Abstract
Transplantation therapy for diabetes is limited by unavailability of donor organs and outcomes complicated by immunosuppressive drug toxicity. Xenotransplantation is a strategy to overcome supply problems. Implantation of tissue obtained early during embryogenesis is a way to reduce transplant immunogenicity. Insulin-producing cells originating from embryonic pig pancreas obtained very early following pancreatic primordium formation [embryonic day 28 (E28)] engraft long-term in inbred diabetic Lewis or Zucker Diabetic Fatty (ZDF) rats or rhesus macaques. Endocrine cells originating from embryonic pig pancreas transplanted in host mesentery migrate to mesenteric lymph nodes, engraft, normalize glucose tolerance in rats and improve glucose tolerance in rhesus macaques without the need for immune suppression. Engraftment of primordia is permissive for engraftment of an insulin-expressing cell component from porcine islets implanted subsequently without immune suppression. Similarities between findings in inbred rat and non-human primate hosts bode well for successful translation to humans of what could be a novel xenotransplantation strategy for the treatment of diabetes.
Keywords: beta cell, diabetes mellitus, non-human primates, transplantation, xenotransplantation
Introduction
Immunological aspects of strategies for xenotransplantation in humans are best modeled using old world monkeys or baboons as hosts. This is because the non-human primates share uniquely with humans a complex and redundant immune system that includes the capacity for humoral rejection of vascularized implants originating from pigs, a frequently used donor-species, outbred diversity and MHC expression patterns known to present barriers to long-term graft survival.1 Research employing non-human primates is limited by its expense, and the paucity of facilities that can provide the highly specialized resources and expertise required to meet the physical and emotional needs of these close human relatives. For this reason, inbred rodents, more widely available than non-human primates and less expensive to maintain, are commonly employed to generate data preliminary to non-human primate studies.2 Unfortunately, immunological differences between rodents and primates can result in an inability to translate findings made in the former to members of the latter species. In addition, rats and mice housed in a laboratory setting are sedentary, obese and glucose intolerant. These factors may confound interpretation of data generated to ascertain whether one or another therapeutic intervention, successful in rats, will correct metabolic abnormalities such as those of diabetes mellitus in humans.1,2
Diabetes is a common, disabling, and deadly metabolic disease that has reached epidemic proportions worldwide.3,4 Among adults in the USA, diabetes is the leading cause of new cases of blindness, kidney failure, and amputations of feet and legs not related to accidents or injury. Transplantation therapy for diabetes mellitus represents the best way to replace organ function in humans and the only way to prevent long-term complications. Unfortunately it is limited by unavailability of human donor organs and outcomes are complicated by immunosuppressive drug toxicity. There are approximately 1,000 pancreas and islet transplants performed per year in the USA, where the diabetic population exceeds 20 million individuals.5 Transplantation is rarely used for type 2 diabetes which is almost 20 times more common than type 1 disease.4,5 When performed, whole pancreas or islet transplantation requires use of potent immune suppressive medications that have significant complications.1,4,5
Pig insulin works well in humans. For that reason, the pig could be a physiologically suitable β cell donor.1 The severity of humoral rejection effectively precludes the use of pigs, as whole pancreas donors. However, because they are vascularized by the host post-transplantation, pancreatic cellular transplants, that include embryonic pancreas and isolated islets, are not subject to humoral rejection.4 Experience with non-transgenic or transgenic pig to primate islet or neonatal islet transplantation in immune suppressed non-human primates shows that sustained insulin independence can be achieved, but only through the use of agents that are not approved for human use or that result in a high level of morbidity and mortality1,4,6-8 As reviewed previously in Organogenesis,6-8 the use of embryonic tissue (kidney or pancreas) for transplantation offers theoretical advantages relative to transplantation of either stem cells or adult differentiated organs. Approximately a decade ago, we observed that embryonic pig kidney, transplanted across a wide xenogeneic barrier into immune-suppressed rodent (inbred mouse or rat) mesentery undergoes differentiation into an anatomically precise renal organ at the transplant site.9,10 When we attempted the same approach using embryonic pig pancreas rather than kidney, we discovered using two inbred rat strains as hosts [Lewis rats rendered diabetic using streptozotocin (STZ)10-13 a model for type 1 diabetes in humans or ZDF rats,14 a model for type 2 diabetes] that unlike kidney, embryonic pancreas does not differentiate in situ into an anatomically precise pancreas. Rather: (1) pancreas differentiates selectively in that only endocrine cells survive; (2) endocrine cells migrate into host mesenteric lymph nodes from where they release insulin in a glucose-regulated manner; (3) host immune suppression, required for engraftment of transplanted embryonic pig kidney into mice or rats is not required for engraftment of early-stage embryonic pancreas; and (4) engraftment of embryonic pig pancreas permits engraftment of a cellular component originating from adult pig islets transplanted subsequently.
Employing protocols developed for embryonic-pig to STZ- diabetic Lewis rat pancreas transplantation and recapitulated in ZDF rat hosts, we showed that embryonic pig pancreas engrafts, and improves glucose tolerance in STZ-diabetic rhesus macaques without the need for immune suppression.15,16 Here we review the successful adaptation of pig-into-diabetic rat transplantation technology to a pig-into-diabetic non-human primate model. Similarities of data generated in rat and non-human primate hosts suggest that, whatever the mechanism may be that permits engraftment and function without an immune-suppression requirement, it will be applicable to diabetic humans and may translate into a novel xenotransplantation therapy.
