Abstract
Introduction
To address transplant organ shortage, a promising strategy is to decellularize kidneys in a manner that the scaffold retains signals for seeded pluripotent precursor cells to differentiate and recapitulate native structures: matrix-to-cell signaling followed by cell-cell and cell-matrix interactions, thereby remodeling and replacing the original matrix. This would reduce scaffold antigenicity and enable xeno-allografts.
Results
DAPI-labeled cells in arterial vessels and glomeruli were positive for both endothelial lineage markers, BsLB4 and VEGFR2. Rat scaffold’s basement membrane demonstrated immunolabeling with anti-mouse laminin β1. Labeling intensified over time with 14 day incubations.
Conclusion
We provide new evidence for matrix-to-cell signaling in acellular whole organ scaffolds that induces differentiation of pluripotent precursor cells to endothelial lineage. Production of mouse basement membrane supports remodeling of host (rat)-derived scaffolds and thereby warrants further investigation as a promising approach for xenotransplantation.
Methods
We previously showed that murine embryonic stem cells arterially seeded into acellular rat whole kidney scaffolds multiply and demonstrate morphologic, immunohistochemical and gene expression evidence for differentiation. Vascular cell endothelialization was now further tested by endothelial specific BsLB4 lectin and anti-VEGFR2 (Flk1) antibodies. Remodeling of the matrix basement membranes from rat to mouse (“murinization”) was assessed by a monoclonal antibody specific for mouse laminin β1 chain.
Keywords: scaffolds, embryonic stem cells, ontogeny, glomerulogenesis, collagen IV
Introduction
The shortage of organs for transplantation has led to expanding interest in the use of tissue regeneration technology. Rather than de novo organ growth,1-3 there has been recent successful repopulation of acellular scaffolds derived from a variety of non-renal tissues.4-8 At issue, however, has been how to seed decellularized complex organs with mature or precursor cells so as to reproduce intricate architectures: multiple cell types properly configured and with appropriate cell-to-cell alignment. We have hypothesized that properly prepared scaffolds retain extracellular matrix constituents that can signal pluripotent precursor cells for appropriate site-specific differentiation. A key concept is that the maturing cells would then produce their own matrices and signals that would induce normal ontogeny. Thus, matrix-to-cell would be followed by cell-to-cell, then cell-to-matrix signaling resulting in a cycle of differentiation and tissue maturation. We have predicted that this remodeling of the scaffold matrix would have a secondary advantage of attenuating its original immunogenicity. We believe this would be advantageous for the purposes of transplantation in that rejection could be minimized when donor scaffold antigens are replaced by precursor cells derived from the patient in need of the allograft. The remodeling of the acellular scaffold and resulting attenuation of donor tissue antigenicity may thereby be particularly beneficial for xenotransplantation.
We have previously established9 that whole rat kidney organs can be decellularized intact using a sequential protocol involving ionic and nonionic detergents, mechanical, osmotic and enzymatic steps. Pluripotent murine embryonic stem cells (ESCs) were seeded arterially or retrograde (via ureters) and were shown to adhere and multiply within the scaffold. We also have had preliminary evidence that pumped pulsatile perfusion systems facilitate the growth and, we believe, provide a mechanical shear stress signal that is important for proper differentiation. ESCs infused into the renal artery reached and then filled the small vessels, arterioles and glomeruli. This was followed by apoptosis of cells in the core of the vessels, resulting in lumen formation. The cells that remained were those that lined the scaffold basement membranes and assumed an endothelial appearance. Much of our earlier work focused on demonstrating that the ESCs not only multiplied but also appropriately differentiated.10-12 Our previous findings included: (1) gross histologic evidence for endothelialization, (2) immunohistochemical staining for PAX2, cytokeratins and Ksp cadherin and (3) genetic evidence by PCR for expression of PAX2 and Ksp cadherin.
In this study we sought to provide further evidence that vascularly seeded pluripotent mouse ESCs were (1) differentiating into endothelial cells and (2) producing their own basement membrane matrix that remodeled the mature rat kidney scaffold. Thus, we anticipated that endothelialization of mouse pluripotent precursor cells would be associated with “murinization” of what began as a rat scaffold. Specifically, we tested for the appearance of immunohistochemical markers of endothelial differentiation and evidence for rat basement membrane transformation by incorporation of mouse laminin and type IV collagen.
