Abstract
Transmission of HIV-1 during breastfeeding is a significant source of new pediatric infections in sub-Saharan Africa. Breast milk from HIV-positive mothers contains both cell-free and cell-associated virus; however, the impact of breast milk on HIV-1 infectivity remains poorly understood. In the present study, breast milk was collected from HIV-positive and HIV-negative Tanzanian women attending antenatal clinics in Dar es Salaam. Milk was analyzed for activity in vitro against both cell-free and cell-associated HIV-1. Potent inhibition of cell-free R5 and X4 HIV-1 occurred in the presence of milk from all donors regardless of HIV-1 serostatus. Inhibition of cell-free HIV-1 infection positively correlated with milk levels of sialyl-LewisX from HIV-positive donors. In contrast, milk from 8 of 16 subjects enhanced infection with cell-associated HIV-1 regardless of donor serostatus. Milk from two of these subjects contained high levels of multiple pro-inflammatory cytokines including TNFα, IL-1β, IL-6, IL-8, MIP-1α, MIP-1β, MCP-1 and IP-10, and enhanced cell-associated HIV-1 infection at dilutions as high as 1∶500. These findings indicate that breast milk contains innate factors with divergent activity against cell-free and cell-associated HIV-1 in vitro. Enhancement of cell-associated HIV-1 infection by breast milk may be associated with inflammatory conditions in the mother and may contribute to infant infection during breastfeeding.
Introduction
Transmission of HIV-1 from mother to child during breastfeeding results from the presence of both cell-free virus and HIV-infected cells in the milk [1], [2]. The mechanisms associated with HIV-1 breast milk transmission not well understood; however, the low incidence of infection among most breastfeeding infants of HIV-1 seropositive mothers suggests that HIV-1 transmission is relatively inefficient and supports a protective role for breast milk in preventing viral infection [3]. In clinical studies, components in breast milk, including IL-15 [4], long-chain fatty acids [5] and erythropoietin [6] have been linked to lower rates of post-natal HIV-1 transmission. In vitro, breast milk mucin (MUC1) effectively blocks binding and transfer of virus from dendritic cells (DC) to CD4+ T cells [7], [8] and inhibits HIV-1 infection [9], [10]. The inhibitory effects of MUC1 are attributed to a rich array of repeating LewisX motifs, consistent with a direct role for LewisX in preventing binding of HIV-1 to DC-SIGN [7], [8]. Human milk oligosaccharides and glycans, which are abundant in breast milk, constitute another form of innate immunity against infection with pathogens, including HIV-1 [11]–[13].
In the milieu of breast milk, less is known about the impact of milk factors on cell-associated HIV-1. In contrast to cell-free HIV-1 infection, cell-associated infection arises from direct cell-to-cell transfer of virus to susceptible target cells. Cell-to-cell spread of HIV-1 in culture is significantly more efficient than spread of cell-free virus and involves formation of a transient yet complex virological synapse [14]–[17], which is more difficult to neutralize [15].
Administration of highly active antiretroviral therapy (HAART) to HIV-positive women for the prevention of mother-to-child transmission (PMTCT) during breastfeeding is associated with a rapid decrease in the levels of cell-free HIV-1 RNA in breast milk [18] and a dramatic reduction in HIV transmission [19]–[23]. Transmission rates of less than 2% have been reported in clinical studies of HAART for PMTCT [19]–[21], confirming the effectiveness of this intervention. The reason for residual transmission of HIV-1 in this setting is not clear; however, recent studies have documented transient periods of viremia among women receiving HAART for PMTCT based on intermittent detection of HIV-1 RNA in maternal plasma and breast milk [24]. The presence of a cell-associated reservoir for HIV-1 in breast milk is also suggested by two independent clinical studies documenting the persistence of proviral DNA in breast milk from women receiving HAART during breastfeeding [18], [25]. Taken together, these findings suggest distinct mechanisms that determine the persistence and infectivity of cell-free and cell-associated HIV-1 in breast milk.
In the present study, we sought to evaluate the impact of breast milk on infection of CD4+ target cells by cell-free and cell-associated HIV-1 in vitro. Our findings suggest that breast milk may have divergent activities against cell-free HIV-1 as compared to cell-associated virus, and this may have implications for understanding the pathogenesis of HIV-1 transmission during breastfeeding and in the setting of HAART for PMTCT.
Materials and Methods
Ethics Statement
Protocols for this study were approved by the Institutional Review Boards from the Muhimbili University of Health and Allied Sciences (MUHAS, Dar es Salaam, Tanzania) and Dartmouth College (Hanover, NH). Written informed consent was obtained from all donors prior to collection of milk samples.
Breast Milk Donors
Breast milk was obtained from 20 women (10 HIV-positive and 10 HIV-negative) enrolled in the antenatal clinic of Muhimbili National Hospital (MNH) in Dar es Salaam, Tanzania, where routine counseling and testing for HIV infection is performed. The HIV-positive milk donors were enrolled in the PMTCT clinic, which is affiliated with the regular antenatal clinic at MNH. Information about methods for preventing mother-to-child transmission of HIV-1 and infant feeding options were made available to HIV-positive milk donors through PMTCT counseling sessions conducted by a nurse at the clinic.
To be eligible for the study, women had to have healthy infants who were breastfeeding without difficulty. Breastfeeding mothers with reported signs and symptoms of mastitis were excluded. A medication history was obtained and included information about administration of single-dose Nevirapine (sdNVP) during delivery and/or ARV treatment received at any time prior to donating breast milk.
Breast Milk Samples
Milk was collected from either the left or the right breast by manual self-expression. Samples were collected ≥1 hr after the infant last breastfed from the designated side. Milk was collected into sterile plastic tubes and immediately stored and transported at 4°C. Initial processing was performed in the laboratory at MNH within 4 hrs of collection. Whole breast milk was centrifuged at 10,000×g for 10 min to separate the cell pellet from the fluid phase of the milk. The cell pellet was washed three times using sterile PBS and stored at −80°C. The lipid layer of the milk was removed manually and the skim milk fraction was aliquoted into 1 mL cryovials and stored at −80°C. The skim milk and cell pellets were shipped on dry ice to The Geisel School of Medicine at Dartmouth for further analyses. Before use in experiments, each aliquot of skim milk was again centrifuged at 10,000×g for 5 min to remove any residual lipid and sterile-filtered through a 0.22 micron Millex-GV® filter (Millipore, Billerica, MA). Individual milk samples were not pooled for any experiments.