Transplantation of embryonic pig pancreas in diabetic rats and rhesus macaques
The importance of obtaining primordia from embryos in timed-pregnant sows
Figure 1A illustrates a pancreas dissected from an embryonic day 28 (E28) Yorkshire pig embryo. The dorsal pancreas (dp) and ventral pancreas (vp) components originating from the duodenum (duo) are separate at this stage of embryonic development. An anti-insulin antibody-stained section of E28 pig pancreas shows a relatively undifferentiated organ that contains some insulin positive cells (Fig. 1B, arrow). Figure 1C is a negative control for which normal serum was substituted for the anti-insulin antibody containing serum used to generate data shown in Figure 1B.
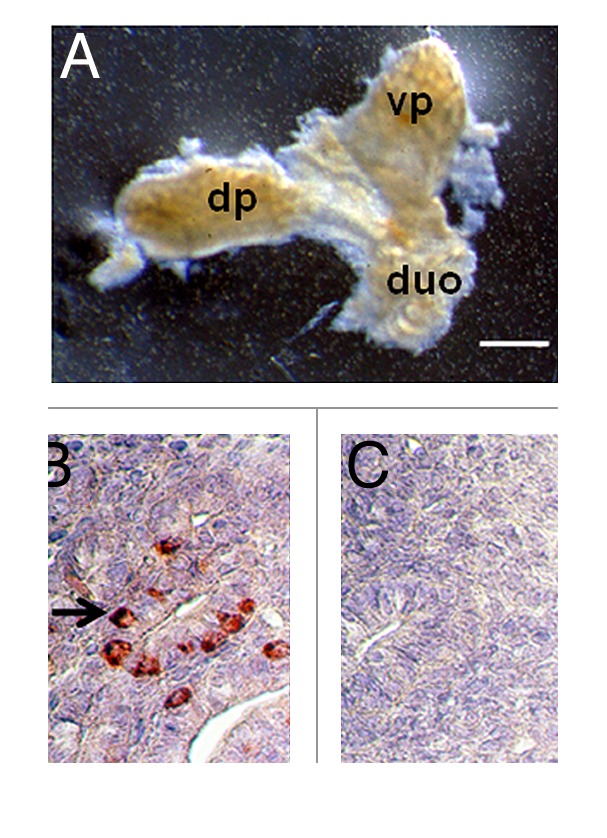
Figure 1. (A) Photograph of a pancreatic primordium freshly dissected from an E28 pig embryo and (B and C) photomicrographs of sections originating from E28 primordia stained using anti-insulin antibodies (B) or control serum (C). dp, dorsal pancreas; vp, ventral pancreas; duo, duodenum. Arrow delineates insulin-positive cells (B). Scale bars 10 um (A) and 1 um (B and C).
We have shown using diabetic rats as hosts that E28 or E29 pig pancreas engrafts successfully without the need for host immune suppression following transplantation in mesentery (Fig. 2). However, E35 pig pancreas undergoes rejection.10 While we have not determined whether E30-E34 pig pancreas has a fate similar to organs obtained on E35, we always use E28 pig pancreas as a source for transplantation because it engrafts predictably. It follows that the importance of obtaining accurately and precisely-timed pregnant Yorkshire sows from which to obtain E28 embryos is critical to the success of experiments.
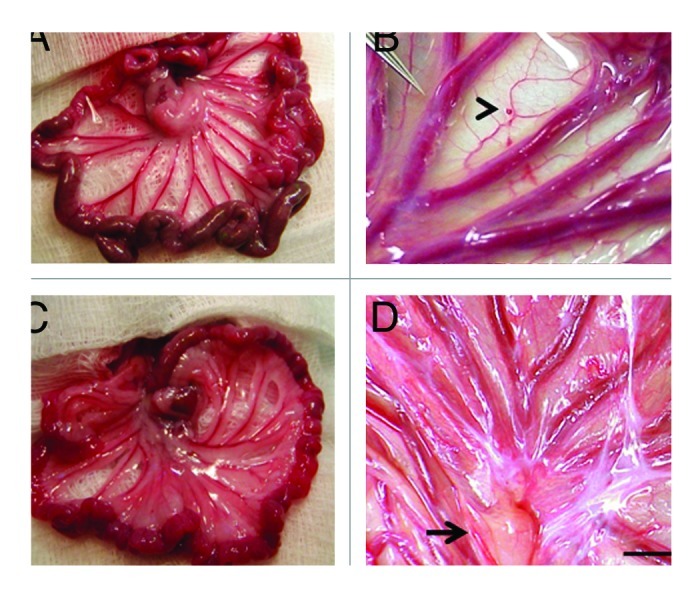
Figure 2. Photographs of mesentery from a rat (A and B) or a rhesus macaque (C and D) several weeks following sham-transplantation (A) or transplantation of E28 pig pancreatic primordia (B) or immediately following the transplantation of an E28 pig pancreatic primordium (C arrowhead) or several weeks following transplantation (D). Arrow, lymph node (D). Scale bar 1 cm (D). Reproduced with permission.11,15
Transplantation between sheets of mesentery
Following incubation for 45 min in vitro of dissected E28 pig pancreatic primordia with selected growth factors and cytokines, we implant individual dorsal and ventral components, within a tunnel dissected between tissue planes of the mesentery in diabetic rat or non-human primate hosts. Figure 2A and B shows mesentery from STZ-diabetic Lewis rats. Relative to an age-matched sham-transplanted diabetic control (Fig. 2A), the mesentery in transplanted rats is opalescent (Fig. 2B) reflecting an increased quantity of fat and proliferation of lymphatic vessels. Figure 2C shows the mesentery of a STZ-diabetic rhesus macaque at the time of transplantation. A primordium between sheets of mesentery is delineated (arrowhead). Figure 2D shows the mesentery post-transplantation. As in rats, accumulation of fat and proliferation of lymphatic vessels are observed in mesentery post-transplantation in non-human primates. A large lymph node is delineated in Figure 2D (arrow).