Results
Similar to our previously reported findings (Fig. 1),9 the decellularization protocol yielded an intact scaffold architecture for the blood vessels, glomeruli and tubules by light microscopy. This was consistent with arterially seeded cells staying within the confines of the afferent vasculature and essentially all glomeruli in the renal cortex without extravasation into the interstitium or Bowman’s space. As previously described, with progressive incubations these cells populated the vascular structures, extending from the afferent arterioles and glomerular tufts to the efferent arterioles and peritubular capillary networks. Once the adequacy of this seeding was verified by light microscopy, we proceeded with the specialized studies characterizing the cells and scaffold as described below.
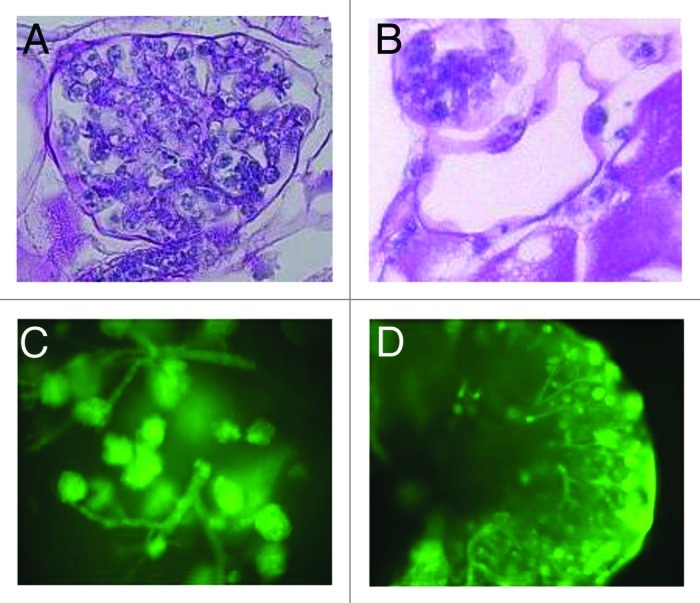
Figure 1. (A and B) Distinct morphologies of ES cells delivered into the renal artery of decellularized kidney scaffolds after 10 d of whole organ culture (PAS stain) with (A) cells filling the glomerular capillary tufts (400x) and (B) flat-appearing cells lining vascular structures (200x). (C and D) Distribution of green fluorescent protein (GFP)-labeled murine ES cells after thick section culture showing cell growth and migration patterns into a vascular tree (C) at day 4 (100x) and formation of complex branching networks (D) (40x) at day 6. Reproduced with permission from reference 9.
Characterization of cells
Cells in the rat scaffold’s glomeruli and blood vessels showed lectin-specific (BsLB4) staining for murine endothelial cells (Fig. 2) and the presence of VEGFR2 (Fig. 3). These findings were increasingly intense for incubations from approximately 4 to 14 d long (Fig. 3A and B). Notably, the presence of these endothelial-specific differentiation markers were found exclusively within the vasculature and glomeruli and areas corresponding to tubular basement membranes were negative (Fig. 3). Unstained parts of the sections (Fig. 2) confirm the murine specificity of the immunolabeling.
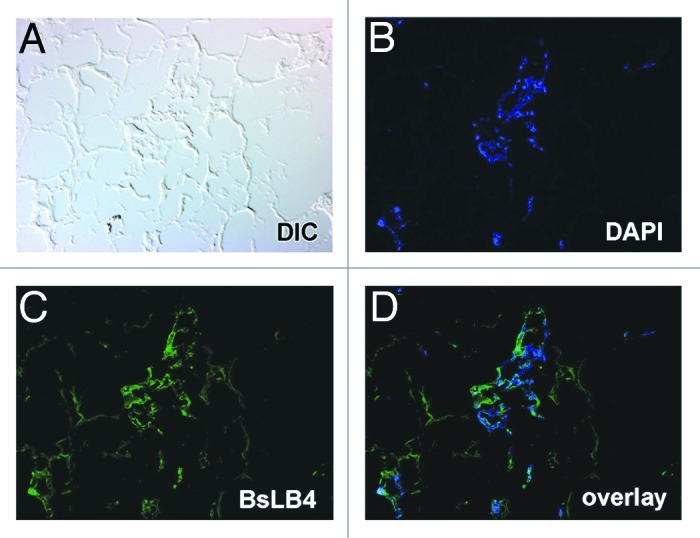
Figure 2. Section of decellularized rat kidney scaffold seeded with ESCs. (A) A differential interference contrast microscopy image of a glomerular region of the scaffold. (B) Incubated with the nuclear label DAPI (blue), showing a glomerular structure and several vascular profiles. Confirmation of expression of vascular endothelial markers is shown in (C), in which the endothelial-specific lectin, BsLB4 (green), binds to many of the same cells marked by DAPI [see merged image in (D)].
