- to 20-fold higher titers post-4th immunization (Figure 1A). In contrast, a significant T cell response to β2GPI was not detected until after the 4th immunization (Figure 1B). Of nine mice immunized with β2GPI/LPS, five showed significant T cell proliferation to β2GPI, while no β2GPI-specific T cell response was observed in mice immunized with LPS alone (Figure 1B). While it may seem paradoxical that anti-β2GPI IgG is observed prior to the detection of a β2GPI-specific T cell response, the number of β2GPI-reactive T cell activated following the first two immunizations is likely small and insufficient for detection in vitro.
Figure 1.
β2GPI-dependent Ab and T cell responses in mice immunized with β2GPI/LPS. (A) Anti-β2GPI and ACL IgG were detected by ELISA in plasma (diluted as indicated) from LPS- or β2GPI/LPS-immunized mice. β2GPI/LPS-immunized mice produced high titers of anti-β2GPI ( p < 0.0001) and ACL ( p < 0.02) IgG, compared to LPS-immunized mice, after the 3rd immunization. (B) Proliferation of splenic T cells from LPS- or β2GPI/LPS-immunized mice in response to various Ag was measured. Proliferation to β2GPI (alone or bound to PS/PC) was significant in β2GPI/LPS-, but not LPS-immunized, mice after the 4th, but not the 2nd, immunization (β2GPI, p < 0.02; β2GPI-PS/PC, p < 0.03). (C) Th1 and Th2 cytokines produced by T cells from β2GPI/LPS-immunized mice post-4th immunization in response to various Ag were quantitated. LPS-immunized mice (not shown) showed cytokine levels similar to those of controls (medium, HSA, or PL). IL-2 and IFN-γ production in response to β2GPI, alone or bound to PS/PC, was significant (p < 0.05) compared to LPS-immunized mice, while IL-4 and IL-10 production did not differ from controls. Each bar represents the mean concentration (pg/ml) ± SEM for each group (n = 4–10).
T cells induced by immunization with β2GPI/LPS respond to native or PL-bound β2GPI
To determine whether the binding of β2GPI to anionic PL alters T cell responses to this protein, PL vesicles consisting of 30% PS and 70% PC (PS/PC), or PC alone, were preincubated with β2GPI (15 μg/ml). Unbound β2GPI was removed. β2GPI binding to anionic PL (PS/PC) was 4.7 μg/ml and, as expected, greater than that to neutral PL (PC; 2.7 μg/ml). T cells from β2GPI/LPS-immunized mice proliferated similarly to native or PS/PC-bound β2GPI, despite the much lower concentration of β2GPI in the PS/PC-bound Ag preparation (4.7 vs. 15 μg/ml; Figure 1B). In contrast, no proliferation was seen in response to PC-bound β2GPI.
IL-2 responses in the β2GPI/LPS- and LPS-immunized mice paralleled the proliferative responses (Figure 1C). Mice (6/9) immunized with β2GPI/LPS produced a significant IL-2 response ( p < 0.05) to either PS/PC-bound or native β2GPI, but not to PC-bound β2GPI. Together, these data demonstrate that T cells from β2GPI/LPS-immunized mice respond to β2GPI in its native form or bound to anionic PL.
β2GPI-Reactive T cells induced by β2GPI and LPS demonstrate a Th1-biased response
Th polarization of the T cell response is an important indicator of its origin and contribution to disease pathogenesis. To investigate the Th polarization of the β2GPI-specific response, we evaluated Th1 (IFN-γ) and Th2 (IL-4, IL-10) cytokine production in response to Ag (Figure 1C). Mice immunized with β2GPI/LPS produced significant levels of IFN-γ in response to either native or PS/PC-bound β2GPI, but not PC-bound β2GPI. In contrast, the same mice produced insignificant levels of IL-4 and IL-10. This profile is characteristic of a Th1 type response, and is consistent with findings in APS patients. Several studies have shown IFN-γ production by β2GPI-reactive T cells [2,7,8] from APS patients, and one study found Th1-polarization in APS patients, but not NHD [9].
Innate immune activation is necessary for the generation of a potent T cell response to native β2GPI
We show here that T cells from mice immunized with β2GPI and LPS proliferated in response to native β2GPI, alone or bound to anionic PL. These T cells exhibited a Th1 profile, producing IL-2 and IFN-γ, but not IL-4 and IL-10, in response to these Ag. Of note, β2GPI-reactive T cell responses were detectable in vitro only after the 4th immunization, while anti-β2GPI and ACL IgG were clearly observed by the second immunization. These data suggest that β2GPI-reactive T cell may be difficult to detect without persistent re-stimulation with Ag, possibly in the context of LPS.
Potential physiological sources of anionic PL to which β2GPI might bind include apo cells, endothelial cells, oxLDL, and activated platelets. While interaction of endogenous β2GPI with anionic PL should be nonimmunogenic, the presence of a concomitant innate or inflammatory stimulus could lead to an altered immune response to this self-protein. In this case, interaction of β2GPI with anionic PL could result in the presentation of novel T cell epitopes [8] or even modulation of the Ag presentation pathway [10–12]. Both putative mechanisms might lead to activation of T cells specific for epitopes not observed in nonautoimmune individuals.
