Summary
Background and objectives
Anti-heat shock protein (HSP)60 autoantibodies are associated with atherosclerosis and are known to affect endothelial cells in vitro. However, their role in thrombus formation remains unclear. We hypothesized that anti-HSP60 autoantibodies could potentiate thrombosis, and evaluated the effect of anti-murine HSP60 antibodies in a ferric chloride (FeCl3)-induced murine model of carotid artery injury.
Methods
Anti-HSP60, or control, IgG was administered to BALB/c mice 48 h prior to inducing carotid artery injury, and blood flow was monitored using an ultrasound probe.
Results
Thrombus formation was more rapid and stable in anti-HSP60 IGG-treated mice than in controls (blood flow = 1.7% ± 0.6% vs. 34% ± 12.6%, P = 0.0157). Occlusion was complete in all anti-HSP60 IgG-treated mice (13/13), with no reperfusion being observed. In contrast, 64% (9/14) of control mice had complete occlusion, with reperfusion occurring in 6/9 mice. Thrombi were significantly larger in anti-HSP60 IgG-treated mice (P = 0.0001), and contained four-fold more inflammatory cells (P = 0.0281) than in controls. Non-injured contralateral arteries of anti-HSP60 IgG-treated mice were also affected, exhibiting abnormal endothelial cell morphology and significantly greater von Willebrand factor (VWF) and P-selectin expression than control mice (P = 0.0024 and P = 0.001, respectively).
Conclusions
In summary, the presence of circulating anti-HSP60 autoantibodies resulted in increased P-selectin and VWF expression and altered cell morphology in endothelial cells lining uninjured carotid arteries, and promoted thrombosis and inflammatory cell recruitment in FeCl3-injured carotid arteries. These findings suggest that anti-HSP60 autoantibodies may constitute an important prothrombotic risk factor in cardiovascular disease in human vascular disease.
Keywords: autoimmunity, endothelial cells, heat shock proteins, infection, thrombosis, von Willebrand factor
Introduction
Immunity to the heat shock protein (HSP)60 family has been implicated in endothelial cell stress/activation and the development of atherosclerosis [1]. This family of proteins shows considerable sequence homology among species, and immune reactions to HSP60 have been widely described in infections due to microorganisms that express HSP65 [2]. Anti-HSP65 antibodies, induced in response to these pathogens, can cross react through molecular mimicry [2] with self-HSP60 expressed on endothelial cells [3–6]. Elevated levels of these anti-HSP60 autoantibodies have been associated with progression and severity of atherosclerosis [7–14], as well as with thrombotic events in the context of systemic lupus erythematosus [15]. Furthermore, anti-HSP60 autoantibodies have been shown to bind to HSP60 expressed on the surface of cultured endothelial cells, and to induce cytotoxicity [3,16,17] or apoptosis [15,18] in these cells.
Thrombotic occlusion of the major arteries supplying the heart and brain typically occurs in the setting of pre-existing atherosclerosis [19]. The sudden transition from a stable, often clinically silent, state to overt thrombosis is thought to follow plaque rupture and denudation of endothelial cells, which exposes the thrombogenic subendothelial matrix to circulating blood [20,21]. Few studies have assessed the pathogenic effects of anti-HSP60 autoantibodies in vivo, and fewer still their role in thrombosis. On the basis of the known effects of anti-HSP60 autoantibodies in vitro [3,15–18] and their association with atherosclerosis and thrombosis in vivo [22], we hypothesized that these antibodies can potentiate thrombus formation. We tested this hypothesis in a murine model of carotid artery thrombosis, in which BALB/c mice were treated with either anti-HSP60 autoantibodies, generated against mycobacterial HSP65, or control IgG. Our findings demonstrate that systemic exposure to anti-HSP60 autoantibodies promotes an altered endothelial cell phenotype and enhances thrombus formation in a murine model of carotid artery injury, supporting a prothrombotic role for these autoantibodies.
Materials and methods
Mice
Animal experiments were approved by the McGill University and the Montreal Heart Institute Animal Care Committees. Specific pathogen-free female BALB/c mice (Harlan Sprague Dawley, Indianapolis, IN, USA; 10–12 weeks) were used in all experiments.
Production and detection of anti-murine HSP60 autoantibodies
Mice were injected subcutaneously with either mycobacterial HSP65 [Stressgen/Assay Designs, Inc., Ann Arbor, MI, USA; 10 μg in 100 μL of 0.01mol L−1 phosphate-buffered saline (PBS), pH 7.3] or PBS, emulsified in complete Freund’s adjuvant (CFA), every 2 weeks for a total of three immunizations, and bled 12–14 days after each immunization. Sera (diluted 1/100) were tested for the presence of anti-mycobacterial HSP65 and anti-murine HSP60 antibodies by enzyme-linked immunosorbent assay [15], with the following modifications: (i) the antigens were eithermycobacterialHSP65 or recombinant murine HSP60 (Stressgen/AssayDesigns, Inc.); (ii) antibodies were detected using peroxidase-conjugated goat anti-mouse IgG (Southern Biotechnology Associates, Inc., Birmingham, AL, USA); and (iii) plates were developed with TMB Substrate Reagent (BD Pharmingen, Oakville, ON, Canada), and the OD405 was read (EL800 reader, Bio-Tek Instruments, Winooski, VT, USA). IgG was isolated from pooled sera of either HSP65-immunized mice with reactivity to murine HSP60 (anti-HSP60 IgG) or PBS-immunized mice (control IgG) using protein A Sepharose CL-4B (Sigma- Aldrich), and quantitated by Micro BCA assay (Pierce, Rockford, IL, USA).