Engraftment in mesenteric lymph nodes and tissues along a lymphatic distribution
Shown in Figure 3A–D, are tissue sections of mesenteric lymph nodes from a STZ-diabetic Lewis rat into which E28 pig pancreatic primordia had been transplanted previously in mesentery stained using anti-insulin antibody (Fig. 3A and C), or control antiserum (Fig. 3B and D). Insulin positive cells are located predominantly in medullary sinus. Individual cells are evident in Figure 3C that do not stain in sections stained using control antiserum (Fig. 3D). Figure 3E-H show photomicrographs originating from a mesenteric lymph node of a STZ-diabetic rhesus macaque previously transplanted with E28 pig pancreatic primordia in mesentery. Sections E and G are stained with an anti-insulin antibody. Sections F and H are incubated with control serum. Individual cells with β cell morphology that stain positive (red) are delineated by arrows. No positive-staining cells are found in sections treated with control serum.
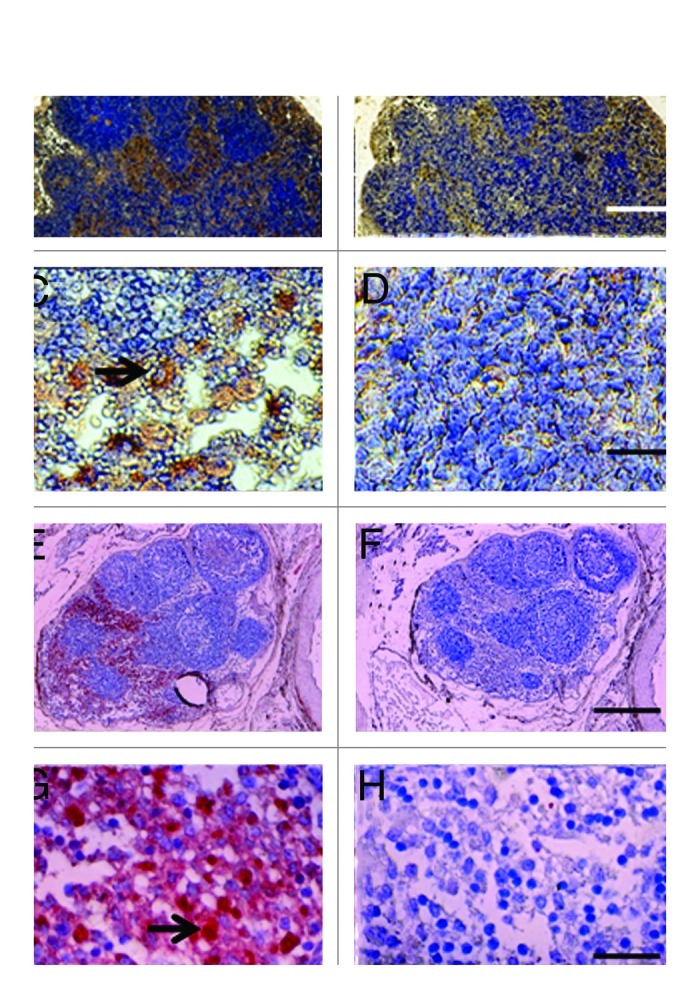
Figure 3. Photomicrographs of mesenteric lymph nodes from a STZ-diabetic Lewis rat (A-D) or STZ diabetic rhesus macaque (E-H) post-transplantation of E28 pig pancreatic primordia. Sections A,C, E and G are stained with an anti-insulin antibody. Sections B,D,F and H are stained using a control serum. Arrows (C and G) insulin positive cells. Scale bars 80 um (A and B); 30 um (C and D); 120 um (E and F) and 20 um (G and H). Reproduced with permission.13,15
Engraftment of pig tissue in the mesenteric lymph nodes of Lewis rats and primates as shown using immune histochemistry (Fig. 3) is documented using in situ hybridization for porcine proinsulin mRNA. Shown in Figure 4 are tissue sections from a diabetic rat (Fig. 4A and B) or rhesus macaque (Fig. 4C and D) into which E28 pig pancreatic primordia had been transplanted previously. Cells expressing porcine mRNA stain with use of an antisense probe to porcine proinsulin mRNA (Fig. 4B and D), but not a sense probe (Fig. 4A and C).
