Abstract
Background
In the Peruvian Amazon, Plasmodium falciparum and Plasmodium vivax malaria are endemic in rural areas, where microscopy is not available. Malaria rapid diagnostic tests (RDTs) provide quick and accurate diagnosis. However, pfhrp2 gene deletions may limit the use of histidine-rich protein-2 (PfHRP2) detecting RDTs. Further, cross-reactions of P. falciparum with P. vivax-specific test lines and vice versa may impair diagnostic specificity.
Methods
Thirteen RDT products were evaluated on 179 prospectively collected malaria positive samples. Species diagnosis was performed by microscopy and confirmed by PCR. Pfhrp2 gene deletions were assessed by PCR.
Results
Sensitivity for P. falciparum diagnosis was lower for PfHRP2 compared to P. falciparum-specific Plasmodium lactate dehydrogenase (Pf-pLDH)- detecting RDTs (71.6% vs. 98.7%, p<0.001). Most (19/21) false negative PfHRP2 results were associated with pfhrp2 gene deletions (25.7% of 74 P. falciparum samples). Diagnostic sensitivity for P. vivax (101 samples) was excellent, except for two products. In 10/12 P. vivax-detecting RDT products, cross-reactions with the PfHRP2 or Pf-pLDH line occurred at a median frequency of 2.5% (range 0%–10.9%) of P. vivax samples assessed. In two RDT products, two and one P. falciparum samples respectively cross-reacted with the Pv-pLDH line. Two Pf-pLDH/pan-pLDH-detecting RDTs showed excellent sensitivity with few (1.0%) cross-reactions but showed faint Pf-pLDH lines in 24.7% and 38.9% of P. falciparum samples.
Conclusion
PfHRP2-detecting RDTs are not suitable in the Peruvian Amazon due to pfhrp2 gene deletions. Two Pf-pLDH-detecting RDTs performed excellently and are promising RDTs for this region although faint test lines are of concern.
Introduction
In Peru, malaria is mainly endemic in the Amazon region, where it is the primary cause of morbidity in adults and the fourth in children [1]. According to the recommendations of the World Health Organization (WHO), diagnosis and treatment should be based on parasitological confirmation by either microscopy or malaria rapid diagnostic tests (RDTs) [2]. In Peru, most cases occur in rural areas where no microscopy is available. Currently, thick blood films (TBFs) of malaria suspected patients are sent for analysis to the most nearby health center, but this process takes several days and patients are often treated presumptively [3]. In such conditions RDTs could be useful, providing quick and accurate diagnosis, thereby leading to timely and correct treatment and reducing the severity and economic burden of disease. Besides, use of RDTs in the Peruvian Amazon has been demonstrated to be cost-effective [4].
RDTs are handheld cassettes detecting malaria parasites by an antigen-antibody reaction on a nitrocellulose strip which become visible as blue or cherry-red test lines. There are several detection antibodies, directed to different antigens: histidine-rich protein-2 (PfHRP2) and Plasmodium falciparum-specific Plasmodium lactate dehydrogenase (Pf-pLDH) for P. falciparum; Plasmodium vivax-specific pLDH (Pv-pLDH) for P. vivax, and pan-pLDH and aldolase which are common to all four Plasmodium species.
The occurrence of both P. vivax and P. falciparum in Peru requires an RDT type that detects and differentiates between both species as they require different treatment [2]. However, cross-reactions may occur, i.e. the presence of a visible P. falciparum test line among P. vivax samples and vice versa [5], [6], due to genuine antigen-antibody interactions or non-specific bindings [7]. In addition, P. falciparum parasites lacking the pfhrp2 and pfhrp3 genes, -encoding PfHRP2 and the related protein PfHRP3 respectively- have been recently described in Peru [8] indicating that the use of PfHRP2 detecting RDTs may be limited [8]. Previous evaluations of two PfHRP2 detecting RDTs in Peru demonstrated sensitivity for P. falciparum diagnosis of 95% [9] and 53.5% [10].
The aims of the present study were to assess diagnostic accuracy of a panel of different RDT products for malaria diagnosis in the Peruvian Amazon, with particular focus on the impact of pfhrp2 and pfhrp3 gene deletions on diagnostic sensitivity and of cross-reactions on diagnostic specificity.
Methods
Ethics statement
The study was approved by the Ethical Review Board of the Universidad Peruana Cayetano Heredia, Lima, Peru (Code SIDISI: 55587 and 55239). All patients with a positive TBF, performed as part of routine patient care, were included after signing informed consent. Written informed consent was obtained from the patient himself in the case of adults or from the parent/guardian in case of a minor (<18 years).