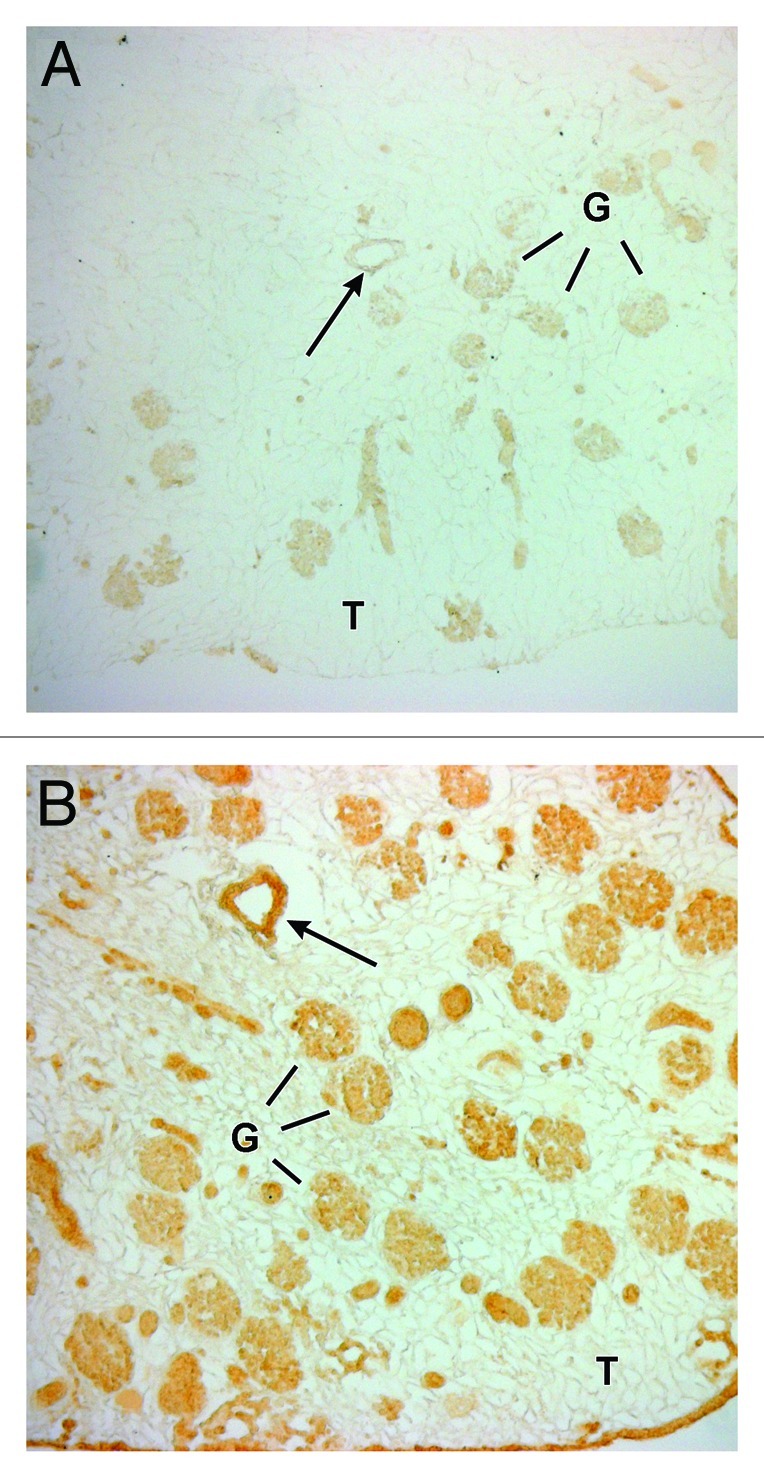
Figure 3. Decellularized rat kidney scaffolds after 4 d (A) and 14 d (B) after seeding with mouse ESCs, immunolabeled with the endothelial specific, anti-VEGFR2 IgG, and processed for horseradish peroxidase immunohistochemistry. There were progressively increased levels of anti-VEGFR2 labeling at the longer incubation time-point (micrographs were taken using identical image capture settings). Intense peroxidase reaction product can be seen along the intimal surface of a blood vessel (arrows) and variable labeling among several glomerular structures (G). Tubules (T) are negative.