HIV-1 Isolates
HIV-1 isolates used for this study included strains with tropism for CCR5 (R5, HIV-1BaL) and CXCR4 (X4, HIV-1HC4). Virus stocks were propagated in phytohemagglutinin (PHA)-activated peripheral blood mononuclear cells (PBMC) and titered on TZM-bl cells (NIH AIDS Research and Reference Reagent Program, contributed by Dr. John C. Kappes, Dr. Xiaoyun Wu and Tranzyme, Inc.).
Cell-free HIV-1 Infectivity Assays
The effect of breast milk on cell-free HIV-1 infection was determined by measuring viral Tat-driven activation of the HIV-1 LTR and luciferase expression in TZM-bl cells as previously described [26]. In brief, TZM-bl cells were plated in 96-well plates at a density of 1×104 cells/well in 100 µl of Dulbecco’s Modified Eagle’s Medium (DMEM) containing 1% fetal bovine serum (FBS), antibiotics and amphotericin B. Cell-free HIV-1 (100 TCID50) was incubated with five-fold serial dilutions of milk for 15 min prior to addition to TZM-bl cells. Each milk sample was tested in triplicate wells of the plate. The cells were exposed to the mixture of milk and HIV-1 for 24 hrs at 37°C. Following this incubation, the cells were washed and assessed for viability using a methane thiosulfonate (MTS)-based solution (CellTiter 96® Aqueous One Solution Cell Proliferation Assay, Promega, WI) according to the manufacturer’s instructions. The cells were then lysed and luciferase activity was measured in cell lysates using the Bright-Glo™ Luciferase Assay System (Promega, WI). Luciferase activity was quantified in Relative Light Units (RLU) using a LMaxII384 luminometer (Molecular Devices, Sunnyvale, CA). Baseline activation of luciferase expression with media or milk alone (in the absence of added HIV-1) was also determined. Percent (%) inhibition or enhancement of HIV-1 infection was calculated by the following formula: 1-([RLU milk + HIV] – [RLU milk alone])/([RLU media +HIV] – [RLU media alone]) × 100%.
Cell-associated HIV-1 Infectivity Assays
To assess the effects of breast milk on cell-associated HIV-1 infection, HIV-infected peripheral blood CD4+ T lymphocytes were co-cultured with TZM-bl cells in the presence or absence of milk as described [26]. The levels of luciferase activity were then quantified as an indicator of HIV-1 infection of the TZM-bl target cells. In brief, primary CD4+ T lymphocytes were first enriched from PBMC and activated for 48 hr with PHA. Cells were then washed and resuspended in fresh media containing 100 U/mL of interleukin-2 (IL-2), followed by infection with HIV-1BAL for 5 days at 37°C prior to use. One day before the experiment, TZM-bl cells were seeded into the wells of a 96-well plate (1×104 cells/well) and allowed to adhere overnight. On the day of the experiment, serial dilutions of milk were added to designated wells of TZM-bl cells, followed by the addition of washed, HIV-infected CD4+ T lymphocytes at a final density of 1×105 lymphocytes per well. After 24 hrs, the cells were lysed directly in the wells with Beta-Glo reagent (Promega, Madison WI) and luciferase activity was quantified. Controls included TZM-bl cells co-cultured with uninfected CD4+ T lymphocytes in the presence of either media or milk. Additionally, to control for any cell-free HIV-1 released from the infected lymphocytes during co-culture, an equivalent number of HIV-infected CD4+ lymphocytes was seeded into wells in the absence of TZM-bl target cells. The supernatant from these wells, containing cell-free virus, was collected after 24 hrs and added to TZM-bl cells. The effect of breast milk on TZM-bl infection by this cell-free virus was also evaluated.
Multiplex and ELISA Cytokine Assays
The levels of cytokines, chemokines and growth factors in breast milk samples were measured using commercially available multiplex assays (Bio-Rad Laboratories, Hercules, CA) in conjunction with the DartLab Immune Monitoring Core Facility at The Geisel School of Medicine at Dartmouth. Results were quantified based on individual standard curves generated for each cytokine and the data analyzed using Bio-Plex Manager™ software. SDF-1α was measured in breast milk using a commercially available ELISA (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions.
LewisX ELISA
Measurement of LewisX in breast milk was carried out by standard ELISA. Milk samples were diluted 1∶10 in 0.2 M NaHCO3 buffer, pH 9.6. The diluted samples (100 µl/well) were added to ELISA plates (Maxisorp plates, Nunc) and incubated overnight at 4°C. The plates were washed three times with Tris-buffered saline (TBS)/0.05% Tween 20 [TBST]) buffer and blocked with TBS/1% BSA for 30 min at room temperature. After washing with TBST, the plates were incubated with either mouse anti-human LewisX IgM or mouse anti-human sialyl- LewisX IgM monoclonal antibodies (Calbiochem, San Diego, CA) for 2 hr at room temperature, followed by washing with TBST and detection with goat anti-mouse IgM-horseradish peroxidase conjugate (Thermo Scientific/Pierce Biotechnology, Rockford, IL). After washing to remove unbound antibodies, the plates were developed using the 1-Step™ Turbo TMB-ELISA reagent (Thermo Scientific/Pierce Biotechnology) at room temperature. The reaction was stopped after 30 min by the addition of 1 M H2SO4 and the plates were read on an ELISA plate reader at A450 nm.