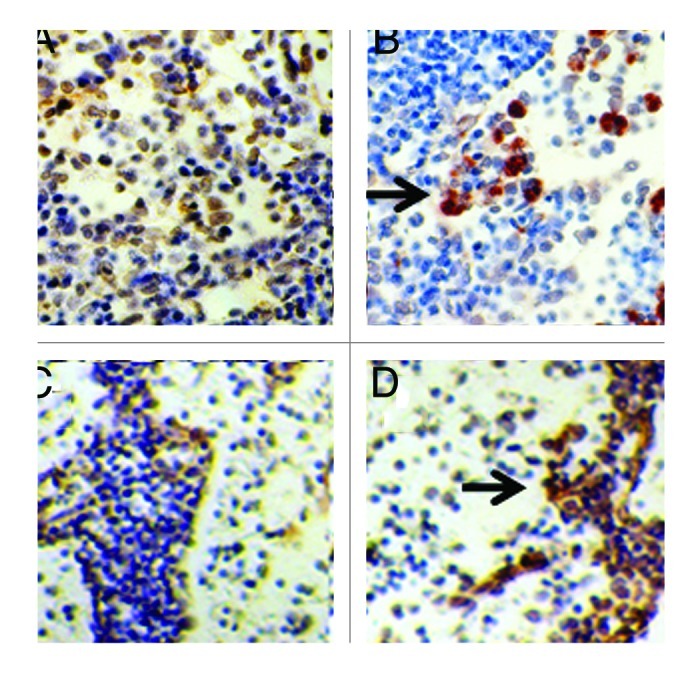
Figure 4. In situ hybridization was performed using pig proinsulin sense (A and C) or antisense probes (B and D) on sections of mesenteric lymph node originating from a STZ-diabetic Lewis rat (A and B) or a STZ-diabetic rhesus macaque (C and D). Arrows delineate cells to which the antisense probe is hybridized (B and D). Scale bar 30 um. Reproduced with permission.12,15
Normalization or improvement of glucose intolerance following transplantation of E28 pig pancreatic primordia
Figure 5 shows the results of intravenous glucose tolerance testing in Lewis rats (A) or rhesus macaques (B) prior to administration of STZ (Pre-STZ) and in the same animals following induction of STZ-diabetes (Post-STZ) and after transplantation of E28 pig pancreatic primordia (Post TX). Glucose tolerance is normalized in nonimmune-suppressed diabetic rats (Fig. 5A) and nearly normalized in diabetic rhesus macaques (Fig. 5B) following transplantation of E28 pig pancreatic primordia.
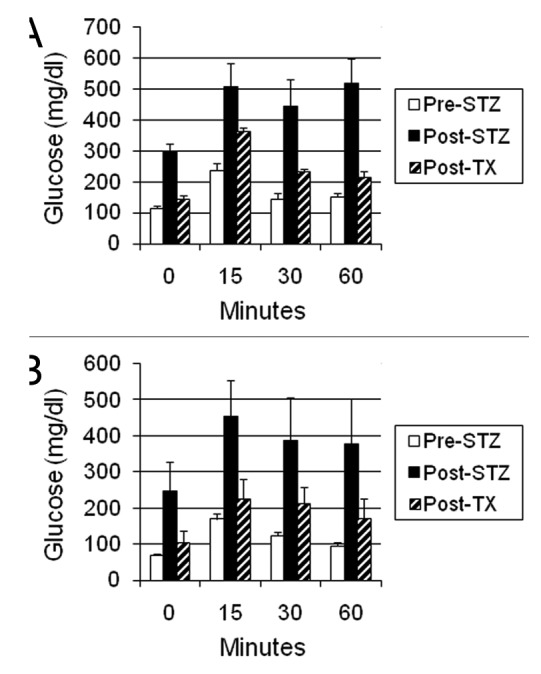
Figure 5. Intravenous glucose tolerance in: (A) Lewis rats (n = 4); or (B) rhesus macaques (n = 3). Glucose in peripheral venous blood was measured prior to intravenous infusion of dextrose (Time 0) and at several times after infusion, in animals prior to administration of STZ (Pre-STZ); 5 d following administration of STZ (Post STZ); or following transplantation of: (A) 5–8; or (B) 20–40 E28 pig pancreatic primordia in mesentery (Post-TX). Data are shown as mean ± SE. Reproduced with permission.13,16
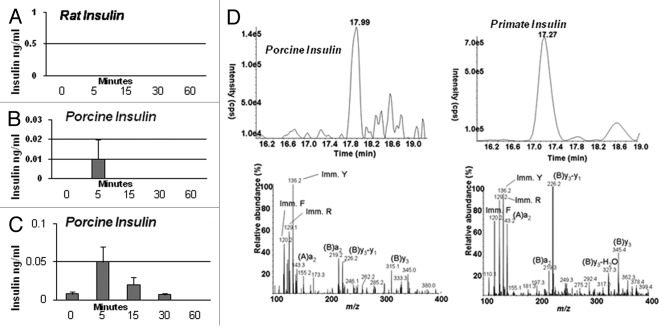
No rat insulin can be detected following transplantation of E28 pig pancreatic primordia in diabetic rats (Fig. 6A). Rather porcine insulin circulates following glucose infusion (Fig. 6B and C). Interestingly, levels are higher in arterial (Fig. 6C) than in venous blood (Fig. 6B), consistent with secretion into mesenteric lymphatics and entry into the arterial circulation via the thoracic duct, left subclavian vein and aorta. While circulating rat insulin can be distinguished from porcine insulin using an enzyme-linked immunosorbent assay (ELISA) no antibody can distinguish porcine from primate (or human) insulin. Accordingly we distinguished between the two in the circulation of rhesus macaques using sequential high performance liquid chromatography, immunoaffinity chromatography and mass spectrometry.15 No rhesus macaque insulin (< 0.1 ng/ml) can be detected post-transplantation of E28 pig pancreatic primordia in diabetic rhesus macaques. Rather porcine insulin is detectable in plasma (Fig. 6D).