Study site and population
Several health centers around Iquitos (Figure 1) were included. Malaria in the Peruvian Amazon is perennial with a peak during the rainy season (November – May) and an incidence of 10–50 malaria cases per 1000 inhabitants per year [11]. Patients were included by either passive case detection (symptomatic patients presenting at the health centers) or active case detection (outreach teams performing malaria screening in epidemic communities). All patients with a positive TBF were included after signing informed consent. Previous antimalarial treatment, symptoms and travel history were recorded.
Figure 1. Map of included health centers.
The village of Atalaya (−3.58, −73.75), located 59 km to the West of Iquitos, is not displayed on the map.
Samples
EDTA anti-coagulated venous blood samples were drawn and transported to the laboratory of San Juan where RDTs were performed. After RDT performance, samples were aliquoted and stored at −20°C, usually within 24 hours (range 2–72 hours) after sample collection, pending further analysis.
Malaria rapid diagnostic tests
Thirteen RDT products detecting several target antigens were selected (Table 1), based on good performance as documented by the WHO/Foundation for Innovative New Diagnostics (FIND) malaria RDT evaluation program [12], [13] or recent release on the market.
Table 1. Overview of RDT products and their lot numbers.
| Product name | Manufacturer/distributor | Further referred to as | Target antigen Pf | Target antigen pan/Pv | Lot numbers | Recommended storage temperature |
| ADVANTAGE Mal Card | J. Mitra & Co., New Dehli, India | Advantage | Pf-pLDH | pan-pLDH | ACM171110 | 4–30°C |
| AZOG Malaria Pf/Pv | AZOG, Inc. New Jersey, USA | AZOG | PfHRP2 | Pv-pLDH | 58LAB017 | 2–30°C |
| CareStart™ Malaria Pf-pLDH/pLDH (Pf/PAN) Combo | Access Bio, Inc. New Jersey, USA | CareStart pLDH | Pf-pLDH | pan-pLDH | A10IL | 4–30°C |
| CareStart™ Malaria HRP2/Pv-pLDH (Pf/Pv) Combo | Access Bio, Inc. New Jersey, USA | CareStart Pf/Pv | PfHRP2 | Pv-pLDH | J10IV | 4–30°C |
| Falcivax Rapid Test for Malaria Pv/Pf | Zephyr Biomedicals, Verna, India | Falcivax | PfHRP2 | Pv-pLDH | 81098 | 4–30°C |
| First Response Ag malaria pLDH/HRP2 combo test | Premier Medical Corporation Daman, India | First Response | PfHRP2 | pan-pLDH | 69I0610 | 4–30°C |
| Onsite Pf/Pv Ag rapid test | CTK Biotech, Inc. San Diego, USA | Onsite | PfHRP2 | Pv-pLDH | F0810G2 | 2–30°C |
| PARACHECK Pf® (device) | Orchid Biomedical Systems Verna, India | Paracheck | PfHRP2 | - | 31795, 31797 | 4–45°C |
| Parascreen Rapid Test for Malaria Pan/Pf | Zephyr Biomedicals, Verna, India | Parascreen | PfHRP2 | pan-pLDH | 101176 | 4–30°C |
| SD Bioline Malaria Antigen test | Standard diagnostic, Hagal-dong, Korea | SDFK40 | Pf-pLDH | pan-pLDH | MLRDT1001, MLRDT1002 | 1–40°C |
| SD Bioline Malaria Antigen P.f/pan | Standard diagnostic, Hagal-dong, Korea | SDFK60 | PfHRP2 | pan-pLDH | 90026, 90017, 90096 | 1–40°C |
| SD Bioline Malaria Antigen P.f/P.v | Standard diagnostic, Hagal-dong, Korea | SDFK80 | PfHRP2 | Pv-pLDH | 145015, 145016 | 1–40°C |
| SD Bioline Malaria Antigen P.f | Standard diagnostic, Hagal-dong, Korea | SDFK90 | PfHRP2 and Pf-pLDH* | - | MFRDT1001, MFRDT1002 | 1–40°C |
SDFK90 contains 2 test lines specific for P. falciparum.
Both SDFK90 and Paracheck detect only P. falciparum and were included for evaluation of P. falciparum diagnosis. SDFK90 was only performed on P. falciparum samples and mixed infections. RDTs were purchased at the Institute of Tropical Medicine (ITM), Belgium and shipped to Peru. For logistic reasons (delays of delivery and shipment), some RDTs had to be performed on stored samples, in these cases median period of sample storage was 51 days (range 29–131 days).
Test procedures
RDTs were performed according to the manufacturer's instructions except that the supplied transfer device was replaced by a micropipette. The first observer read test results within the specified reading time, the second and, when available, third observer within 10 additional minutes. Observers were blinded to each other's readings. In case of absence of the control line the test was repeated. A scoring system of five categories was used to assess line intensities [14]. Test results were based on consensus agreement in case of three observers. In all other cases, the result of the first observer was considered.