Anti-HSP60 antibody treatment and ferric chloride (FeCl3) carotid injury model
Naïve mice were injected intravenously with 100 μl (1 mg/ml in PBS) of anti-HSP60 or control IgG that had been 0.22 μm-filtered immediately prior to injection, to ensure sterility and lack of IgG aggregates. Forty-eight hours later, the right carotid artery of anesthetized mice was injured with FeCl3 according to a standardized protocol [23]; the left artery served as a control. Briefly, aminiature ultrasound flow probe (0.5 VB 552; Transonic Systems, Ithaca, NY, USA), interfaced with a flow meter (T206; Transonic Systems) and a computer-based data acquisition program (Iox 2.2.17.19, Emka, Falls Church, VA, USA), was positioned around the artery. After stabilization of the blood flow, a 0.5 × 1.0-mm strip of filter paper soaked in 6% FeCl3 was applied to the surface of the adventitia for 3 min. After removal of the strip, monitoring of carotid blood flow was resumed, and was continued for 23 min.
Histology and immunohistochemistry
Carotid arteries were excised and fixed in Tissue Fix (Biopharm Inc., Laval, Canada) upon completion of the blood flow measurements. Carotid artery segments were then embedded in paraffin, sectioned at 6 μm, and stained with either hematoxylin and eosin [24], Verhoeff, or monoclonal antivon Willebrand factor (VWF) or P-selectin antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) [25]. Samples were visualized using an Olympus BX60 microscope (Olympus Imaging America Inc., Center Valley, PA, USA), and computerized morphometric analyses were performed using a Retiga 2000R camera (QImaging Corporation, Surrey, BC, Canada) and IMAGE PRO PLUS 6.2 software (Media Cybernetics, Bethesda, MD, USA).
Statistical analysis
For blood flow studies, point-by-point and area under the curve analyses were compared for each dataset using t-tests and Wilcoxon tests. For other experiments, statistical significance was determined by a two-tailed unpaired t-test with Welch correction, using INSTAT 3.0 (GraphPad Software Inc., San Diego, CA, USA).
Results
Immunization with mycobacterial HSP65 induces a break in tolerance to self-HSP60
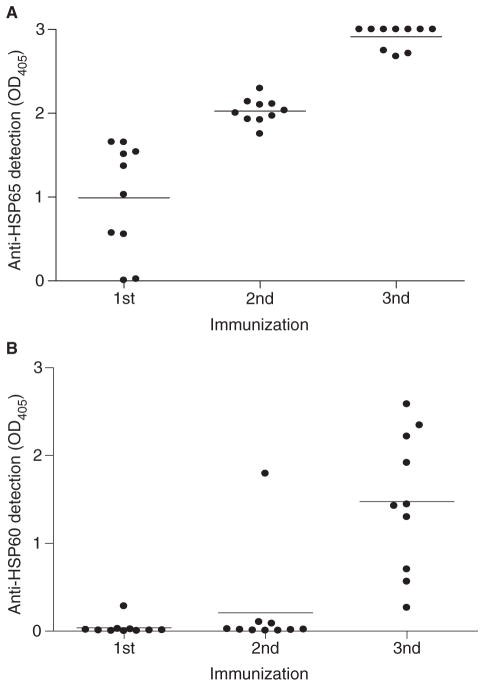
It has been proposed that infection with microbes expressing HSP65 can trigger an anti-self-HSP60 response through molecular mimicry [2]. To test this hypothesis directly, we immunized healthy, non-autoimmune BALB/cmice repeatedly with mycobacterial HSP65 and, following each immunization, tested their sera for the presence of anti-HSP65 (Fig. 1A) and murine anti-self-HSP60 (Fig. 1B) antibodies. As expected, HSP65 immunization induced a strong anti-HSP65 antibody response following the first immunization (Fig. 1A). In contrast, anti-HSP60 autoantibodies took longer to develop, and were detected in one animal as early as following the second immunization and in all mice following the third immunization (Fig. 1B). During the approximately 2-month period during which these mice were immunized and bled for antibody analysis, we did not observe any overt morbidity or mortality, although histology was not performed on tissues from these mice. Neither anti-HSP65 nor anti-HSP60 antibodies were detected in control mice immunized with PBS and CFA only (data not shown). These data clearly demonstrate that repeated exposure of non-autoimmune mice to mycobacterial HSP65 can induce a break in immune tolerance to self-HSP60, and that exposure to mycobacteria may induce anti-HSP60 autoantibodies.
Fig. 1.
Repeated immunization with mycobacterial heat shock protein (HSP)65 induces a break in tolerance to murine self-HSP60 in BALB/c mice. BALB/c mice were immunized with mycobacterial HSP65 in the presence of complete Freund’s adjuvant (CFA). Sera from immunized mice were evaluated for IgG antibody to (A) mycobacterial HSP65 or (B) murine HSP60. Each point represents the mean of duplicate samples from an individual mouse, and the bar represents the mean binding (OD405) for the entire group of mice (n = 10). These data are representative of five independent experiments.
Anti-HSP60 antibodies promote and stabilize thrombus formation
We selected the FeCl3-induced model of carotid artery injury, because it entails a localized and relatively non-invasive induction of thrombosis, and permits highly quantifiable monitoring of thrombus formation. To ensure that the model would be sensitive enough to detect subtle prothrombotic changes, we evaluated several concentrations of FeCl3 (5%, 6%, and 7.5%) to determine the optimal concentration for inducing a partial and unstable occlusion (Fig. S1). We chose 6% FeCl3 for our remaining experiments, as this concentration led to greater stability and reproducibility than 5% FeCl3, but seemed to be more sensitive to modulation of thrombogenesis than 7.5% FeCl3.