Figure 6. (A-C) Levels of insulin in transplanted STZ diabetic Lewis rats (n = 4) measured during intravenous glucose tolerance testing. Data are shown and mean ± SE. D Left: Chromatogram (top) and product ion mass spectrum (bottom) of porcine insulin (precursor ion [M+5H]5+m/z 1156.3) extracted from 2 ml plasma obtained 5 min after IV glucose administration to a STZ-diabetic transplanted rhesus macaque. The retention time and diagnostic product ions derived from the 5-fold charged precursor ion unambiguously identify porcine insulin. (D) Right: chromatogram (top) and product ion mass spectrum (bottom) of human/macaque insulin (precursor ion [M+5H]5+m/z 1162.3) extracted from 1 ml of non-diabetic rhesus macaque plasma. The retention time and diagnostic product ions derived from the 5-fold charged precursor ion unambiguously identify human/macaque insulin. Reproduced with permission.13,15
The ZDF rat is an inbred strain derived from a colony of Zucker fatty rats. ZDF and Zucker fatty animals have an autosomal recessive mutation in the gene that encodes the leptin receptor. Homozygous Zucker fatty rats manifest hyperphagia, obesity and severe insulin resistance, but are generally normoglycemic. ZDF homozygous males become hyperglycemic beginning at approximately 7 weeks of age and thereafter spontaneously develop overt diabetes. Homozygous females become overtly diabetic beginning at age 6–8 weeks only if maintained on a diabetogenic high fat diet. In ZDF males and females, hyperglycemia occurs concomitant with markedly elevated levels of circulating insulin and failure of insulin secretion in response to a glucose challenge, mimicking the pathophysiology of human type 2 diabetes mellitus.
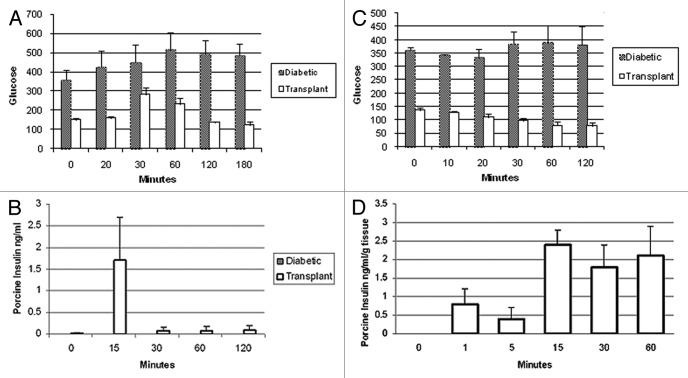
Glucose intolerance is corrected in both male and female ZDF rats following transplantation of E28 pig pancreatic primordia in mesentery. Figure 7A shows the results of oral glucose tolerance testing in diabetic male ZDF rats prior to (Diabetic) and following transplantation (Transplant). Levels of porcine insulin in the venous circulation following an oral glucose load are shown in Figure 7B. Levels of glucose (markedly elevated in diabetic ZDF rats) do not fall after intravenous administration of exogenous insulin to male ZDF rats reflecting insulin resistance. In contrast normalized glucose levels fall after intravenous insulin is injected in transplanted male ZDF rats, consistent with restored insulin sensitivity (Fig. 7C). To show that pig β cells within transplanted diabetic ZDF rats are capable of releasing insulin in response to glucose, we incubated mesenteric lymph nodes from rats into which pig pancreatic primordia in a solution containing 3 mM glucose. We took aliquots immediately prior to (time 0) and following addition of glucose to incubations so as render the glucose concentration 20 mM. As illustrated in Figure 7D no porcine insulin could be detected at time 0. However, porcine insulin was detectable by 1 min after increasing the glucose level, consistent with a first phase insulin release characteristic of β cells.
Figure 7. (A) Levels of glucose (mg/dl) measured over 180 min after an oral glucose load in male ZDF rats (n = 4) prior to transplantation of E28 pig pancreatic primordia (diabetic) or following transplantation (Transplant); (B) levels of porcine insulin (ng/ml) measured over 120 min after an oral glucose load in transplanted ZDF rats (n = 4). (C) Levels of glucose (mg/dl) measured over 120 min after i.v. insulin was administered to ZDF rats (n = 4); (D) levels of porcine insulin (ng/ml/g tissue wet weight) measured over 60 min in a solution containing mesenteric lymph node tissue from diabetic ZDF rats into which pig pancreatic primordia had been transplanted previously (n = 4). Immediately after measurements were made at time 0, glucose was added so as to increase the concentration from 3 mM to 20 mM, a range over which insulin secretion is stimulated in vivo. Data are expressed as mean ± SE. Reproduced with permission.14
Whereas rats the glucose tolerance of which is normalized following transplantation of 5–8 E28 pig pancreatic primordia are rendered insulin independent for the remainder of their lives, macaques transplanted with 20–40 primordia remain dependent on exogenous insulin (albeit as doses lower than required prior to transplantation) to maintain euglycemia.15 The most likely explanation for the differential success between rats and macaques is that macaques weigh 20 times as much as rats. A STZ-diabetic rat can be rendered normoglycemic lifelong with no exogenous insulin requirement by transplantation of 5–8 pig pancreatic primordia. Extrapolating, it would take 100–200 primordia to render a diabetic macaque independent of exogenous insulin. This would require the sacrifice of about 10–20 pregnant sows and multiple surgeries with the attendant complications.8
In lieu of increasing the numbers of transplanted primordia or transplant surgeries in diabetic rhesus macaques, we embarked on a series of experiments to determine whether porcine islets, a more easily obtainable and possibly more robust source of insulin-producing cells, could be substituted for animals in which embryonic pig pancreas already had engrafted. To this end, we implanted adult porcine islets beneath the capsule of one kidney from STZ-diabetic Lewis rats or macaques that several weeks earlier had been transplanted with E28 pig pancreatic primordia in mesentery.13,16 We employed the renal subcapsular site for islet implantation so that we could differentiate engrafted porcine tissue originating from the islets from tissue originating from prior mesenteric E28 pig pancreatic transplants, that never engraft in host kidney. In this setting, the contralateral (non-transplanted) kidney served as a control as did kidneys from rats or macaques implanted with islets without prior transplantation of E28 pig pancreatic primordia in mesentery. As in experiments demonstrating engraftment of cells originating from E28 pig pancreatic primordia transplanted in mesentery of rats or macaques, we employed multiple techniques to ascertain whether cells from porcine islets engraft in kidney including immune histochemistry for insulin, in situ hybridization specific for porcine proinsulin mRNA, and fluorescent in situ hybridization for pig X chromosomes.