Microscopy
At the laboratory of San Juan, species and parasite density were determined by TBF microscopy, assuming a white blood cell count of 8,000/µl [15]. For quality control (QC), 20% randomly selected slides, including those with interpretive problems, discordant RDT results, negative slides and suspected mixed infections were reexamined by two blinded expert microscopists at ITM. For parasite density the results of the first microscopist were considered except when QC indicated a density of more than two fold difference with the original count, in such cases mean of the two QC readings was considered.
DNA extraction
DNA was extracted from 200 µl whole blood using QIAamp DNA blood Mini kit (QIAGEN, Venlo, The Netherlands), according to the manufacturer's instructions except for a dilution in 100 µl instead of 200 µl elution buffer.
Species-specific PCR
In case of discordances between RDT and microscopy or between initial and QC microscopy, real-time PCR (P. falciparum/P. vivax) was performed [16] which was considered conclusive.
Assessment of pfhrp2 and pfhrp3 gene deletions
Confirmed P. falciparum samples were assessed for pfhrp2 and pfhrp3 gene deletions by conventional PCR using primers and conditions as described elsewhere [8], [17]. For pfhrp2, two amplifications were performed: one of entire exon 2 (encoding PfHRP2) and another across exon 1 and exon 2 (exon1–2). Samples were considered lacking the pfhrp2 gene when both amplifications failed to generate a PCR product. For pfhrp3, a single amplification of entire exon 2 was performed.
PfHRP2 ELISA
The presence of PfHRP2 protein in whole blood samples was determined by enzyme linked immune sorbent assay (ELISA, Standard Diagnostic, Hagal-Dong, Korea) according to the manufacturer's instructions. ELISA was performed in all samples with P. falciparum infection, mixed infections and in P. vivax samples generating visible PfHRP2 lines.
Statistical analysis
Diagnostic sensitivity (calculated with 95% confidence intervals (C.I.)) of the RDT products was defined as the number of P. falciparum or P. vivax samples with a visible P. falciparum-specific or Pv-/pan-pLDH test line respectively (regardless of the presence of another test line), divided by the total number of P. falciparum or P. vivax samples respectively. Mixed infections were not included for calculation. Cross-reactions were defined as P. falciparum samples generating a visible Pv-pLDH line or P. vivax samples generating a visible PfHRP2 or Pf-pLDH line.
Proportions were assessed for statistical significance using the Chi-square test or, in case of small sample size, the Fisher-exact test. A p-value<0.05 was considered significant.
Interobserver agreement was determined by kappa values (κ) for positive and negative readings and line intensity readings between the first pair of observers.
Additional analysis
All microscopically confirmed P. falciparum samples that did not show a visible PfHRP2 line in more than one RDT product were repeated two times with all PfHRP2-detecting RDTs.
Results
Patients and samples
From December 2010–July 2011, 182 patients were included, in three patients malaria was not confirmed by microscopy nor by PCR. Final sample collection consisted of P. falciparum (n = 74), P. vivax (n = 101) and four mixed infections. The collected samples comprised 5% of all P. falciparum and P. vivax infections reported in Loreto region in that time period [1], [18]. Data of demography and parasite density are shown in Table 2. Nineteen patients, including the two asymptomatic cases, were included through active case detection performed once in Tarapoto (n = 5) and once in Atalaya (n = 14).
Table 2. Patient data and parasite density of the final sample collection.
| P. falciparum (n = 74) | P. vivax (n = 101) | Mixed infection (n = 4) | |
| Sample collection period | Dec 2010–Jul 2011 | Dec 2010–Mar 2011 | Dec 2010–Mar 2011 |
| Male gender | 41 (55.4%) | 52 (51.5%) | 4 (100%) |
| Age, median years (range) | 27.5 (4–74) | 29 (2–76) | 31.5 (4–47) |
| Children <15 years, number (%) | 16 (21.6%) | 24 (23.8%) | 1 (25%) |
| Median parasite density/µl (range) | 4,971.5 (0–78,208) | 5,080 (255–58,880) | 9,527.5 (5,204–22,321) |
| Asymptomatic patients (number) | 1 (1.4%) | 1 (1.0%) | 0 (0.0%) |
| Antimalarial treatment past 2 weeks | 4 (5.4%)* | 0 (0.0%) | 0 (0.0%) |
artesunate + mefloquine since 2 days (n = 1), chloroquine since 2 days (n = 2), full course of chloroquine/primaquine (n = 1) at least >1 week ago (exact date not known).
Diagnostic sensitivity of the RDT products
PfHRP2-detecting RDTs had significantly lower sensitivity for P. falciparum diagnosis compared to Pf-pLDH-detecting RDTs (p<0.0001, Table 3), due to a subset of samples that consequently failed to generate a PfHRP2 line in all PfHRP2 RDT products tested, see results below.