To examine the effect of anti-HSP60 autoantibodies on thrombus formation, purified IgG antibodies from anti-HSP60 autoantibody-producing or control mice were injected into naïve BALB/c mice 48 h prior to FeCl3-induced carotid artery injury. Passive transfer of anti-HSP60 antibodies avoids confounding factors that could result from immunization with HSP65 itself. Mice treated with control IgG had blood flow values similar to those of vehicle-treated mice, both before and after FeCl3-induced injury (Fig. S1), and occluded similarly [64% (9/14) vs. 67% (4/6); data not shown]. We conclude that control IgG had no detectable effect on blood flow or thrombus formation.
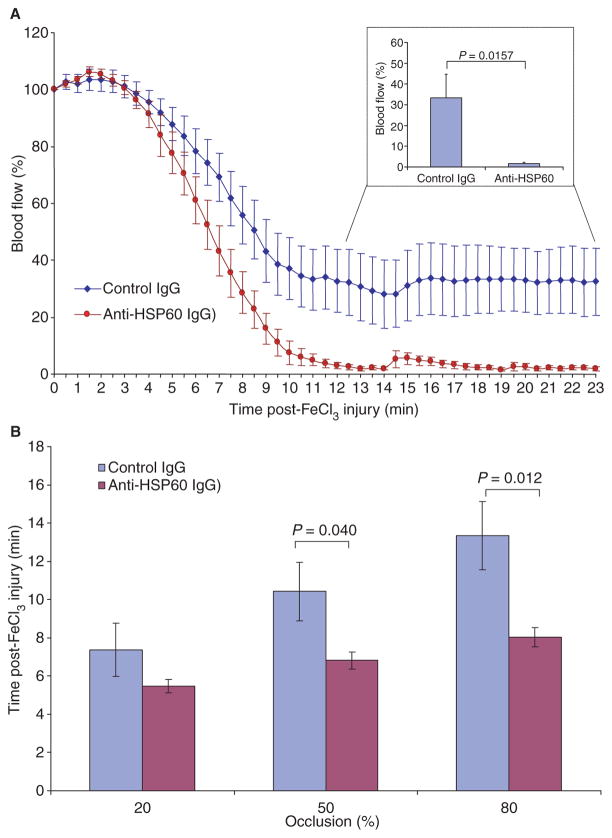
In marked contrast, passive transfer of anti-HSP60 IgG had a major effect on blood flow and thrombus formation. Whereas the control IgG-treated and anti-HSP60 IgG-treated groups did not differ for the first 5 min following FeCl3 injury (Fig. 2A), significant differences in blood flow were detected from 7 min onward. Anti-HSP60 IgG-treated mice achieved 50% and 80% occlusion significantly faster than did control IgG-treated mice (P = 0.040 and P = 0.012, respectively) (Fig. 2B). Moreover, although blood flow reduction was observed in all mice, complete occlusion of the carotid artery was present in 64% (9/14) of control mice, as compared to 100% of anti-HSP60 IgG-treated mice. Thus, passive transfer of anti-HSP60, but not control, IgG increases both the rapidity and extent of occlusion following FeCl3-induced injury of the carotid artery.
Fig. 2.
Anti-heat shock protein (HSP)60 antibodies promote thrombus stabilization in a murine model of carotid injury. (A) Blood flow was monitored (23 min) following application of 6% FeCl3 to the carotid arteries of mice injected with anti-HSP60 IgG or control IgG. Initial blood flow through the carotid artery was considered to be maximal (100%). Points represent the mean value for each group of mice, and bars represent standard error of the mean (SEM). The graph inset represents blood flow (mean and SEM) during the thrombus stabilization phase for mice treated with phosphate-buffered saline, control IgG, or anti-HSP60 IgG. (B) The time required to achieve 20%, 50% or 80% occlusion was determined from the curves in (A), and the mean time and SEM for each group are indicated (50% and 80%occlusion occurred significantly faster in anti-HSP60 IgG-treated mice than in control IgG-treated mice).
Among the various effects of anti-HSP60 IgG in our model, its most striking was on thrombus stabilization. Anti-HSP60 IgG-treated mice showed complete and stable occlusion (blood flow = 1.7% ± 0.6%) in all animals by 12 min, whereas the minimum blood flow achieved in control IgG-treated mice was 34.0% ± 12.6% (P = 0.0157) (Fig. 2A, inset). Moreover, no reperfusion was observed in the anti-HSP60 IgG-treated mice up to the end of monitoring (23 min), indicating extreme stability of the thrombus. In contrast, reperfusion was observed in 67% of control IgG-treated mice (Fig. 2A). Taken together, these results indicate that anti-HSP60 IgG autoantibodies enhance thrombus formation, leading to the more rapid generation of a thrombus that is both more occlusive and more stable.
Anti-HSP60 antibodies promote the generation of larger thrombi
To analyze further the characteristics of the thrombi and underlying artery, FeCl3-injured carotid arteries from four anti- HSP60 IgG-treated and four control IgG-treated mice were subjected to histologic and immunohistochemical analysis, and computer-assisted planimetry. The mean cross-sectional area of thrombi observed in the carotid arteries of anti-HSP60 IgG-treated mice was significantly larger than that of thrombi in control IgG-treated mice (0.33 ± 0.011 mm2 vs. 0.05 ± 0.014 mm2, P = 0.0001) (Fig. 3A–E). To evaluate the effect of thrombus formation on the walls of affected carotid arteries, Verhoeff staining for elastic fibers was performed. The elastic laminae of the carotid arteries treated with control IgG appeared normal, as indicated by the wavy structure of the lamina (Fig. 3C). In contrast, carotid arteries from anti-HSP60 IgG-treated mice appeared distended, and the majority of the elastic fibers were stretched, unfolded, and almost linear (Fig. 3D). Taken together, these results indicate that treatment with anti-HSP60 IgG favors the formation of a larger thrombus, which expands the walls of the carotid artery and results in highly stretched elastic laminae.