Quantitative Real-time PCR Assay
Real-time PCR was used to quantify HIV-1 proviral DNA in cell pellets from individual breast milk samples. Total cellular genomic DNA was isolated from milk cell pellets using a Qiagen blood minikit (Qiagen, Valencia CA). DNA samples were then assayed by real-time PCR for HIV-1 proviral DNA using primers and molecular beacons with broad specificity for different HIV-1 genotypes [27]. Conserved sequences within the HIV-1 gag gene were amplified with primers gagF (5′-ATAATCCACCTATCCCAGTAGGAGAAAT-3′ and gagR (5′-TTTGGTCCTTGTCTTATG TCCAGAATG-3′) and detected using the molecular beacon HIV-1/FAM, 5′GCGAGCCTGGGATTAAATAAAATAGTAAGAATGTATAGCGCTCGC-3′ with the quencher DABCYL. Proviral DNA copy number was quantified based on a standard curve generated from 10-fold serial dilution (from 108 to 101 copies) of an HIV-1 plasmid of known copy number. The number of cell equivalents was quantified by amplification of CCR5 sequences using the primers CCR5-590 (5′-CTTCATCATCCTCCTGACAATCG-3′) and CCR5rc890 (5′- GATTCCCGAGTAGCAGATGACC-3′) and the molecular beacon CCR5/FAM, CGAAGCTTGGGTGGTGGCTGTGTTTGCTTCG with the quencher DABCYL. All PCR reactions were carried out in duplicate in a final volume of 50 µl using HotStart-IT Probe qPCR master mix for real-time PCR (USB Corporation, Cleveland OH). Cycling conditions for both CCR5 and HIV quantification were denaturation (95°C, 5 min) followed by 45 cycles of amplification (95°C, 15 sec; 50°C, 30 sec; 72°C, 30 sec) using an Applied Biosystems 7300 Real-Time PCR System.
Statistical Analysis
GraphPad Prism v.4 (GraphPad Software, Inc, La Jolla, CA) was used to calculate t-tests and Mann-Whitney U tests. A one-way and two-way analysis of variance (ANOVA) was used to perform group comparisons. Student t-test was used to compare means ± standard error of the mean. Mann-Whitney U test was used to compare the median percent inhibition of HIV-1 and concentration of cytokines present in the milk from the HIV-positive and HIV-negative donors. Spearman’s correlations were used to correlate the concentrations of specific cytokines and the inhibitory activity against cell-free HIV-1. A p-value of ≤0.05 was used to indicate significance.
Results
Characteristics of Tanzanian Breast Milk Donors
Breast milk was obtained from a total of 20 HIV-positive and HIV-negative mothers enrolled in the antenatal clinic at MNH. The HIV-positive women were in regular attendance at the PMTCT clinic, where they received counseling on strategies for preventing HIV-1 transmission to their infants. Although the HIV-positive women were enrolled in the PMTCT clinic, none reported having taken either sdNVP or any combination of ARV therapy. They acknowledged being offered medication at delivery, but declined to take it.
Women with clinical mastitis were excluded from the study based on physical evaluation and specific questions about the presence of pain, swelling and discharge from the breasts. The characteristics of the milk donors are summarized (Table 1). All milk samples were obtained from women within 42 days post-partum. Among these, colostrum samples were collected within three days post-partum from two HIV-positive and two HIV-negative donors. The average time of breast milk collection post-partum from HIV-positive donors (12.4 days) was less than that of HIV-negative donors (19.3 days); however, this difference was not statistically significant. This difference was likely due to close follow-up of HIV-positive women enrolled in the PMTCT clinic as compared to healthy HIV-negative women who had no medical indication for more frequent follow-up.
Table 1. Characteristics of Tanzanian breast milk donors.
| HIV-positive (n = 10) | HIV-negative (n = 10) | |
| Average age of mother (yrs) | 27.5 (range 16–40) | 26.5 (range 21–37) |
| PMTCT medication taken | No | N/A |
| ARV medication taken | No | N/A |
| Symptomatic breast illness | No | No |
| Average time of milk collection (days post-partum) | 12.4 (range 1–28) | 19.3 (range 2–42) |
Breast Milk from HIV-positive and HIV-negative Donors Inhibits Cell-free R5 HIV-1
To determine if breast milk from HIV-positive women affects cell-free HIV-1 infection in vitro, TZM-bl cells were infected with R5 HIV-1BaL in the presence of five-fold serial dilutions (from 1∶4 to 1∶2500) of milk. The effect of mature milk from HIV-positive (n = 8) and HIV-negative (n = 8) donors was evaluated. Significant inhibition of cell-free HIV-1BaL was observed using milk from both HIV-positive donors (dotted line) and HIV-negative donors (solid line) beginning at a 1∶4 dilution (Figure 1). At this dilution, the median percent inhibition of HIV-1 was 85% (range 68%–90.5%) for HIV-positive donors and 64% (range 61%–75.5%) for HIV-negative donors. Although there was a trend toward greater inhibitory activity associated with milk from HIV-positive donors as compared to HIV-negative donors, this difference did not reach statistical significance. The inhibitory activity of breast milk from all donors decreased in direct relation to further dilution of the milk. Only minimal inhibitory activity was observed at a 1∶2500 dilution using milk from either HIV-positive or HIV-negative donors.
Figure 1. Inhibition of cell-free HIV-1 by breast milk.
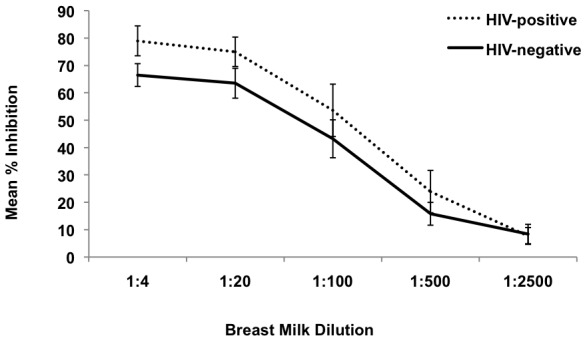
Mature breast milk from HIV-positive (n = 8) and HIV-negative (n = 8) donors was evaluated for inhibition of cell-free HIV-1 in TZM-bl cell assays. Cells were infected with R5 HIV-1BaL in the presence of five-fold serial dilutions of breast milk. Percent inhibition was calculated relative to control cultures infected with HIV-1BaL in the absence of added breast milk. HIV-inhibitory activity of breast milk from HIV-positive (dotted line) and HIV-negative (solid line) donors is shown (mean ± SD).