Figure 8 shows sections from a kidney of a STZ-diabetic rat (Fig. Eight A and B) or rhesus macaque (Fig. 8C and D) implanted with porcine islets following transplantation of E28 pig pancreatic primordia in mesentery. Sections are stained using anti-insulin antibodies (Fig. 8A and C) or control serum (Fig. 8B and D). Cells that stain for insulin (Fig. 8A and C), but not with control serum (Fig. 8B and D) are present in an expanded renal subcapsular space. Nuclei of cells in the subcapsular space hybridize to antisense robes for porcine proinsulin mRNA, but not sense probes and cell nuclei hybridize to a probe for the porcine X chromosomes.13,16 Neither cells that stain for insulin nor cells to which a the probe for porcine proinsulin mRNA binds are present in contralateral (non-implanted) kidneys of STZ diabetic rats or macaques in which E28 pig pancreatic primordia were transplanted previously in mesentery, or in kidneys from STZ-diabetic rats or macaques into which porcine islets are implanted without prior transplantation of E28 pig pancreatic primordia in mesentery.
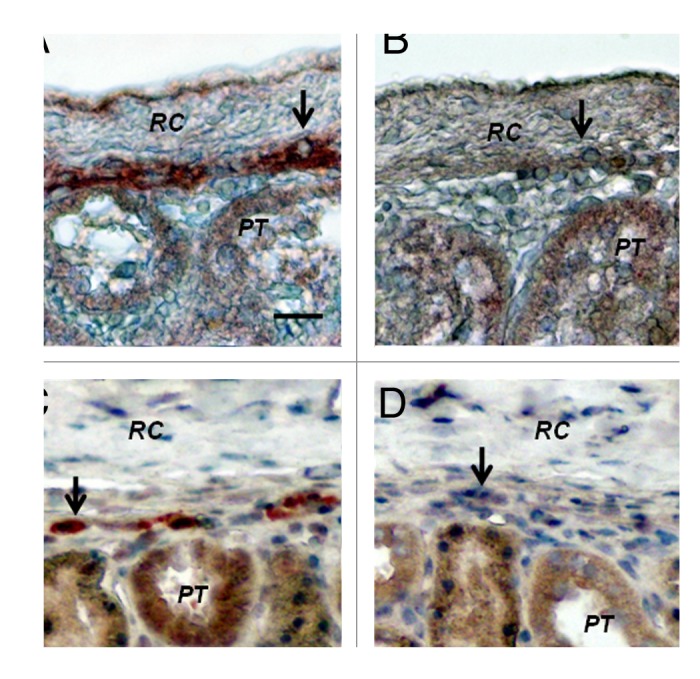
Figure 8. Sections of the islet-implanted kidney from a STZ-diabetic Lewis rat (A and B) or rhesus macaque (C and D) transplanted with E28 pig pancreatic primordia in mesentery followed by porcine islets in the renal subcapsular space stained using anti-insulin antibodies (A and C) or control antiserum (B and D). PT, proximal tubule. RC, renal capsule. Arrows, positively staining cells (A and C); negatively staining cells (B and D). Scale bar 10 um. Reproduced with permission.13,16
Cells from porcine islets do not survive post-implantation in rat or macaque kidneys without prior transplantation of E28 pig pancreatic primordia transplantation in mesentery. Whole porcine islets do not engraft in kidneys. Rather, an endocrine (β cell) component originating from porcine islets does so.13,16 Ours is the first report describing sustained survival of such cells following transplantation of porcine islets in non-immune suppressed primates.16 Glucose tolerance in diabetic rats not fully- normalized by prior transplantation of E28 pig pancreatic primordia in mesentery, is corrected following subsequent implantation of porcine islets in kidney.13 In contrast, correction is not observed following E28 pig pancreatic primordia transplantation and porcine islets implantation in macaques, consistent with previous observations that glucose tolerance is more difficult to correct in macaques than in rats.8 It may be that the mass of engrafted cells originating from porcine islets implanted in kidney (perhaps derived from a numerically small stem cell component within the islets) is insufficient to impact on control of circulating glucose in an animal as large as a macaque.