Fig. 3.
Anti-heat shock protein (HSP)60 antibodies promote the generation of larger thrombi. Representative histologic transverse sections of carotid arteries injured with 6%FeCl3, stained with Verhoeff, illustrate the size of the thrombus and the structure of the elastic laminae of the vessels. The size of the thrombi in the carotid arteries of anti-HSP60 IgG-treated mice (B, D) was visually greater than that of those in control IgG-treated mice (A, C). In addition, the elastic fibers were stretched and unfolded in the anti-HSP60 IgG-treated mice (B, D), whereas those isolated from the control IgG-treated group (A, C) appeared normal, as indicated by the wavy folded structure of the elastic laminae. (E) The cross-sectional area of the thrombus was measured by computer-assisted planimetry, and the thrombus area for each carotid artery was calculated inmm2 by standard formulae. The mean thrombus size and standard error of the mean for each group are indicated. All data are representative of four mice from each group. (C) and (D) show the segment of the artery [indicated by the square in (A) and (B), respectively] at higher magnification [(A, B) original magnification, ×20; (C, D) original magnification, ×100]. Scale bars: 0.05 mm.
Anti-HSP60 antibodies favor inflammatory cell recruitment to the site of the thrombus
We further studied the morphology of thrombi from control and anti-HSP60 IgG-treated mice by hematoxylin and eosin staining. Thrombi from control IgG-treated mice were bland and contained predominantly fibrin and platelets (Fig. 4A), with only scattered, rare inflammatory cells (Fig. 4A,C). In striking contrast, thrombi occluding the injured carotid arteries of anti-HSP60 IgG-treated mice contained focal accumulations of inflammatory cells, especially neutrophils, located in close apposition to the vessel wall (Fig. 4B,D,E). Indeed, four-fold more inflammatory cells were observed in thrombi from anti-HSP60 IgG-treated mice than in thrombi from control mice, as assessed by the mean number of nuclei per thrombus (88 ± 17 vs. 17 ± 4, P = 0.0281) (Fig. 4F). These data suggest that anti-HSP60 antibodies favor the recruitment of inflammatory cells, in particular neutrophils, to the site of thrombus formation.
Fig. 4.
Anti-heat shock protein (HSP)60 antibodies favor inflammatory cell recruitment to the site of the thrombus. Representative histologic transverse sections of carotid arteries injured with 6% FeCl3, stained with hematoxylin and eosin, illustrate the structure of the thrombus and the presence of inflammatory cells. Few inflammatory cells were detected in the thrombi from control IgG-treated mice (A, C), as compared to a larger number of clustered cells in the thrombi of anti-HSP60 IgG-treated mice (B, D, E). (F) The number of inflammatory cells recruited into the thrombi was evaluated by counting the number of nuclei in a transverse section of the thrombus, using a computer-assisted enumeration method. The mean value and standard error of the mean for each group are indicated. All data are representative of four mice from each group. (D) and (E) show the segments of the artery [indicated by the squares on the right and left, respectively, in (B)] at higher magnification [(A, B) original magnification, ×20; (C, D, E) original magnification, ×40]. Scale bars: 0.05 mm.
Anti-HSP60 antibodies induce expression of VWF and P-selectin by endothelial cells
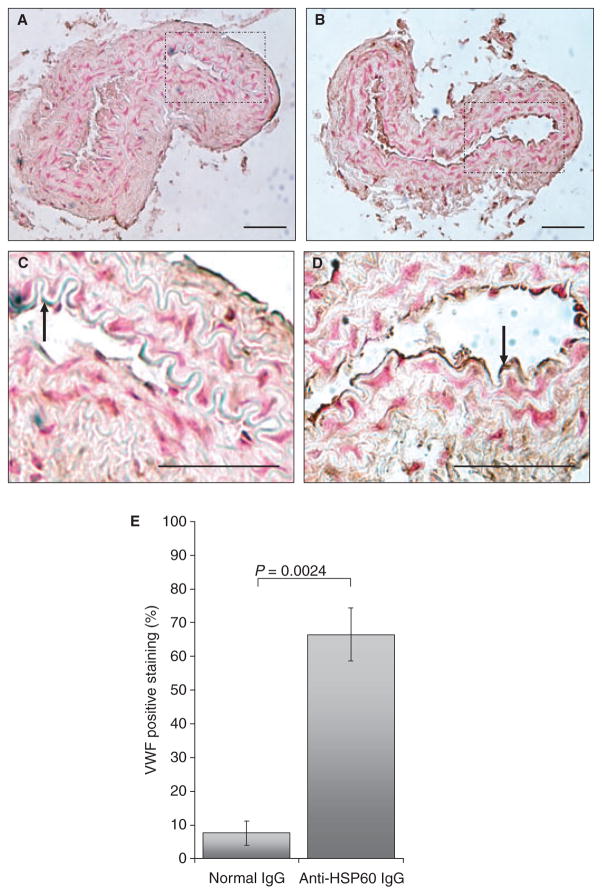
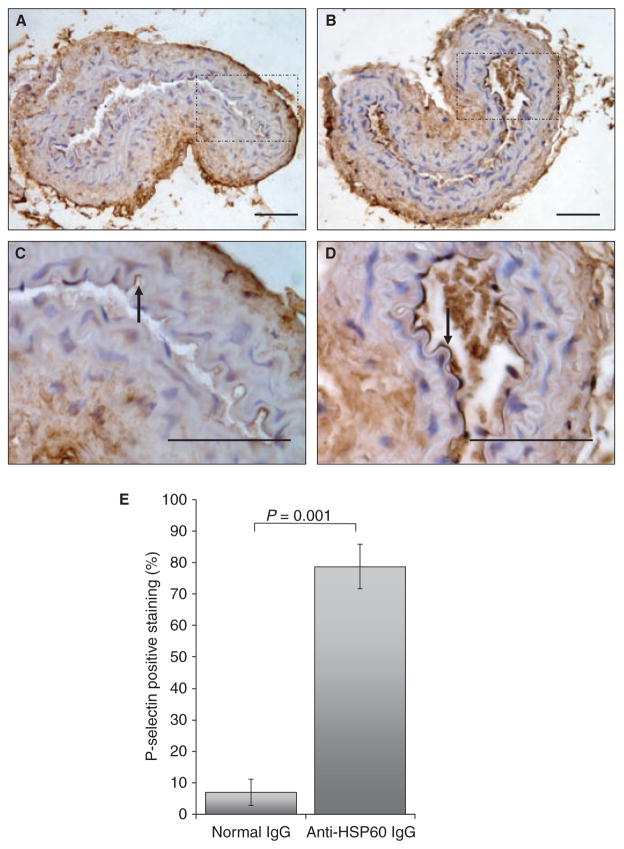