Table 3. Sensitivity, faint line intensity and cross-reactions of the different RDT products for detection of P. falciparum and P. vivax.
| % Sensitivity (95% C.I.) | % of positive test lines with faint intensity* | Number of cross-reactions (%) | ||||
| RDT product | P. falciparum (n = 74) | P. vivax (n = 101) | PfHRP2/Pf-pLDH | Pv-/pan-pLDH† | P. vivax with PfHRP2/Pf-pLDH test line | P. falciparum with Pv-pLDH test line |
| PfHRP2-detecting RDT | ||||||
| Paracheck | 70.3 (58.5–80.3) | - | 5.8 | - | 0 (0.0) | - |
| PfHRP2 and pan-pLDH detecting RDTs | ||||||
| First Response | 71.6 (60.0–81.5) | 100.0 (94.6–100.0) | 3.8 | 2.0 | 3 (3.0) | - |
| Parascreen | 71.6 (60.0–81.5) | 89.1 (81.4–94.4) | 1.9 | 21.1 | 7 (6.9) | - |
| SDFK60 | 71.6 (60.0–81.5) | 100.0 (94.6–100.0) | 7.1 | 4.0 | 5 (5.0) | - |
| PfHRP2and Pv-pLDH detecting RDTs | ||||||
| AZOG | 71.6 (60.0–81.5) | 87.1 (79.0–93.0) | 17.0 | 79.5 | 2 (2.0) | 0 (0.0) |
| CareStart Pf/Pv | 71.6 (60.0–81.5) | 100.0 (94.6–100.0) | 5.7 | 10.9 | 11 (10.9) | 0 (0.0) |
| Falcivax | 71.6 (60.0–81.5) | 100.0 (94.6–100.0) | 1.9 | 7.9 | 5 (5.0) | 0 (0.0) |
| Onsite | 71.6 (60.0–81.5) | 100.0 (94.6–100.0) | 5.7 | 4.0 | 0 (0.0) | 2 (2.7) |
| SDFK80 | 71.6 (60.0–81.5) | 100.0 (94.6–100.0) | 1.9 | 0.0 | 1 (1.0) | 1 (1.4) |
| Pf-pLDH and pan-pLDH detecting RDTs | ||||||
| Advantage | 98.7 (92.7–100.0) | 100.0 (94.6–100.0) | 24.7 | 4.0 | 1 (1.0) | - |
| CareStart pLDH | 98.7 (92.7–100.0) | 99.0 (94.6–100.0) | 9.6 | 8.0 | 10 (9.9) | - |
| SDFK40 | 97.3 (90.6–99.7) | 100.0 (94.6–100.0) | 38.9 | 1.0 | 1 (1.0) | - |
| PfHRP2 and Pf-pLDH detecting RDT | ||||||
| SDFK90 PfHRP2 line | 71.6 (60.0–81.5) | - | 1.9 | - | - | - |
| SDFK90 Pf-pLDH line | 98.7 (92.7–100.0) | - | 40.5 | - | - | - |
cross-reactions excluded.
only P. vivax samples were considered.
For P. vivax diagnosis, most RDTs performed equally well, except for AZOG (detecting Pv-pLDH) and Parascreen (detecting pan-pLDH) (Table 3), which failed to detect P. vivax samples at a median parasite density of 1,075/µl (range 255–4,532/µl) and 600.5/µl (range 255–10,720/µl) respectively.
The mixed infections were detected by all RDT products except for AZOG which displayed a single PfHRP2 line for a sample consisting predominantly of P. falciparum parasites.
For P. falciparum, faint test line intensities occurred more frequently among Pf-pLDH compared to PfHRP2-detecting RDTs (p<0.001, Table 3). For P. vivax, no overall difference in proportion of faint test lines was observed between Pv-pLDH versus pan-pLDH-detecting RDTs.
Failure of P. falciparum diagnosis by PfHRP2-detecting RDTs and pfhrp2 gene deletions
All PfHRP2-detecting RDTs failed to diagnose 21 P. falciparum samples (Table 4), whereas the Pf-pLDH-detecting RDTs detected all of them. Most samples (19/21) were lacking pfhrp2 (no amplification of exon1–2 and exon2). The remaining two samples (PI151 and PI156) generated PCR products for pfhrp2 exon1–2 and exon2. Pfhrp2 gene deletions occurred at both low and high parasite densities (Table 4) and all patients were symptomatic. PfHRP2 ELISA of the 21 samples confirmed the absence of PfHRP2, with only one sample (PI26) showing a weak positive result (optical density ten-fold lower than other ELISA positive samples).