Perspectives
The studies reviewed above represent one of two independent decade-long efforts (ours based at Washington University6-8 and the other at the Weizmann Institute of Science, Rehovot Israel17,18) to adapt xenotransplantation of embryonic pig pancreas as a novel therapy for diabetes mellitus in humans. Hecht et al.17 (Weizmann Institute) applied findings generated in mice (Tchorsh-Yutsis et al.)18 to a non-human primate model. The mouse studies had demonstrated the curative potential of such implants in diabetic mice under relatively tolerable immune suppression. A working hypothesis, suggesting that embryonic pig pancreas transplantation would not be adversely affected by a humoral response was based on two observations: (1) hyperacute and acute rejection are mainly mediated by complement activation in blood vessels, mediated by preformed anti-pig antibodies recognizing carbohydrates expressed on endothelial cells; and (2) data from the mouse studies showed that the embryonic pancreas implants predominantly induce host-type vasculature to support their growth and development in the recipient, resulting in an organ comprised mainly of porcine epithelial cells and host endothelium. It was found that that porcine E42 embryonic pancreatic tissue can correct hyperglycemia in diabetic Cynomolgous monkeys under a tolerable immune suppression protocol and concluded that long-term proliferative capacity of these grafts, their ability to induce revascularization by host endothelium, and their reduced immunogenicity, suggest that porcine embryonic xenotransplantation could offer an attractive replacement therapy for diabetes.17
Host immune suppression was required for successful engraftment of embryonic pig pancreas in rodents18 or non-human primates17 in the studies of Tchorsh-Yutsis et al. and Hecht et al. It is likely that one or more factors in the methodology we employ different from that used by these investigators17,18 is critical for engraftment without an immune suppression requirement. We have speculated regarding what the factors might be.19 First, the developmental stage of donor pig embryos from which primordia are obtained impacts on the host immune response. We have shown that E35 pig pancreatic primordia are rejected in Lewis rats following transplantation employing conditions under which E28 or E29 pig pancreatic primordia engraft.10 While we have no experience transplanting E42 pig pancreatic primordia in rats, the preferred stage for studies described by Tchorsh-Yutsis et al. and Hecht et al., we would expect them to reject in the absence of host immune-suppression based on our experience with E35 pancreatic primordia. Second, it is likely that that the incubation of embryonic pancreas with one or more growth factors and cytokines we employ prior to transplantation alters the host immune response. Tchorsh-Yutsis et al. Hecht et al. do not employ such agents. Third, the transplantation site and technique probably impact on the host immune response. We interpose pancreatic primordia between sheets of mesentery. Tchorsh-Yutsis et al. and Hecht et al., transplant into pockets of omentum and secure using suture. The omentum ‘pocket’ or ‘pouch’ transplantation site was developed for transplantation of syngeneic islets into rats20 and adapted for transplantation of allogenetic islets into non-human primates21 the omental anatomy of which is comparable to that of humans.22
The potential for use of the mesenteric transplant site to enable dissemination of cells along a lymphatic distribution and engraftment in mesenteric lymph nodes was confirmed recently in fumarylacetoacetate mice (Fah-/-), a model for the human liver disease tyrosinemia type I. Intra peritoneal transplantation of liver cells from congenic wild-type mice resulted in correction of metabolic abnormalities and long-term survival of the animals that otherwise would have died. Transplantation of liver cells into the peritoneal cavity allowed the hepatocytes to migrate into the lymphatic system, enter mesenteric lymph nodes and expand under homeostatic mechanisms driven by the liver. When ectopic liver tissue reached the required balance for hepatic function, proliferation ceased resulting in 20 to 40 “hepatized” mesenteric lymph nodes that represented 70% of the original liver mass. The authors speculate that the highly vascularized nature of the lymph nodes supports the efficient engraftment and massive expansion of ectopic tissue and can be compared with a well-designed in vivo bioreactor.23 Little is known about the factors that regulate the differentiation, migration and immunogenicity of cells transplanted within mesentery. Delineation of such factors could enable optimization of engraftment and expansion of endocrine cells originating from E28 pig pancreatic primordia distributed similarly via the lymphatic route.
Acknowledgments
Supported by George M. O’Brien Center DK079333.