Together, these results demonstrate potent HIV-1 inhibitory activity against cell-free R5 HIV-1 in mature milk from both HIV-positive and HIV-negative subjects. Similar results were obtained against cell-free HIV-1BaL using colostrum samples obtained from two HIV-positive and two HIV-negative donors. Compared to mature milk, colostrum had more potent inhibitory activity at a 1∶500 dilution (50% versus 24%). However, the inhibitory activity of colostrum was significantly decreased at a 1∶2500 dilution, a finding that was similar to that observed for mature milk.
Additional experiments were performed to compare the inhibitory activity of breast milk against HIV-1 isolates with difference tropism. Individual milk samples were evaluated against R5-tropic (HIV-1BaL) and X4-tropic (HIV-1HC4) strains of HIV-1. Among HIV-positive donors, HIV-inhibitory activity in breast milk ranged from 45–96% against HIV-1BaL, and from 25–94% against HIV-1HC4. Overall, milk from each donor was able to inhibit both strains of cell-free HIV-1, and there was a significant correlation between the ability to inhibit R5 and X4 strains (R2 = 0.596, p = 0.025).
Similar findings were observed using breast milk from HIV-negative donors, demonstrating a significant correlation between the HIV-inhibitory activity against cell-free R5 and X4 HIV-1 (R2 = 0.599, p = 0.024). Again, there was a trend toward higher inhibitory activity among milk samples from the HIV-positive donors as compared to HIV-negative donors, similar to our earlier observation (Fig. 1); however, this difference was not statistically significant.
For three HIV-positive donors, sequential breast milk samples were obtained 2 weeks apart. When assayed for HIV-inhibitory activity, milk from 2 of the 3 donors maintained a consistent level of inhibition against cell-free R5 HIV-1BAL between the first and second time points, while the inhibitory activity of milk from the third donor declined (data not shown).
Levels of Breast Milk MIP-1α, MIP-1β, RANTES and SDF-1α do not Correlate with Cell-free HIV-inhibitory Activity
We next explored whether the HIV-inhibitory activity of breast milk against cell-free HIV-1 was associated with increased concentrations of the ligands for the HIV-1 co-receptors, CCR5 and CXCR4. Levels of MIP-1α, MIP-1β, RANTES and SDF-1α were quantified in the milk of HIV-positive and HIV-negative subjects. The concentration of milk MIP-1α was very low in the majority of samples tested, with mean concentrations of 42.1 pg/ml and 80.1 pg/ml for HIV-positive and HIV-negative donors, respectively. There was no significant difference in the mean level of MIP-1β, (998.3 vs 842.1 pg/ml, Mann-Whitney, p = 0.21) or RANTES (173.2 vs 146.1 pg/ml, Mann-Whitney, p = 0.66), when comparing milk samples from HIV-positive versus HIV-negative donors. The mean concentration of SDF-1α was significantly higher in the milk of HIV-positive donors as compared to HIV-negative donors (1,525 vs 1,189 pg/ml; Mann-Whitney, p = 0.009).
To determine if the levels of breast milk MIP-1α, MIP-1β, RANTES or SDF-1α correlate with inhibitory activity against cell-free HIV-1 in vitro, concentrations of each factor were compared to the percent HIV-inhibition for the same sample. Spearman’s correlations were performed for MIP-1α, MIP-1β, RANTES and SDF-1α and the inhibitory activity of all milk samples from both HIV-positive and HIV-negative donors. The results of these comparisons revealed no significant correlation between the level of MIP-1α, MIP-1β, RANTES or SDF-1α in milk and cell-free HIV-inhibitory activity for all donors, independent of HIV-1 serostatus. Additionally, there was no correlation between the concentration of MIP-1α, MIP-1β, RANTES or SDF-1α in milk and the percent inhibitory activity against cell-free HIV-1 when comparing HIV-positive and HIV-negative donors in separate analyses.
Breast Milk Contains Elevated Levels of Inflammatory Cytokines
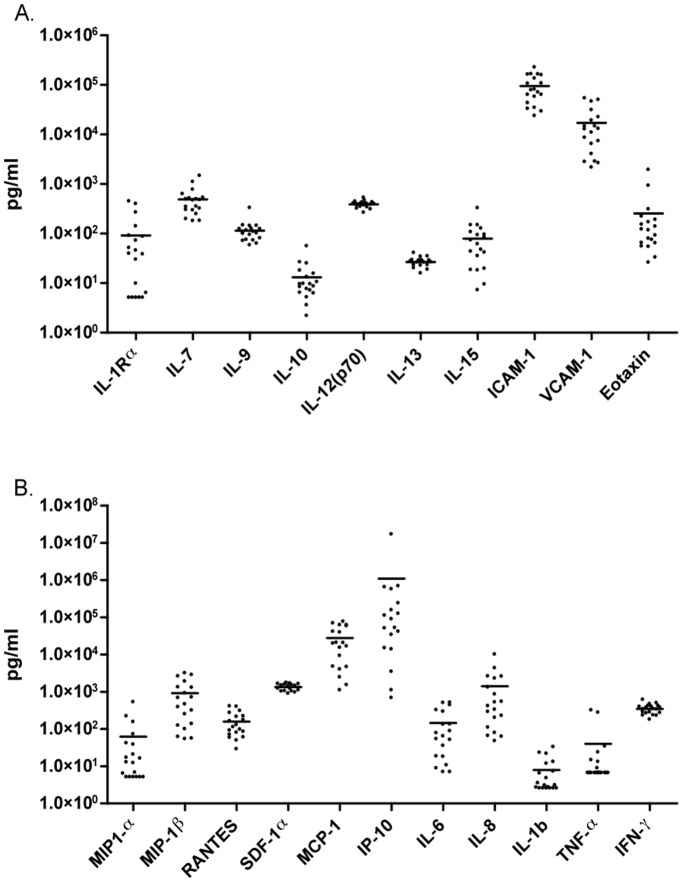
Breast milk from both HIV-positive and HIV-negative donors was further evaluated for the presence of an expanded range of cytokines, chemokines and growth factors using a multiplex panel. Among milk samples from all donors, considerable variation was observed in the range of concentrations of the factors measured (Figure 2). In particular, IL-6, IL-8, MIP-1α, MIP-1β, MCP-1 and IP-10 had ≥1 log variation among donors. Of note, subject 002+ (HIV-positive) and subject 002- (HIV-negative) were remarkable for having significantly higher concentrations of multiple inflammatory factors, including TNFα, IL-1β, IL-6, IL-8, MIP-1α, MIP-1β, MCP-1 and IP-10 in their milk as compared to other donors. No significant differences were observed in the levels of individual immune factors among milk samples from HIV-positive and HIV-negative donors, and no significant correlations were found between the level of individual cytokines in milk and inhibition of cell-free HIV-1.