To examine the potential effect of anti-HSP60 IgG on uninjured arteries, the contralateral carotid arteries (n = 4 for each experimental group), which had not been exposed to FeCl3, were examined for cell morphology as well as VWF and P-selectin surface expression. Normal flat endothelial cell morphology was observed in the carotid arteries from control IgG-treated mice (Fig. 5A), whereas endothelial cells from carotid arteries of anti-HSP60 IgG-treated mice exhibited mild karyomegaly (enlarged nuclei), as shown by rounding of the cells and thinning of their cytoplasm (Fig. 5B). Possible early endothelial cell detachment from the basement membrane was also observed in one specimen (Fig. 5C). Importantly, VWF was detected on a significantly greater area of the endothelial surface in the intact contralateral carotid arteries from the anti-HSP60 IgG-treated group than in those from the control IgG-treated group (67% ± 8% vs. 8% ± 3%, P = 0.0024) (Fig. 5C,D,E). Moreover, the intensity of the staining by VWF-positive endothelial cells was greater in carotid arteries from anti-HSP60 IgG-treated mice, indicating that more VWF was expressed per cell. P-selectin, an adhesion molecule and a marker of endothelial activation, was also detected on a significantly greater area of the endothelial surface in intact contralateral carotid arteries from anti-HSP60 IgG-treated mice than in those from control IgG-treated mice (78% ± 14% vs. 7% ± 8%, P = 0.001) (Fig. 6C,D,E). Altered endothelial cell morphology and increased VWF and P-selectin expression may reflect a broader effect of anti-HSP60 autoantibodies on endothelial cell function.
Fig. 5.
Anti-heat shock protein (HSP)60 antibodies induce endothelial von Willebrand factor (VWF) overexpression. Representative histologic transverse sections of intact carotid arteries, contralateral to the arteries injured with 6%FeCl3, were stained for VWF. VWF detection is represented by dark brown staining on the surface of endothelial cells [examples of positive cells are indicated by arrows in (C) and (D)]. Note that VWF staining is more widespread and intense in intact carotid arteries from anti-HSP60 IgG-treated mice (B, D) than in intact carotid arteries from control IgG-treated mice (A, C). (E) The percentage of positive staining for VWF was evaluated by determining the ratio of the endothelial surface stained with anti-VWF (mm2) to the total endothelial surface (mm2), using computer-assisted technology. The mean value and standard error of the mean for each group are indicated. All data are representative of four mice from each group. (C) and (D) show the segments of the artery [indicated by the squares in (A) and (B), respectively] at higher magnification [(A, B) original magnification, ×20; (C, D) original magnification, ×40]. Scale bars: 0.05 mm.
Fig. 6.
Anti-heat shock protein (HSP)60 antibodies induce endothelial P-selectin overexpression. Representative histologic transverse sections of intact carotid arteries, contralateral to the arteries injured with 6%FeCl3, were stained for P-selectin. P-selectin detection is represented by dark brown staining on the surface of endothelial cells [examples of positive cells are indicated by arrows in (C) and (D)]. Note that P-selectin staining is more widespread and intense in intact carotid arteries from anti-HSP60 IgG-treated mice (B, D) than in intact carotid arteries from control IgG-treated mice (A, C). (E) The percentage of positive staining for P-selectin was evaluated by determining the ratio of the endothelial surface stained with anti-P-selectin (mm2) to the total endothelial surface (mm2), using computer-assisted technology. The mean value and standard error of the mean for each group are indicated. All data are representative of four mice from each group. (C) and (D) show the segments of the artery [indicated by the squares in (A) and (B), respectively] at higher magnification [(A, B) original magnification, ×20; (C, D) original magnification, ×40]. Scale bars: 0.05 mm.