Glossary
Abbreviations:
- E
embryonic day
- ELISA
enzyme-linked immunosorbent assay
- STZ
streptozotocin
- ZDF rats
Zucker Diabetic Fatty rats
Footnotes
Previously published online: www.landesbioscience.com/journals/organogenesis/article/20930
References
- 1.Hering BJ, Walawalkar N. Pig-to-nonhuman primate islet xenotransplantation. Transpl Immunol. 2009;21:81–6. doi: 10.1016/j.trim.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Martin B, Ji S, Maudsley S, Mattson MP. “Control” laboratory rodents are metabolically morbid: why it matters. Proc Natl Acad Sci U S A. 2010;107:6127–33. doi: 10.1073/pnas.0912955107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonow RO, Gheorghiade M. The diabetes epidemic: a national and global crisis. Am J Med. 2004;116(Suppl 5A):2S–10S. doi: 10.1016/j.amjmed.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 4.Bottino R, Trucco M. Multifaceted therapeutic approaches for a multigenic disease. Diabetes. 2005;54(Suppl 2):S79–86. doi: 10.2337/diabetes.54.suppl_2.S79. [DOI] [PubMed] [Google Scholar]
- 5.McCullough KP, Keith DS, Meyer KH, Stock PG, Brayman KL, Leichtman AB. Kidney and pancreas transplantation in the United States, 1998-2007: access for patients with diabetes and end-stage renal disease. Am J Transplant. 2009;9:894–906. doi: 10.1111/j.1600-6143.2009.02566.x. [DOI] [PubMed] [Google Scholar]
- 6.Hammerman MR. Pancreas and kidney transplantation using embryonic donor organs. Organogenesis. 2004;1:3–13. doi: 10.4161/org.1.1.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammerman MR. Organogenesis of kidney and endocrine pancreas: the window opens. Organogenesis. 2007;3:59–66. doi: 10.4161/org.3.2.5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hammerman MR. Organogenetic tolerance. Organogenesis. 2010;6:270–5. doi: 10.4161/org.6.4.13283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rogers SA, Talcott M, Hammerman MR. Transplantation of pig metanephroi. ASAIO J. 2003;49:48–52. doi: 10.1097/00002480-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Rogers SA, Liapis H, Hammerman MR. Normalization of glucose post-transplantation of pig pancreatic anlagen into non-immunosuppressed diabetic rats depends on obtaining anlagen prior to embryonic day 35. Transpl Immunol. 2005;14:67–75. doi: 10.1016/j.trim.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Rogers SA, Chen F, Talcott M, Hammerman MR. Islet cell engraftment and control of diabetes in rats following transplantation of pig pancreatic anlagen. Am J Physiol. 2004;286:E502–9. doi: 10.1152/ajpendo.00445.2003. [DOI] [PubMed] [Google Scholar]
- 12.Rogers SA, Hammerman MR. Normalization of glucose post-transplantation into diabetic rats of pig pancreatic primordia preserved in vitro. Organogenesis. 2008;4:48–51. doi: 10.4161/org.5747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rogers SA, Mohanakumar T, Liapis H, Hammerman MR. Engraftment of cells from porcine islets of Langerhans and normalization of glucose tolerance following transplantation of pig pancreatic primordia in nonimmune-suppressed diabetic rats. Am J Pathol. 2010;177:854–64. doi: 10.2353/ajpath.2010.091193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rogers SA, Chen F, Talcott M, Liapis H, Hammerman MR. Glucose tolerance normalization following transplantation of pig pancreatic primordia into non-immunosuppressed diabetic ZDF rats. Transpl Immunol. 2006;16:176–84. doi: 10.1016/j.trim.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Rogers SA, Chen F, Talcott MR, Faulkner C, Thomas JM, Thevis M, et al. Long-term engraftment following transplantation of pig pancreatic primordia into non-immunosuppressed diabetic rhesus macaques. Xenotransplantation. 2007;14:591–602. doi: 10.1111/j.1399-3089.2007.00429.x. [DOI] [PubMed] [Google Scholar]
- 16.Rogers SA, Tripathi P, Mohanakumar T, Liapis H, Chen F, Talcott MR, et al. Engraftment of cells from porcine islets of Langerhans following transplantation of pig pancreatic primordia in non-immunosuppressed diabetic rhesus macaques. Organogenesis. 2011;7:154–62. doi: 10.4161/org.7.3.16522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hecht G, Eventov-Friedman S, Rosen C, Shezen E, Tchorsh D, Aronovich A, et al. Embryonic pig pancreatic tissue for the treatment of diabetes in a nonhuman primate model. Proc Natl Acad Sci U S A. 2009;106:8659–64. doi: 10.1073/pnas.0812253106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tchorsh-Yutsis D, Hecht G, Aronovich A, Shezen E, Klionsky Y, Rosen C, et al. Pig embryonic pancreatic tissue as a source for transplantation in diabetes: transient treatment with anti-LFA1, anti-CD48, and FTY720 enables long-term graft maintenance in mice with only mild ongoing immunosuppression. Diabetes. 2009;58:1585–94. doi: 10.2337/db09-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammerman MR. Engraftment of insulin-producing cells from porcine islets in non-immune suppressed rats or non-human primates transplanted previously with embryonic pig pancreas. J Transplantation. 2011;2011:261352. doi: 10.1155/2011/261352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kin T, Korbutt GS, Rajotte RV. Survival and metabolic function of syngeneic rat islet grafts transplanted in the omental pouch. Am J Transplant. 2003;3:281–5. doi: 10.1034/j.1600-6143.2003.00049.x. [DOI] [PubMed] [Google Scholar]
- 21.Kenyon NS, Berman DM, O’Neil JJ, Kenyon M, Vasconcellos AV, Curry W, et al. Survival and function of allogeneic islets implanted in an omental pouch in cynomolgus monkeys (Macaca fascicularis) Xenotransplantation. 2007;14:406. [Google Scholar]
- 22.Chaffanjon PC, Kenyon NM, Ricordi C, Kenyon NS. Omental anatomy of non-human primates. Surg Radiol Anat. 2005;27:287–91. doi: 10.1007/s00276-005-0329-4. [DOI] [PubMed] [Google Scholar]
- 23.Hoppo T, Komori J, Manohar R, Stolz DB, Lagasse E. Rescue of lethal hepatic failure by hepatized lymph nodes in mice. Gastroenterology. 2011;140:656–66, e2. doi: 10.1053/j.gastro.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]