Table 4. P. falciparum samples not detected by PfHRP2-detecting RDTs: pfhrp2 and pfhrp3 PCR results and PfHRP2 ELISA results.
| Sample and patient information | |||||||
| Sample number | Sex | Age | Parasite density (/µl) | pfhrp2 exon 1–2 | pfhrp2 exon 2 | pfhrp3 exon 2 | PfHRP2 ELISA |
| PI138 | f | 56 | 0* | − | − | − | − |
| PI139 | m | 6 | 79 | − | − | − | − |
| PI137 | m | 41 | 80 | − | − | − | − |
| PI136 | m | 53 | 270 | − | − | − | − |
| PI 24 | f | 20 | 752 | − | − | − | − |
| PI113 | f | 30 | 876 | − | − | − | − |
| PI142 | m | 21 | 1,000 | − | − | − | − |
| PI151 | m | 28 | 1,222 | + | + | − | − |
| PI 18 | f | 12 | 1,400 | − | − | − | − |
| PI 78 | m | 37 | 2,808 | − | − | − | − |
| PI156 | f | 36 | 3,480 | + | + | + | − |
| PI135 | m | 7 | 4,784 | − | − | − | − |
| PI153 | m | 70 | 5,080 | − | − | − | − |
| PI140 | f | 20 | 5,640 | − | − | − | − |
| PI 26 | m | 48 | 7,227 | − | − | − | +/− |
| PI 27 | f | 67 | 7,840 | − | − | − | − |
| PI163 | m | 65 | 16,552 | − | − | − | − |
| PI 81 | m | 46 | 18,800 | − | − | − | − |
| PI148 | f | 34 | 19,600 | − | − | − | − |
| PI 74 | m | 38 | 22,560 | − | − | − | − |
| PI 65 | m | 27 | 43,089 | − | − | − | − |
+ = positive, − = negative, +/− = weak positive.
This sample contained only gametocytes.
Pfhrp2: percentage of samples with gene deletions and geographic origin
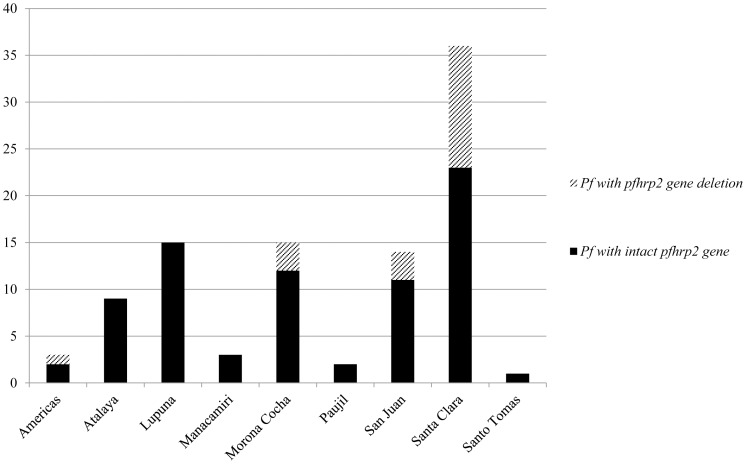
Pfhrp2 gene deletions occurred among 19 (25.7%) P. falciparum samples. Thirteen (68.4%) were obtained from patients presenting at the health center of Santa Clara (Figure 2), with most patients living in Tarapoto (8/13, 61.5%). The remaining six were distributed among three other health centers (Figure 2). Pfhrp2 gene deletions were found throughout the study period and sometimes P. falciparum samples with and without pfhrp2 gene deletions were found simultaneously in the same village. One patient with a pfhrp2 gene deletion diagnosed at Morona Cocha had been travelling to Angamos (close to the Brazilian border) during the month previous to sampling.
Figure 2. Number of P. falciparum samples containing or lacking the pfhrp2 gene per health center.
The village of Atalaya is not a health center, but is displayed separately as all samples in Atalaya were collected by an outreach team during an epidemic.
Pfhrp3 gene deletion
In total 34 (43.6%) P. falciparum samples lacked the pfhrp3 gene: they included all samples lacking the pfhrp2 gene (n = 19) as well as 15 additional samples which contained pfhrp2, and which were correctly diagnosed by all PfHRP2-detecting RDTs.
Occurrence of cross reactions
In most (10/12) RDT products that were assessed with P. vivax samples, P. falciparum test lines (either PfHRP2 or Pf-pLDH) were visible at a median frequency of 2.5% (range 1.0%–10.9%). In total, 27 (26.7%) P. vivax samples were involved. In all of these samples, mixed infection with P. falciparum was excluded by PCR and none of the patients had reported P. falciparum infection in the month prior to sampling. In six of these samples however, HRP2 ELISA yielded a weak positive result. There was no apparent relation between parasite density and the occurrence of cross-reactions (range 255–58,880/µl).