Figure 2. Immune factors in breast milk from Tanzanian donors.
The concentrations of multiple immune factors including cytokines, chemokines and growth factors were measured in breast milk samples using a multiplex panel. Data for each factor is expressed as concentration (pg/mL) and is presented for all donors irrespective of HIV serostatus.
Sialyl-LewisX in Breast Milk from HIV-positive Donors Correlates with Inhibition of Cell-free HIV-1
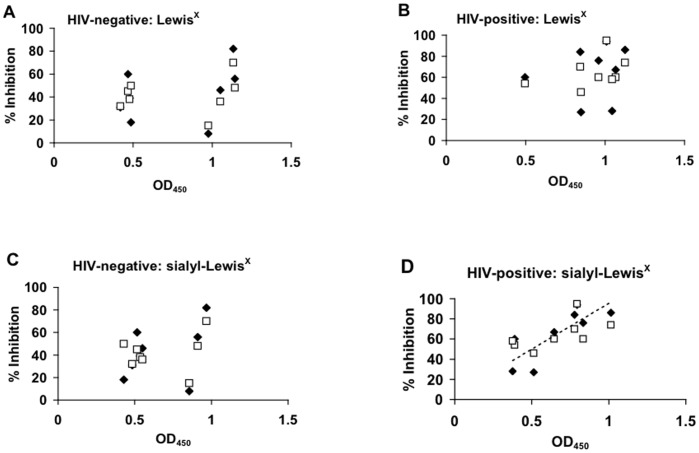
Previous studies have indicated a role for the blood group antigen LewisX in blocking the interaction between HIV-1gp120 and DC-SIGN expressed on dendritic cells (DC) [8]. Similarly, epithelial-derived MUC1 in breast milk, which contains multiple repeating LewisX motifs, has been implicated in preventing cell-free HIV-1 transmission to DC and infection of CD4+ T cells [8]–[10]. In the present study, levels of LewisX and sialyl-LewisX were measured in the breast milk of Tanzanian donors. Results demonstrate the presence of both LewisX and sialyl-LewisX in the milk of all donors, with distinct clustering of low and high expression patterns among individual donors (Figure 3). When compared with HIV-inhibitory activity against cell-free R5- or X4-tropic virus, no correlation was found between the levels of milk LewisX or sialyl-LewisX among HIV-negative donors or the levels of LewisX among HIV-positive donors (Fig. 3A–C). However, a significant correlation was observed among HIV-positive donors between the levels of sialyl-LewisX in milk and cell-free HIV-inhibitory activity (R2 = 0.657) (Fig. 3D). These results support a possible role for sialyl-LewisX in breast milk in preventing cell-free HIV-1 infection of susceptible CD4+ target cells.
Figure 3. LewisX and sialyl-LewisX in breast milk.
Levels of LewisX and sialyl-LewisX were measured by ELISA in breast milk samples from both HIV-positive and HIV-negative donors. The results were then compared to cell-free HIV-inhibitory activity for the same samples. Data is expressed as the percent inhibition of cell-free R5 (□) and X4 (♦) HIV-1 relative to the levels of either LewisX or sialyl-LewisX measured for each sample.
Breast Milk Enhances Cell-associated HIV-1 Infection in vitro
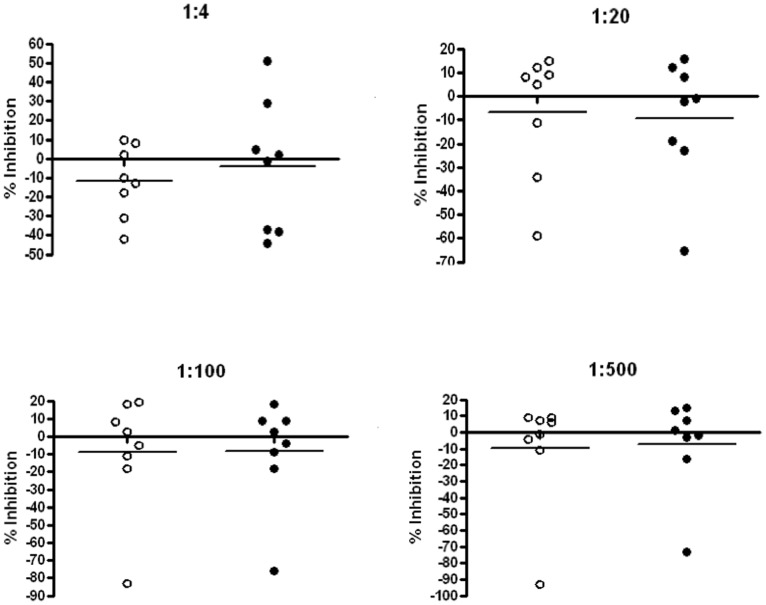
In further experiments, we assessed the ability of breast milk to inhibit cell-associated HIV-1 infection in vitro. The effect of milk at dilutions of 1∶4, 1∶20, 1∶100 and 1∶500 was evaluated in TZM-bl cells co-cultured with HIV-infected primary CD4+ T lymphocytes. In contrast to our results using cell-free virus, inhibition of cell-associated HIV-1 was observed with milk from only 2 of 16 donors, and then only at the lowest dilution (1∶4) (Figure 4). Moreover, significant enhancement of cell-associated HIV-1 infection was observed in the presence of milk from both HIV-positive and HIV-negative donors. Even at dilutions as high as 1∶500, milk from two donors, Subject 002+ (HIV-positive) and Subject 002- (HIV-negative), significantly enhanced cell-associated HIV-1 infection (Fig. 4). As noted earlier, milk from these donors had significantly higher levels of multiple inflammatory factors. No correlation was found between the levels of LewisX or sialyl-LewisX in milk samples and the observed enhancing activity against cell-associated HIV-1 (data not shown), suggesting that distinct factors influence the impact of breast milk on cell-free versus cell-associated virus.