Discussion
Conventional cardiovascular risk factors only partially predict the occurrence of atherosclerosis and thrombosis. Chronic infection and autoimmune reactions, especially to HSPs, may constitute another important variable in the pathogenesis of these diseases [26]. We demonstrate here that anti-HSP60 autoantibodies can arise in response to mycobacterial HSP65, and that these autoantibodies can potentiate thrombus formation. Furthermore, our findings show that anti-HSP60 autoantibodies affect both the uninjured endothelium and the developing thrombus, resulting in more rapid, extensive and stable thrombus formation. Although it is not known how anti-HSP60 autoantibodies promote thrombosis, our data suggest two potential mechanisms: (i) induction of a procoagulant endothelial cell phenotype, in which VWF and P-selectin expression may play a prominent role; and (ii) initiation of inflammation, with recruitment of inflammatory cells and release of inflammatory mediators.
We provide clear evidence that endothelial cells exposed to circulating anti-HSP60 autoantibodies overexpress VWF, a marker of endothelial cell dysfunction and of procoagulant activity [27]. VWF mediates platelet adhesion and platelet aggregation, and serves as a plasma carrier for factor VIII. In addition to its known role in aggregation of activated platelets, VWF may also be involved in activation-independent aggregation of platelets, a novel mechanism that has been demonstrated to initiate thrombus formation under high hemodynamic forces, such as may occur when the vascular lumen is restricted by a growing thrombus [28]. Finally, VWF has been described at the edges of forming thrombi, and may help to recruit platelets [29]. These VWF-dependent mechanisms may at least partially explain the striking differences in thrombus formation observed between anti-HSP60 IgG-treated and control IgG-treated mice.
We clearly demonstrate that endothelium exposed to circulating anti-HSP60 autoantibodies overexpresses the adhesion molecule P-selectin, a marker of endothelial cell activation [30]. The primary ligand for P-selectin is P-selectin glycoprotein ligand-1 (PSGL-1), which is constitutively found on all leukocytes and platelets. P-selectin is largely responsible for the rolling phase of the leukocyte adhesion cascade, through the transient interactions between P-selectin and PSGL-1. Moreover, P-selectin is also able to mediate the heterotypic aggregation of platelets on stimulated endothelium [30]. These P-selectin-related processes may also partly explain the inflammatory cell recruitment observed in the thrombus of anti-HSP60 IgG-treated mice, as well as the large size of these thrombi.
Inflammation may be another mechanism by which anti-HSP60 antibodies promote thrombosis. Endothelial cell-bound anti-HSP60 antibodies can recruit inflammatory cells, such as neutrophils and macrophages, whose secreted products can further activate platelets and the coagulation cascade. Activated platelets themselves may also contribute to recruitment of inflammatory cells. Finally, it is possible that anti-HSP60 autoantibodies affect endothelial cell function through mechanisms that are independent of VWF and inflammatory mediators, such as increased sensitivity to FeCl3 treatment. It seems likely that anti-HSP60 autoantibodies mediate their effects in our model through more than one mechanism.
The cellular antigen targeted by anti-HSP60 autoantibodies remains unknown, but is presumably located on the endothelium. Although mammalian HSP60 is generally considered to be an intracellular protein expressed only on the surface of cells that have been activated or stressed [31], endothelial cells in culture that are otherwise intact are known to express surface HSP60 [6] and to be recognized by anti-HSP60 autoantibodies [15], perhaps from a basal level of stress caused by in vitro culturing conditions. Thus, it is possible that surface expression of HSP60 may occur during normal endothelial cell turnover on morphologically intact cells, explaining the effect of anti- HSP60 treatment on uninjured contralalateral carotid arteries in our mice. Alternatively, it has been shown that a subset of anti-HSP60 antibodies can interact with several cell proteins (e.g. CD49f, CD51, CD61, and CD151) expressed on the surface of non-stressed endothelial cells [18]. In either case, anti- HSP60 antibodies might initiate an amplification loop, in which binding to endothelial cells could favor HSP60 expression on neighboring endothelial cells and the spread of anti- HSP60-induced endothelial dysfunction.
In summary, our findings demonstrate unequivocally the pathogenic potential of anti-HSP60 autoantibodies to accelerate thrombosis in healthy, non-autoimmune mice. Although other studies have examined the effect of anti-HSP60 autoantibodies on atherosclerosis [22], we believe ours to be the first to evaluate the effect of anti-HSP60 antibodies on thrombogenesis. Previous studies have suggested a link between anti-HSP60 autoantibodies and thrombosis in patients with systemic lupus erythematosus, although the numbers of patients were small [15]. Here, we present direct evidence that circulating anti-HSP60 autoantibodies result in altered endothelial cell morphology and promote expression of VWF and P-selectin in the absence of exogenous injury to the arterial wall. Furthermore, these antibodies promote thrombosis and inflammatory cell recruitment in FeCl3-injured arteries. Further studies are needed to clarify the role of anti-HSP60 autoantibodies as a clinical predictor of thrombotic events, as well as the mechanisms by which anti-HSP60 antibodies mediate their prothrombotic effects.
Supplementary Material
Acknowledgments
The authors are grateful to J. Correa for performing the statistical analysis on the blood flow data, to D. Lauzier, M.-H. Clavet-Lanthier and J.-F. Tanguay for their expertise and assistance with histology studies, and to P. Amireault for numerous discussions and comments about the manuscript.
This work was supported by Canadian Institutes of Health Research operating grants [MOP-42391 (J. Rauch), MOP-67101 (J. Rauch), and MOP-82767 (Y. Merhi)], the ‘Réseau en santé cardiovasculaire (RSCV)’ of the ‘Fonds de la recherche en santé du Québec (FRSQ)’ (E. Thorin), a Genzyme Renal Innovations Program Award (Genzyme Corporation) (J. S. Levine), and postdoctoral fellowships from the Research Institute of the McGill University Health Centre (M. Dieudé) and the Department of Medicine of McGill University (M. Dieudé).