Characterization of the scaffold
With regard to the remodeling of the rat scaffold, there was progressively intense labeling from day 4 to day 14 with the murine-specific monoclonal antibody to the laminin β1 chain (Fig. 4, mAb 5A2) in patterns that appeared identical to that seen for the endothelial differentiation markers, BsLB4 and VEGFR2. Similarly, the rat kidney scaffolds tested positive for the presence of mouse α1α2α1 collagen IV (Fig. 5). Consistent with our hypothesis of a recapitulation of normal ontogeny, these mouse basement membrane proteins co-localized with the seeded cells, and were not present in acellular areas of the mature rat scaffold.
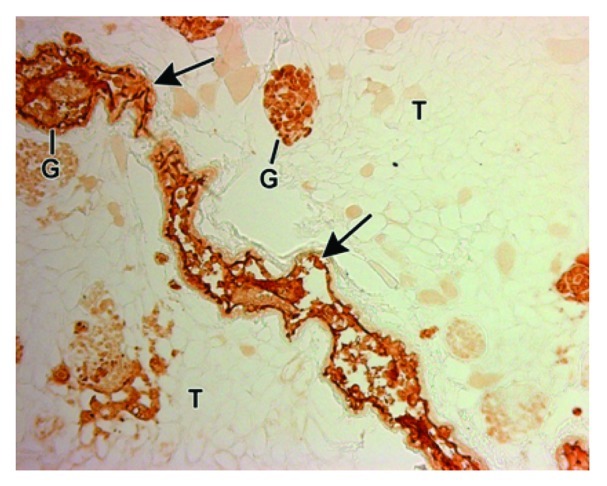
Figure 4. Decellularized rat kidney scaffold seeded with mouse ESCs, immunolabeled with the mouse specific, anti-laminin β1 IgG, and processed for horseradish peroxidase immunohistochemistry. Mouse laminin immunoreactivity can be seen in the arteriole (arrow) and glomeruli (G). Tubules (T) are negative.
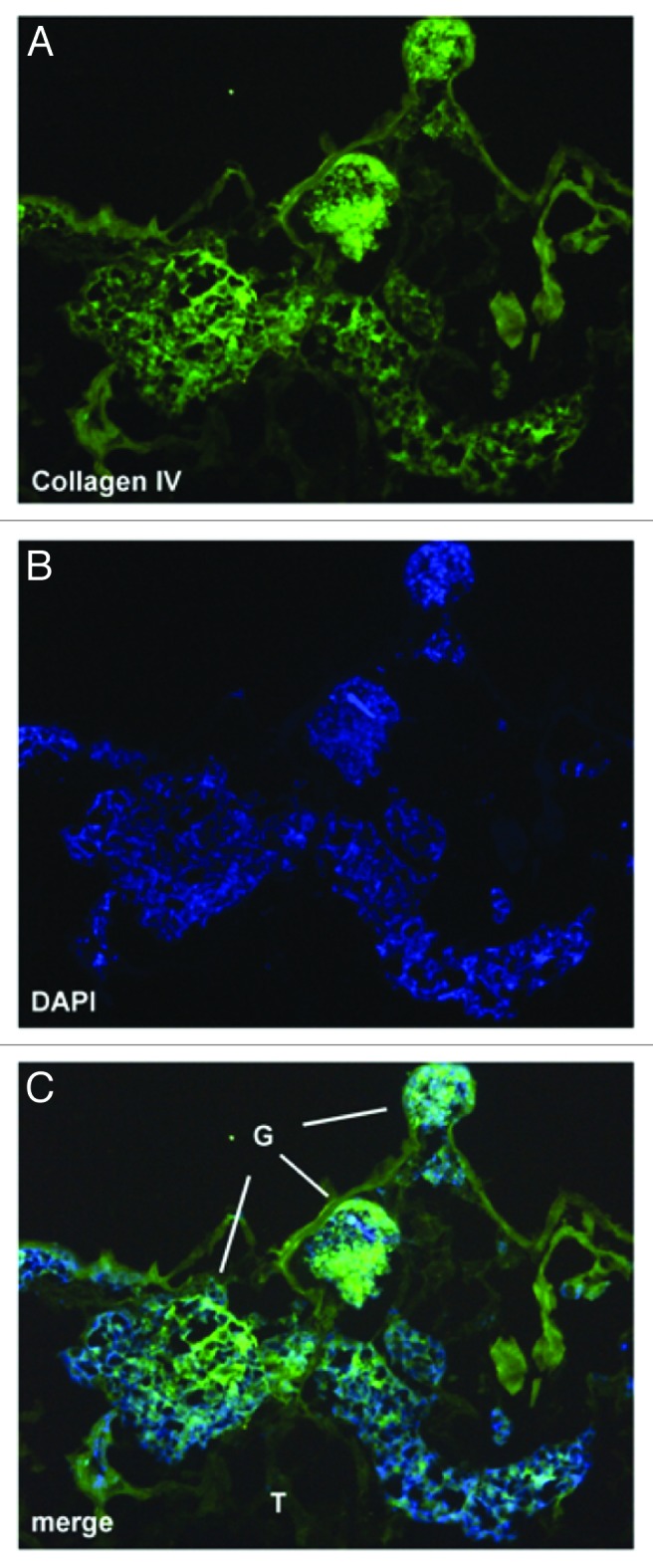
Figure 5. ESC-seeded scaffold immunolabeled with anti-collagen α1α2α1 (IV) (A, green) and DAPI (B, blue). Merged image (C) shows direct correspondence between the seeded cells with basement membrane, type IV collagen.
Discussion
Use of acellular scaffolds has shown great recent promise for tissue regeneration and has been highlighted for cardiac, vascular, urologic, esophageal and airway tissues.4-8 We have had concerns, however, that simple applications of lineage-specific (i.e., endothelial or chondrocyte) cells would not be adequate for complex organs. Kidney generation, for example, would require multiple types of cells in intricate three dimensional heterogeneous arrangements. We believe it