Figure 4. Enhancement of cell-associated HIV-1 infection by breast milk.
Breast milk from HIV-positive and HIV-negative donors was evaluated for activity against cell-associated HIV-1 infection in TZM-bl cell assays. Breast milk was tested at 5-fold serial dilutions (1∶4, 1∶20, 1∶100 and 1∶500). Percent inhibition or enhancement of cell-associated R5 HIV-1BAL is shown for breast milk samples from individual HIV-positive (open circles) and HIV-negative (closed circles) donors.
Detection of Proviral DNA in Breast Milk Cells
To determine whether enhancement of cell-associated HIV infection by breast milk in vitro was associated with the presence of HIV-infected cells in vivo, we used quantitative real-time PCR to assess proviral load in breast milk cells isolated from the HIV-1 positive donors in this study. HIV-1 proviral DNA was detected by real-time PCR in 4 of 9 donors tested. Of those donors with detectable proviral DNA in their breast milk cells, 3 of 4 were found to have breast milk that significantly enhanced cell-associated HIV-1 infection in vitro.
Discussion
The results of this study demonstrate significant inhibition of cell-free HIV-1 by breast milk from both HIV-positive and HIV-negative Tanzanian women. Inhibition of cell-free virus was largely independent of viral tropism; however, there was a trend toward higher levels of inhibition of cell-free HIV-1 with milk from HIV-positive as compared to HIV-negative subjects, suggesting both innate and adaptive immune factors may contribute to the overall anti-viral effect of the milk.
Previously, we found that breast milk from HIV-negative donors in the United States potently inhibited cell-free HIV-1 infection in vitro, suggesting the presence of innate anti-viral factors at relatively high abundance in breast milk [26]. Here, we confirm the inhibitory effect of breast milk on cell-free HIV-1 using samples obtained from both HIV-positive and HIV-negative women from sub-Saharan Africa. The anti-viral activity against cell-free HIV-1 did not correlate with the presence or levels of individual cytokines, chemokines or growth factors in the milk, including factors that serve as blocking ligands for the HIV-1 co-receptors, CCR5 and CXCR4.
However, a correlation was found between HIV-inhibitory activity and the siaylated form of the blood group antigen LewisX in the milk of HIV-positive women. LewisX and MUC1 isolated from breast milk have been shown to inhibit binding of cell-free HIV-1 to DC-SIGN and to prevent transfer of infection to CD4+ T cells [8]–[10]. The observed correlation between breast milk inhibitory activity against cell-free HIV-1 and levels of sialyl-LewisX in milk suggests sialic acid residues may play a key role in blocking cell-free viral infection. The presence of sialic acid motifs at the ends of carbohydrate side chains has been shown to significantly reduce the inherent infectivity of both SIV and HIV-1 [28], [29], while digestion with neuraminindase, which cleaves sialyl moieties, enhances viral infectivity and increases cell-cell syncytium formation in vitro [28].
Despite the finding of potent inhibitory activity against cell-free HIV-1, breast milk from the same subjects had no inhibitory effect on cell-associated HIV infection in vitro. Rather, a significant proportion of milk from individual donors, including both HIV-positive and HIV-negative subjects, enhanced the level of cell-associated HIV-1 infection. Enhancement did not correlate with levels of sialyl-LewisX in the milk or with the presence of individual cytokines or chemokines, indicating that distinct factors in breast milk modulate cell-free versus cell-associated HIV-1 infection.
Enhancement of cell-cell interaction and HIV-1 infection in the presence of breast milk may result from up-regulation of cell surface molecules needed for efficient attachment and interaction of HIV-infected milk cells to target cells in the intestinal epithelium or submucosal tissue. Formation of a virological synapse between HIV-infected and uninfected cells is characterized by a tight adhesive junction, involving HIV-1 gp120 binding to CD4, and membrane interaction with adhesion molecules, including ICAM-1 [30]. Breast milk contains high levels of soluble ICAM-1 (sICAM-1), which might inhibit cell-cell adhesion and modulate cell-associated HIV-1 infection. We measured the levels of sICAM-1 in the milk of subjects in this study, and found a range of concentrations (Fig. 2A), but no correlation with inhibition or enhancement of cell-associated HIV-1 infection. Earlier studies reported no effect of blocking antibodies against ICAM-1 in preventing adhesion of HIV-infected lymphocytic cells to epithelial cells [31], [32], suggesting other molecules are critical to the formation of a tight adhesive junction in this setting.
Alternatively, activation of HIV-1 expression from latently infected cells, and/or enhanced release of virions into the synaptic cleft, may be promoted by the presence of stimulatory factors in breast milk. Cell-associated HIV-1 in breast milk is found primarily in infected CD4+ T lymphocytes and macrophages [33]–[35]. When stimulated in vitro, HIV-infected milk cells secrete higher levels of virus than the comparable population of cells from peripheral blood [35]. Recently, activated milk CD4+ T cells from women receiving HAART were shown to produce HIV-1 in vitro, suggesting that these cells may constitute a reservoir that is relatively refractive to treatment [36].
Activation of HIV-1 transcription is known to be modulated by immune cytokines [37], and it is possible that high levels of inflammatory factors in breast milk may play a role in enhancing HIV-1 release from infected cells. Among the subjects in this study, markedly elevated concentrations of multiple inflammatory factors were observed in milk from two subjects, and milk from these subjects also enhanced infection with cell-associated HIV-1 at dilutions up to 1∶500 (the highest dilution tested).
Inflammatory conditions in the breast, including mastitis, are known to increase the risk of HIV-1 transmission during breastfeeding [38]–[40]. Mothers in this study were screened at enrollment, and those with clinical signs and symptoms of mastitis were excluded. However, subclinical mastitis may also increase the levels of inflammatory factors in breast milk. Studies by Kantarci, et al. [41] found a positive correlation between subclinical mastitis, increased milk Na/K ratios, and higher numbers of HIV-infected cells in milk from HIV-infected Tanzanian mothers who transmitted HIV-1 to their infants through breastfeeding. While anecdotal, our results demonstrate that in vitro exposure of HIV-infected cells to breast milk containing high levels of multiple inflammatory factors can significantly enhance cell-associated HIV-1 infection.