Footnotes
Disclosure of Conflict of Interests
M. A. Gillis, J. F. Théorêt and G. Lajoie state that they have no conflict of interest.
Additional Supporting Information may be found in the online version of this article:
Fig. S1. Stability and reproducibility of blood flow in the FeCl3-induced murine model of carotid injury.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Wick G, Knoflach M, Xu Q. Autoimmune and inflammatory mechanisms in atherosclerosis. Annu Rev Immunol. 2004;22:361–403. doi: 10.1146/annurev.immunol.22.012703.104644. [DOI] [PubMed] [Google Scholar]
- 2.Mayr M, Kiechl S, Willeit J, Wick G, Xu Q. Infections, immunity, and atherosclerosis: associations of antibodies to Chlamydia pneumoniae, Helicobacter pylori, and cytomegalovirus with immune reactions to heat-shock protein 60 and carotid or femoral atherosclerosis. Circulation. 2000;102:833–9. doi: 10.1161/01.cir.102.8.833. [DOI] [PubMed] [Google Scholar]
- 3.Xu Q, Schett G, Seitz CS, Hu Y, Gupta RS, Wick G. Surface staining and cytotoxic activity of heat-shock protein 60 antibody in stressed aortic endothelial cells. Circ Res. 1994;75:1078–85. doi: 10.1161/01.res.75.6.1078. [DOI] [PubMed] [Google Scholar]
- 4.Khan IU, Wallin R, Gupta RS, Kammer GM. Protein kinase A-catalyzed phosphorylation of heat shock protein 60 chaperone regulates its attachment to histone 2B in the T lymphocyte plasma membrane. Proc Natl Acad Sci USA. 1998;95:10425–30. doi: 10.1073/pnas.95.18.10425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfister G, Stroh CM, Perschinka H, Kind M, Knoflach M, Hinterdorfer P, Wick G. Detection of HSP60 on the membrane surface of stressed human endothelial cells by atomic force and confocal microscopy. J Cell Sci. 2005;118:1587–94. doi: 10.1242/jcs.02292. [DOI] [PubMed] [Google Scholar]
- 6.Soltys BJ, Gupta RS. Cell surface localization of the 60 kDa heat shock chaperonin protein (hsp60) in mammalian cells. Cell Biol Int. 1997;21:315–20. doi: 10.1006/cbir.1997.0144. [DOI] [PubMed] [Google Scholar]
- 7.Xu Q, Willeit J, Marosi M, Kleindienst R, Oberhollenzer F, Kiechl S, Stulnig T, Luef G, Wick G. Association of serum antibodies to heat-shock protein 65 with carotid atherosclerosis. Lancet. 1993;341:255–9. doi: 10.1016/0140-6736(93)92613-x. [DOI] [PubMed] [Google Scholar]
- 8.Perschinka H, Mayr M, Millonig G, Mayerl C, van der Zee R, Morrison SG, Morrison RP, Xu Q, Wick G. Cross-reactive B-cell epitopes of microbial and human heat shock protein 60/65 in atherosclerosis. Arterioscler Thromb Vasc Biol. 2003;23:1060–5. doi: 10.1161/01.ATV.0000071701.62486.49. [DOI] [PubMed] [Google Scholar]
- 9.Xiao Q, Mandal K, Schett G, Mayr M, Wick G, Oberhollenzer F, Willeit J, Kiechl S, Xu Q. Association of serum-soluble heat shock protein 60 with carotid atherosclerosis: clinical significance determined in a follow-up study. Stroke. 2005;36:2571–6. doi: 10.1161/01.STR.0000189632.98944.ab. [DOI] [PubMed] [Google Scholar]
- 10.Zhu J, Quyyumi AA, Rott D, Csako G, Wu H, Halcox J, Epstein SE. Antibodies to human heat-shock protein 60 are associated with the presence and severity of coronary artery disease: evidence for an autoimmune component of atherogenesis. Circulation. 2001;103:1071–5. doi: 10.1161/01.cir.103.8.1071. [DOI] [PubMed] [Google Scholar]
- 11.Kervinen H, Huittinen T, Vaarala O, Leinonen M, Saikku P, Manninen V, Manttari M. Antibodies to human heat shock protein 60, hypertension and dyslipidemia. A study of joint effects on coronary risk. Atherosclerosis. 2003;169:339–44. doi: 10.1016/s0021-9150(03)00229-6. [DOI] [PubMed] [Google Scholar]
- 12.Huittinen T, Leinonen M, Tenkanen L, Manttari M, Virkkunen H, Pitkanen T, Wahlstrom E, Palosuo T, Manninen V, Saikku P. Autoimmunity to human heat shock protein 60, Chlamydia pneumoniae infection, and inflammation in predicting coronary risk. Arterioscler Thromb Vasc Biol. 2002;22:431–7. doi: 10.1161/hq0302.104512. [DOI] [PubMed] [Google Scholar]
- 13.Burian K, Kis Z, Virok D, Endresz V, Prohaszka Z, Duba J, Berencsi K, Boda K, Horvath L, Romics L, Fust G, Gonczol E. Independent and joint effects of antibodies to human heat-shock protein 60 and Chlamydia pneumoniae infection in the development of coronary atherosclerosis. Circulation. 2001;103:1503–8. doi: 10.1161/01.cir.103.11.1503. [DOI] [PubMed] [Google Scholar]