In two RDT products, P. falciparum samples generated a visible Pv-pLDH line: one faint line for SDFK80 (parasite density 78,208/µl); and a faint and medium line for Onsite (parasite density 53,333/µl and 3,480/µl). Mixed infection with P. vivax was excluded by PCR and none of the patients reported recent P. vivax infection.
Interobserver agreement
For positive/negative readings, median κ per RDT product was 1.00 (range 0.84–1.00). For line intensity readings, median κ was 0.87 (range 0.62–0.99).
Discussion
The present study evaluated a panel of RDT products for malaria diagnosis in the Peruvian Amazon. It showed that Pf-pLDH-detecting RDTs performed significantly better for P. falciparum diagnosis compared to PfHRP2-detecting RDTs in this geographical region. The low sensitivity of PfHRP2-detecting RDTs was related to pfhrp2 gene deletions which invariably leaded to false negative PfHRP2 results irrespective of the parasite density. For P. vivax diagnosis all but two RDT products performed well with no overall difference in sensitivity and line intensity between Pv-pLDH and pan-pLDH detecting RDTs. Cross-reactions with the P. falciparum line were observed in 10/12 P. vivax-detecting RDT products at a median frequency of 2.5% (range 1.0%–10.9%) of P. vivax samples assessed. In two RDT products, false positive Pv-pLDH lines were observed in up to 2.7% of P. falciparum samples.
Impact of pfhrp2 gene deletions
The exact incidence of pfhrp2 gene deletions in the Peruvian Amazon is not known. We presently found 25.7% of P. falciparum samples lacking pfhrp2, in a previous study this was 41.0% [8]. In the present study pfhrp2 gene deletions were found at different sites, but not at all health centers. Pfhrp2 gene deletions have however been reported throughout the Peruvian Amazon [8] as well as in Brazil [19] and one of the presently included patients might have acquired infection near the Brazilian border. By consequence, the findings as currently described may be applicable to the whole Amazon region.
The impact of pfhrp2 gene deletions is further highlighted by the fact that all samples lacking pfhrp2 were not detected by any of the PfHRP2-detecting RDT products. In addition, all samples lacking pfhrp2 were found in symptomatic patients and occurred at both high and low parasite densities, in contrast to a previous study [20] which demonstrated pfhrp2 gene deletions only in asymptomatic patients and at low parasite densities. Of note is that in 1998–1999 an evaluation of the PfHRP2-detecting RDT Parasight-F around Iquitos showed sensitivity for P. falciparum diagnosis of 95% [9]. Possibly, pfhrp2 gene deletions have become common in this area only recently.
Discordances between pfhrp2 PCR and PfHRP2 RDT results
For samples PI151 and PI156, the presence of pfhrp2 exon2 was demonstrated by both PCRs but PfHRP2 RDT and ELISA results were negative. Parasite density of both samples was far above the RDT detection threshold and does not explain failure of detection. A mutation or deletion may have occurred, leading to failure of production of the antigen or production of an antigen that is not recognized. Failure of detection of both samples may also be due to errors in transcription or translation, causing low parasite protein expression and consequently failure of detection by RDTs and ELISA [21]. Further research is needed to investigate the occurrence and cause of this phenomenon.
Role of pfhrp3
It has been postulated that PfHRP3 might compensate for absence of PfHRP2 in PfHRP2-detecting diagnosis, due to cross-reaction of PfHRP3 with PfHRP2 antibodies [8], [17]. In the present study this could not be assessed since all pfhrp2 negative samples lacked the pfhrp3 gene as well.
Cross-reactions
In all samples showing cross-reactions, mixed infections were excluded and Plasmodium infection during the month previous to sampling was not reported. In the case of P. vivax samples generating a PfHRP2 line, past subclinical infection with PfHRP2 persistence (caused by slow clearance of PfHRP2 [22]) may have occurred in at least part of the samples, as supported by the weak positive ELISA results in six samples. However, optical density values in these samples were low and PfHRP2 lines were only visible in few RDT products, which makes non-specific reactions a more plausible explanation. In the case of visible Pf-pLDH lines among P. vivax samples and Pv-pLDH lines among P. falciparum samples, genuine antigen-antibody reactions [23] or non-specific reactions [7] may have occurred. Cross-reactions (false positive P. falciparum test lines) among P. vivax samples are particularly relevant in RDTs detecting pan-pLDH: in these cases RDT results are interpreted as P. falciparum infection and the patient will not be treated with primaquine, which is needed to eradicate the liver stages. Conversely, false positive Pv-pLDH test lines among P. falciparum samples indicate mixed P. falciparum/P. vivax infection, which will lead to unnecessary treatment with primaquine.