Further studies are needed to determine the mechanisms of enhancement of cell-associated HIV-1 infection by breast milk. Localized and systemic inflammatory responses in the mother may contribute by increasing the levels of proinflammatory factors in the milk. Among HIV-infected mothers, this may represent an increased risk of virus transmission during breastfeeding. Moreover, the failure of breast milk to block cell-associated HIV-1 may be associated with maintenance of a cellular reservoir of virus in breast milk and may contribute to the residual transmission of HIV-1 to breastfeeding infants among women receiving HAART during lactation.
Acknowledgments
The authors wish to thank Karin Metzner for very helpful assistance and advice with quantitative real-time PCR assays, and Stephanie Dorosko, Susana Asin, Christiane Rollenhagen, Charles Wira, Paul Guyre, Paul Palumbo and Andrew Daubenspeck for helpful scientific discussions and expert advice during the course of this work, and C. Ford von Reyn and Richard Waddell for continued support and guidance through the Dartmouth-Fogarty AITRP. We thank the doctors and nurses at the Muhimbili National Hospital, the Muhimbili University of Health and Allied Sciences, and the PMTCT clinics in Dar es Salaam, Tanzania. We especially thank the Tanzanian mothers who donated breast milk for this study.
Funding Statement
This study was supported by the National Institutes of Health (NIH) AIDS International Training and Research Program/Fogarty International Center at The Geisel School of Medicine at Dartmouth, Howard Hughes Medical Institute Undergraduate Research Award, and a Veterans Affairs Merit Review Award. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Rousseau CM, Nduati RW, Richardson BA, John-Stewart GC, Mbori-Ngacha DA, et al. (2004) Association of levels of HIV-1-infected breast milk cells and risk of mother-to-child transmission. J Infect Dis 190(10): 1880–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rousseau CM, Nduati RW, Richardson BA, Steele MS, John-Stewart GC, et al. (2003) Longitudinal analysis of human immunodeficiency virus type 1 RNA in breast milk and of its relationship to infant infection and maternal disease. J Infect Dis 1 187(5): 741–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aldrovandi GM, Kuhn L (2010) What infants and breasts can teach us about natural protection from HIV infection. J Infect Dis 1 202 Suppl 3: S366–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Walter J, Ghosh MK, Kuhn L, Semrau K, Sinkala M, et al. (2009) High concentrations of interleukin 15 in breast milk are associated with protection against postnatal HIV transmission. J Infect Dis 15 200(10): 1498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Villamor E, Koulinska IN, Furtado J, Baylin A, Aboud S, et al. (2007) Long-chain n-6 polyunsaturated fatty acids in breast milk decrease the risk of HIV transmission through breastfeeding. Am J Clin Nutr 86(3): 682–9. [DOI] [PubMed] [Google Scholar]
- 6. Arsenault JE, Webb AL, Koulinska IN, Aboud S, Fawzi WW, et al. (2010) Association between breast milk erythropoietin and reduced risk of mother-to-child transmission of HIV. J Infect Dis 202(3): 370–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Saeland E, de Jong MA, Nabatov AA, Kalay H, Geijtenbeek TB, et al. (2009) MUC1 in human milk blocks transmission of human immunodeficiency virus from dendritic cells to T cells. Mol Immunol 46(11–12): 2309–16. [DOI] [PubMed] [Google Scholar]
- 8. Naarding MA, Ludwig IS, Groot F, Berkhout B, Geijtenbeek TB, et al. (2005) Lewis X component in human milk binds DC-SIGN and inhibits HIV-1 transfer to CD4+ T lymphocytes. J Clin Invest 115(11): 3256–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Habte HH, de Beer C, Lotz ZE, Tyler MG, Kahn D, et al. (2008) Inhibition of human immunodeficiency virus type 1 activity by purified human breast milk mucin (MUC1) in an inhibition assay. Neonatology 93(3): 162–70. [DOI] [PubMed] [Google Scholar]
- 10. Habte HH, Kotwal GJ, Lotz ZE, Tyler MG, Abrahams M, et al. (2007) Antiviral activity of purified human breast milk mucin. Neonatology 92(2): 96–104. [DOI] [PubMed] [Google Scholar]
- 11. Newburg DS, Linhardt RJ, Ampofo SA, Yolken RH (1995) Human milk glycosaminoglycans inhibit HIV glycoprotein gp120 binding to its host cell CD4 receptor. J Nutr 125(3): 419–24. [DOI] [PubMed] [Google Scholar]
- 12. Viveros-Rogel M, Soto-Ramirez L, Chaturvedi P, Newburg DS, Ruiz-Palacios GM (2004) Inhibition of HIV-1 infection in vitro by human milk sulfated glycolipids and glycosaminoglycans. Adv Exp Med Biol 554: 481–7. [DOI] [PubMed] [Google Scholar]
- 13. Newburg DS (2009) Neonatal protection by an innate immune system of human milk consisting of oligosaccharides and glycans. J Anim Sci 87 13 Suppl: 26–34. [DOI] [PubMed] [Google Scholar]
- 14. Alfsen A, Yu H, Magérus-Chatinet A, Schmitt A, Bomsel M (2005) HIV-1-infected blood mononuclear cells form an integrin- and agrin-dependent viral synapse to induce efficient HIV-1 transcytosis across epithelial cell monolayer. Mol Biol Cell 16(9): 4267–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen P, Hübner W, Spinelli MA, Chen BK (2007) Predominant mode of human immunodeficiency virus transfer between T cells is mediated by sustained Env-dependent neutralization-resistant virological synapses. J Virol 81(22): 12582–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Groot F, Welsch S, Sattentau QJ (2008) Efficient HIV-1 transmission from macrophages to T cells across transient virological synapses. Blood 111(9): 4660–3. [DOI] [PubMed] [Google Scholar]