- 14.Heltai K, Kis Z, Burian K, Endresz V, Veres A, Ludwig E, Gonczol E, Valyi-Nagy I. Elevated antibody levels against Chlamydia pneumoniae, human HSP60 and mycobacterial HSP65 are independent risk factors in myocardial infarction and ischaemic heart disease. Atherosclerosis. 2004;173:339–46. doi: 10.1016/j.atherosclerosis.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 15.Dieude M, Senecal JL, Raymond Y. Induction of endothelial cell apoptosis by heat-shock protein 60-reactive antibodies from anti-endothelial cell autoantibody-positive systemic lupus erythematosus patients. Arthritis Rheum. 2004;50:3221–31. doi: 10.1002/art.20564. [DOI] [PubMed] [Google Scholar]
- 16.Mayr M, Metzler B, Kiechl S, Willeit J, Schett G, Xu Q, Wick G. Endothelial cytotoxicity mediated by serum antibodies to heat shock proteins of Escherichia coli and Chlamydia pneumoniae: immune reactions to heat shock proteins as a possible link between infection and atherosclerosis. Circulation. 1999;99:1560–6. doi: 10.1161/01.cir.99.12.1560. [DOI] [PubMed] [Google Scholar]
- 17.Schett G, Xu Q, Amberger A, van der Zee R, Recheis H, Willeit J, Wick G. Autoantibodies against heat shock protein 60 mediate endothelial cytotoxicity. J Clin Invest. 1995;96:2569–77. doi: 10.1172/JCI118320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bason C, Corrocher R, Lunardi C, Puccetti P, Olivieri O, Girelli D, Navone R, Beri R, Millo E, Margonato A, Martinelli N, Puccetti A. Interaction of antibodies against cytomegalovirus with heat-shock protein 60 in pathogenesis of atherosclerosis. Lancet. 2003;362:1971–7. doi: 10.1016/S0140-6736(03)15016-7. [DOI] [PubMed] [Google Scholar]
- 19.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell CJ, Roger V, Rumsfeld J, Sorlie P, et al. Heart disease and stroke statistics – 2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 20.Davies MJ, Thomas AC. Plaque fissuring – the cause of acute myocardial infarction, sudden ischaemic death, and crescendo angina. Br Heart J. 1985;53:363–73. doi: 10.1136/hrt.53.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuster V, Badimon L, Cohen M, Ambrose JA, Badimon JJ, Chesebro J. Insights into the pathogenesis of acute ischemic syndromes. Circulation. 1988;77:1213–20. doi: 10.1161/01.cir.77.6.1213. [DOI] [PubMed] [Google Scholar]
- 22.Foteinos G, Afzal AR, Mandal K, Jahangiri M, Xu Q. Anti-heat shock protein 60 autoantibodies induce atherosclerosis in apolipoprotein E-deficient mice via endothelial damage. Circulation. 2005;112:1206–13. doi: 10.1161/CIRCULATIONAHA.105.547414. [DOI] [PubMed] [Google Scholar]
- 23.Konstantinides S, Schafer K, Thinnes T, Loskutoff DJ. Plasminogen activator inhibitor-1 and its cofactor vitronectin stabilize arterial thrombi after vascular injury in mice. Circulation. 2001;103:576–83. doi: 10.1161/01.cir.103.4.576. [DOI] [PubMed] [Google Scholar]
- 24.Tanguay JF, Geoffroy P, Dorval JF, Sirois MG. Percutaneous endoluminal arterial cryoenergy improves vascular remodelling after angioplasty. Thromb Haemost. 2004;92:1114–21. doi: 10.1160/TH04-06-0336. [DOI] [PubMed] [Google Scholar]
- 25.Chandrasekar B, Sirois MG, Geoffroy P, Lauzier D, Nattel S, Tanguay JF. Local delivery of 17beta-estradiol improves reendothelialization and decreases inflammation after coronary stenting in a porcine model. Thromb Haemost. 2005;94:1042–7. doi: 10.1160/TH04-12-0823. [DOI] [PubMed] [Google Scholar]
- 26.Ayada K, Yokota K, Kobayashi K, Shoenfeld Y, Matsuura E, Oguma K. Chronic infections and atherosclerosis. Ann NY Acad Sci. 2007;1108:594–602. doi: 10.1196/annals.1422.062. [DOI] [PubMed] [Google Scholar]
- 27.Blann AD. Plasma von Willebrand factor, thrombosis, and the endothelium: the first 30 years. Thromb Haemost. 2006;95:49–55. [PubMed] [Google Scholar]
- 28.Ruggeri ZM. The role of von Willebrand factor in thrombus formation. Thromb Res. 2007;120(Suppl 1):S5–9. doi: 10.1016/j.thromres.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsui H, Sugimoto M, Mizuno T, Tsuji S, Miyata S, Matsuda M, Yoshioka A. Distinct and concerted functions of von Willebrand factor and fibrinogen in mural thrombus growth under high shear flow. Blood. 2002;100:3604–10. doi: 10.1182/blood-2002-02-0508. [DOI] [PubMed] [Google Scholar]
- 30.Chen M, Geng JG. P-selectin mediates adhesion of leukocytes, platelets, and cancer cells in inflammation, thrombosis, and cancer growth and metastasis. Arch Immunol Ther Exp (Warsz) 2006;54:75–84. doi: 10.1007/s00005-006-0010-6. [DOI] [PubMed] [Google Scholar]
- 31.Calderwood SK, Mambula SS, Gray PJ., Jr Extracellular heat shock proteins in cell signaling and immunity. Ann NY Acad Sci. 2007;1113:28–39. doi: 10.1196/annals.1391.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.