Limitations
The present study did not include Plasmodium negative patients, precluding calculation of specificity and positive and negative predictive values. However, it provides relevant data about RDT diagnostic sensitivity and its relation with pfhrp2 gene deletions, based upon which suitable RDTs can be selected. Further, we included a large panel of simple one-step RDT products but did not include RDTs with a more complex procedure such as the previously evaluated OptiMAL [24]. Not all RDTs could be performed on fresh samples, though samples had been stored for a short period and had not been exposed to repeated freezing and thawing. Besides, no apparent differences were found between RDT results on stored versus fresh samples. Finally, observers of RDT results were not always blinded to microscopy results provided by the health center.
Which RDT for the Peruvian Amazon?
From the present study it is clear that PfHRP2-detecting RDTs are not suitable for the Peruvian Amazon, due to the high prevalence of P. falciparum samples lacking the pfhrp2 gene which was invariably associated with false negative results. Pfhrp2 gene deletions occurred at all parasite densities and all patients were symptomatic. The three Pf-pLDH-detecting RDTs - all combining pan-pLDH - performed excellently for P. falciparum and P. vivax diagnosis. Among one of them however, an unacceptably high proportion of P. vivax samples generated cross-reactions with the Pf-pLDH line, impeding its use. For the remaining two, the high number of faint test lines is of concern as especially in field settings faint lines tend to be overlooked or disregarded as negative [25], [26], [27]. Besides, general limitations of Pf-pLDH-detecting RDTs are a lower sensitivity at low parasite densities [7], [12], [14] and less heat stability, although the latter is currently less important than originally described [7], [14] and SDFK40 reports heat stability up to 40°C (Table 1).
Despite the excellent diagnostic accuracy of SDFK40 and Advantage in the present study, prospective field evaluation on all malaria suspected patients is needed to determine positive and negative predictive values and end user performance. In the long term, the development of an RDT targeting both Pf-pLDH and Pv-pLDH could be considered. Such a combination could, besides diagnosing each of both species, also differentiate between P. falciparum and mixed P. falciparum/P. vivax infections, but is not yet commercially available.
Funding Statement
Part of the work performed by JM was funded by the “Steunfonds Marguerite-Marie Delacroix”. Part of the work performed by DG and JB was funded by a National Institute of Health grant (U19 AI089681) and by Directorate General for Development Cooperation (DGCD) of the Belgian Government (framework agreement 3, project 95502). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gobierno regional de Loreto (2011) Análisis de la situación de salud de la región Loreto año 2010. Available: www.diresaloreto.gob.pe. Accessed 2011 Dec 15.
- 2.WHO (2010) Guidelines for the treatment of malaria, second edition. Geneva, Switzerland.
- 3. Durand S, Ramal C, Huilca M, Cabezas C (2005) Oportunidad en el diagnóstico y tratamiento de la malaria en comunidades periurbanas de la amazonía peruana. Rev Peru Med Exp Salud Publica 22 1:47–53. [Google Scholar]
- 4. Rosas Aguirre AM, Llanos Zavalaga LF, Trelles de Belaunde M (2009) Relación costo-efectividad del uso de pruebas rápidas para el diagnóstico de la malaria en la Amazonia peruana. Rev Panam Salud Publica 25 5:377–88. [DOI] [PubMed] [Google Scholar]
- 5. Cho D, Kim KH, Park SC, Kim YK, Lee KN, et al. (2001) Evaluation of rapid immunocapture assays for diagnosis of Plasmodium vivax in Korea. Parasitol Res 87: 445–448. [DOI] [PubMed] [Google Scholar]
- 6. Maltha J, Gillet P, Cnops L, van den Ende J, van Esbroeck M, et al. (2010) Malaria rapid diagnostic tests: Plasmodium falciparum infections with high parasite densities may generate false positive Plasmodium vivax pLDH lines. Malar J 9: 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO (2011) Malaria Rapid Diagnostic Test Performance. Results of WHO product testing of malaria RDTs: Round 3 (2010–2011). Available: http://www.finddiagnostics.org/export/sites/default/resource-centre/reports_brochures/docs/RDTMalariaRd3_web.pdf. Accessed 2011 Dec 5.
- 8. Gamboa D, Ho MF, Bendezu J, Torres K, Chiodini PL, et al. (2010) A large proportion of P. falciparum isolates in the Amazon region of Peru lack pfhrp2 and pfhrp3: implications for malaria rapid diagnostic tests. PLoS One 5: e8091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Forney JR, Wongsrichanalai C, Magill AJ, Craig LG, Sirichaisinthop J, et al. (2003) Devices for rapid diagnosis of Malaria: evaluation of prototype assays that detect Plasmodium falciparum histidine-rich protein 2 and a Plasmodium vivax-specific antigen. Journal of clinical microbiology 41: 2358–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bendezu J, Rosas A, Grande T, Rodriguez H, Llanos-Cuentas A, et al. (2010) Field evaluation of a rapid diagnostic test (Parascreen) for malaria diagnosis in the Peruvian Amazon. Malar J 9: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO (2011) World Malaria Report 2011. Available: http://www.who.int/entity/malaria/world_malaria_report_2011/9789241564403_eng.pdf. Accessed 2012 Mar 28.