- 17. Vasiliver-Shamis G, Dustin ML, Hioe CE (2010) HIV-1 virological synapse is not simply a copycat of the immunological synapse. Viruses 2(5): 1239–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shapiro RL, Ndung'u T, Lockman S, Smeaton LM, Thior I, et al. (2005) Highly active antiretroviral therapy started during pregnancy or postpartum suppresses HIV-1 RNA, but not DNA, in breast milk. J Infect Dis 192(5): 713–9. [DOI] [PubMed] [Google Scholar]
- 19. Shapiro RL, Hughes MD, Ogwu A, Kitch D, Lockman S, et al. (2010) Antiretroviral regimens in pregnancy and breast-feeding in Botswana. N Engl J Med 17 362(24): 2282–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kilewo C, Karlsson K, Ngarina M, Massawe A, Lyamuya E, et al. (2009) Prevention of mother-to-child transmission of HIV-1 through breastfeeding by treating mothers with triple antiretroviral therapy in Dar es Salaam, Tanzania: the Mitra Plus study. J Acquir Immune Defic Syndr 52(3): 406–16. [DOI] [PubMed] [Google Scholar]
- 21. Chama CM, Bello M, Ajayi BA, Zarma S, Gashau W, et al. (2010) The use of highly active antiretroviral therapy for the prevention of mother-to-child transmission of the human immunodeficiency virus in Nigeria. J Obstet Gynaecol 30(4): 362–6. [DOI] [PubMed] [Google Scholar]
- 22. de Vincenzi I (2011) the Kesho Bora Study Group (2011) Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): a randomised controlled trial. Lancet Infect Dis 11(3): 171–80. [DOI] [PubMed] [Google Scholar]
- 23. Chasela CS, Hudgens MG, Jamieson DJ, Kayira D, Hosseinipour MC, et al. (2010) Maternal or infant antiretroviral drugs to reduce HIV-1 transmission. N Engl J Med 362(24): 2271–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Slyker JA, Chung MH, Lehman DA, Kiarie J, Kinuthia J, et al. (2012) Incidence and correlates of HIV-1 RNA detection in the breast milk of women receiving HAART for the prevention of HIV-1 transmission. PloS One 7(1): e29777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lehman DA, Chung MH, John-Stewart GC, Richardson BA, Kiarie J, et al. (2008) HIV-1 persists in breast milk cells despite antiretroviral treatment to prevent mother-to-child transmission. AIDS 22(12): 1475–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lyimo MA, Howell AL, Balandya E, Eszterhas SK, Connor RI (2009) Innate factors in human breast milk inhibit cell-free HIV-1 but not cell-associated HIV-1 infection of CD4+ cells. J Acquir Immune Defic Syndr 51(2): 117–24. [DOI] [PubMed] [Google Scholar]
- 27. Vet JA, Majithia AR, Marras SA, Tyagi S, Dube S, et al. (1999) Multiplex detection of four pathogenic retroviruses using molecular beacons. Proc Natl Acad Sci USA 96(11): 6394–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sun J, Barbeau B, Sato S, Tremblay MJ (2001) Neuraminidase from a bacterial source enhances both HIV-1-mediated syncytium formation and the virus binding/entry process. Virology. 2001 284(1): 26–36. [DOI] [PubMed] [Google Scholar]
- 29. Means RE, Desrosiers RC (2000) Resistance of native, oligomeric envelope on simian immunodeficiency virus to digestion by glycosidases. J Virol 74(23): 11181–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jolly C, Mitar I, Sattentau QJ (2007) Adhesion molecule interactions facilitate human immunodeficiency virus type 1-induced virological synapse formation between T cells. J Virol 81(24): 13916–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pearce-Pratt R, Phillips DM (1996) Sulfated polysaccharides inhibit lymphocyte-to-epithelial transmission of human immunodeficiency virus-1. Biol Reprod 54(1): 173–82. [DOI] [PubMed] [Google Scholar]
- 32. Puigdomènech I, Massanella M, Izquierdo-Useros N, Ruiz-Hernandez R, Curriu M, et al. (2008) HIV transfer between CD4 T cells does not require LFA-1 binding to ICAM-1 and is governed by the interaction of HIV envelope glycoprotein with CD4. Retrovirology 5: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Southern SO (1998) Milk-borne transmission of HIV. Characterization of productively infected cells in breast milk and interactions between milk and saliva. J Hum Virol 1(5): 328–37. [PubMed] [Google Scholar]
- 34. Petitjean G, Becquart P, Tuaillon E, Al Tabaa Y, Valea D, et al. (2007) Isolation and characterization of HIV-1-infected resting CD4+ T lymphocytes in breast milk. J Clin Virol 39(1): 1–8. [DOI] [PubMed] [Google Scholar]
- 35. Becquart P, Petitjean G, Tabaa YA, Valéa D, Huguet MF, et al. (2006) Detection of a large T-cell reservoir able to replicate HIV-1 actively in breast milk. AIDS 26 20(10): 1453–5. [DOI] [PubMed] [Google Scholar]
- 36. Valea D, Tuaillon E, Al Tabaa Y, Rouet F, Rubbo PA, et al. (2011) CD4+ T cells spontaneously producing human immunodeficiency virus type I in breast milk from women with or without antiretroviral drugs. Retrovirology 8: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Alfano M, Poli G (2005) Role of cytokines and chemokines in the regulation of innate immunity and HIV infection. Mol Immunol 42(2): 161–82. [DOI] [PubMed] [Google Scholar]
- 38. Semba RD (2000) Mastitis and transmission of human immunodeficiency virus through breast milk. Ann N Y Acad Sci 918: 156–62. [DOI] [PubMed] [Google Scholar]
- 39. Willumsen JF, Filteau SM, Coutsoudis A, Uebel KE, Newell ML, et al. (2000) Subclinical mastitis as a risk factor for mother-infant HIV transmission. Adv Exp Med Biol 478: 211–23. [DOI] [PubMed] [Google Scholar]
- 40. Lunney KM, Iliff P, Mutasa K, Ntozini R, Magder LS, et al. (2010) Associations between breast milk viral load, mastitis, exclusive breast-feeding, and postnatal transmission of HIV. Clin Infect Dis 50(5): 762–9. [DOI] [PubMed] [Google Scholar]
- 41. Kantarci S, Koulinska IN, Aboud S, Fawzi WW, Villamor E (2007) Subclinical mastitis, cell-associated HIV-1 shedding in breast milk, and breast-feeding transmission of HIV-1. J Acquir Immune Defic Syndr 46(5): 651–4. [DOI] [PubMed] [Google Scholar]