Acknowledgments
This work was supported by Canadian Institutes of Health Research (CIHR) operating grants (MOP-42391; MOP-67101; J.R.); a GRIP Renal Innovations Program Award from Genzyme, Inc. (J.S.L.); a McGill University Health Centre (MUHC) Research Institute Studentship (T.T.); MUHC/Department of Medicine Fellowships (A.R.S., M.D.); a CIHR Fellowship (A.R.S.); a Max Stern Studentship (E.R.); a Fond de la recherche en santé du Québec Studentship (E.R.); and a CIHR Studentship (E.R.).
References
- 1.Hattori N, Kuwana M, Kaburaki J, Mimori T, Ikeda Y, Kawakami Y. TC that are autoreactive to beta2-glycoprotein I in patients with antiphospholipid syndrome and healthy individuals. Arthritis Rheum. 2000;43:65–75. doi: 10.1002/1529-0131(200001)43:1<65::AID-ANR9>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 2.Arai T, Yoshida K, Kaburaki J, Inoko H, Ikeda Y, Kawakami Y, Kuwana M. Autoreactive CD4(+) TC clones to beta2-glycoprotein I in patients with antiphospholipid syndrome: Preferential recognition of the major phospholipid-binding site. Blood. 2001;98:1889–1896. doi: 10.1182/blood.v98.6.1889. [DOI] [PubMed] [Google Scholar]
- 3.Ito H, Matsushita S, Tokano Y, Nishimura H, Tanaka Y, Fujisao S, Mitsuya H, Hashimoto H, Nishimura Y. Analysis of TC responses to the beta 2-glycoprotein I-derived peptide library in patients with anti-beta 2-glycoprotein I Ab-associated autoimmunity. Hum Immunol. 2000;61:366–377. doi: 10.1016/s0198-8859(99)00184-6. [DOI] [PubMed] [Google Scholar]
- 4.Khoruts A, Mondino A, Pape KA, Reiner SL, Jenkins MK. A natural immunological adjuvant enhances TC clonal expansion through a CD28-dependent, interleukin (IL)-2-independent mechanism. J Exp Med. 1998;187:225–236. doi: 10.1084/jem.187.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maxwell JR, Rossi RJ, McSorley SJ, Vella AT. TC clonal conditioning: A phase occurring early after Ag presentation but before clonal expansion is impacted by toll-like receptor stimulation. J Immunol. 2004;172:248–259. doi: 10.4049/jimmunol.172.1.248. [DOI] [PubMed] [Google Scholar]
- 6.Levine JS, Subang R, Nasr SH, Fournier S, Lajoie G, Wither J, Rauch J. Immunization with the apo cell-binding protein, β2-glycoprotein I, in the presence of lipopolysaccharide recapitulates both AAb emergence and nephritis of human SLE. J Immunol. 2006;177:6504–6516. doi: 10.4049/jimmunol.177.9.6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Visvanathan S, McNeil HP. Cellular immunity to beta 2-glycoprotein-1 in patients with the antiphospholipid syndrome. J Immunol. 1999;162:6919–6925. [PubMed] [Google Scholar]
- 8.Kuwana M, Matsuura E, Kobayashi K, Okazaki Y, Kaburaki J, Ikeda Y, Kawakami Y. Binding of beta 2-glycoprotein I to anionic phospholipids facilitates processing and presentation of a cryptic epitope that activates pathogenic autoreactive TC. Blood. 2005;105:1552–1557. doi: 10.1182/blood-2004-08-3145. [DOI] [PubMed] [Google Scholar]
- 9.Karakantza M, Theodorou GL, Meimaris N, Mouzaki A, John E, Andonopoulos AP, Maniatis A. Type 1 and type 2 cytokine-producing CD4 + and CD8 + TC in primary antiphospholipid syndrome. Ann Hematol. 2004;83:704–711. doi: 10.1007/s00277-004-0910-7. [DOI] [PubMed] [Google Scholar]
- 10.Yamaguchi Y, Seta N, Kaburaki J, Kobayashi K, Matsuura E, Kuwana M. Excessive exposure to anionic surfaces maintains AAb response to beta(2)-glycoprotein I in patients with antiphospholipid syndrome. Blood. 2007;110:4312–4318. doi: 10.1182/blood-2007-07-100008. [DOI] [PubMed] [Google Scholar]
- 11.Kajiwara T, Yasuda T, Matsuura E. Intracellular trafficking of beta2-glycoprotein I complexes with lipid vesicles in macrophages: Implications on the development of antiphospholipid syndrome. J Autoimmun. 2007;29:164–173. doi: 10.1016/j.jaut.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Buttari B, Profumo E, Mattei V, Siracusano A, Ortona E, Margutti P, Salvati B, Sorice M, Rigano R. Oxidized beta2-glycoprotein I induces human DC maturation and promotes a T helper type 1 response. Blood. 2005;106:3880–3887. doi: 10.1182/blood-2005-03-1201. [DOI] [PubMed] [Google Scholar]