- 12.WHO (2009) Malaria Rapid Diagnostic Test Performance. Results of WHO product testing of malaria RDTs: Round 1 (2008). Available: http://www.finddiagnostics.org/export/sites/default/media/press/pdf/Full-report-malaria-RDTs.pdf. Accessed 2011 Dec 5.
- 13.WHO (2010) Malaria Rapid Diagnostic Test Performance. Results of WHO product testing of malaria RDTs: Round 2 (2009). Available: http://whqlibdoc.who.int/publications/2010/9789241599467_eng.pdf. Accessed 2011 Dec 5.
- 14. Maltha J, Gillet P, Cnops L, Bottieau E, Van Esbroeck M, et al. (2011) Evaluation of the rapid diagnostic test SDFK40 (Pf-pLDH/pan-pLDH) for the diagnosis of malaria in a non-endemic setting. Malar J 10: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO (2010) Basic Malaria Microscopy - Part I. Learner's Guide, second edition. Geneva, Switzerland. Available: http://www.searo.who.int/LinkFiles/Malaria_malaria_microscopy_Learners_guide2010.pdf. Accessed 2010 Dec 27.
- 16. Cnops L, Jacobs J, Van Esbroeck M (2010) Validation of a four-primer real-time PCR as a diagnostic tool for single and mixed Plasmodium infections. Clin Microbiol Infect [DOI] [PubMed] [Google Scholar]
- 17. Baker J, McCarthy J, Gatton M, Kyle DE, Belizario V, et al. (2005) Genetic diversity of Plasmodium falciparum histidine-rich protein 2 (PfHRP2) and its effect on the performance of PfHRP2-based rapid diagnostic tests. J Infect Dis 192: 870–877. [DOI] [PubMed] [Google Scholar]
- 18.Minesterio de Salud (2011) Boletines epidemiológicas año 2011. Available: http://www.diresaloreto.gob.pe/portal/index.php/boletines-epidemiologicos. Accessed 2011 Dec 22.
- 19. Houze S, Hubert V, Le Pessec G, Le Bras J, Clain J (2011) Combined deletions of pfhrp2 and pfhrp3 genes result in Plasmodium falciparum malaria false-negative rapid diagnostic test. Journal of clinical microbiology 49: 2694–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Koita OA, Doumbo OK, Ouattara A, Tall LK, Konare A, et al. (2012) False-negative rapid diagnostic tests for malaria and deletion of the histidine-rich repeat region of the hrp2 gene. The American journal of tropical medicine and hygiene 86: 194–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baker J, Gatton ML, Peters J, Ho MF, McCarthy JS, et al. (2011) Transcription and expression of Plasmodium falciparum histidine-rich proteins in different stages and strains: implications for rapid diagnostic tests. PLoS One 6: e22593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mayxay M, Pukrittayakamee S, Chotivanich K, Looareesuwan S, White NJ (2001) Persistence of Plasmodium falciparum HRP-2 in successfully treated acute falciparum malaria. Trans R Soc Trop Med Hyg 95: 179–182. [DOI] [PubMed] [Google Scholar]
- 23. Piper RC, Buchanan I, Choi YH, Makler MT (2011) Opportunities for improving pLDH-based malaria diagnostic tests. Malaria journal 10: 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arróspide N, Flores R, Ruíz J (2006) Evaluación de una prueba rápida para el diagnóstico de malaria en áreas endémicas del Perú. Rev Peru Med Exp Salud Publica 23 [Google Scholar]
- 25. Harvey SA, Jennings L, Chinyama M, Masaninga F, Mulholland K, et al. (2008) Improving community health worker use of malaria rapid diagnostic tests in Zambia: package instructions, job aid and job aid-plus-training. Malar J 7: 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mayxay M, Newton PN, Yeung S, Pongvongsa T, Phompida S, et al. (2004) Short communication: An assessment of the use of malaria rapid tests by village health volunteers in rural Laos. Trop Med Int Health 9: 325–329. [DOI] [PubMed] [Google Scholar]
- 27. Rennie W, Phetsouvanh R, Lupisan S, Vanisaveth V, Hongvanthong B, et al. (2007) Minimising human error in malaria rapid diagnosis: clarity of written instructions and health worker performance. Trans R Soc Trop Med Hyg 101: 9–18. [DOI] [PubMed] [Google Scholar]