unlikely that there could be practical engineering methods that would mechanically place so many different types in a spatially appropriate architecture. Instead we have proposed that an appropriately prepared matrix13,14 will retain the signals to trigger site-specific differentiation of pluripotent precursor cells, and thereby launch a cascade of matrix-cell, cell-cell15 and cell-matrix events culminating in cycles of organ differentiation and remodeling of the original scaffold. The concept of matrix-induced cellular differentiation is not new,16-21 and extends back to pioneering work by MJ Bissell.22 In addition, and integral to our hypothesis, is that the ontogeny of the differentiation of the seeded precursor cells will be embryologically correct, as would be the subsequent changes to matrix and basement membranes they produce.
Our study involving the use of matrix signaling has been facilitated and supported by evidence from investigations over the last two decades into kidney organogenesis,23-26 and in particular the embryology of renal basement membrane formation.27-29 The contribution of each of three major cell types (mesangial, endothelial and podocytes) to basement membrane laminin30,31 and collagen has in large part been elucidated. In particular, it has become clear that there is a well-defined temporal sequence of collagen IV and laminin isoforms and that the sequential replacement of immature proteins is required for the maturation of normal adult glomeruli.32 Early in development, glomerular basement membranes form from a fusion of a layer of immature collagen IV α1α2α1 produced by the endothelial cells with another from podocytes.25,33-36 This is subsequently replaced by the more proteolysis-resistant collagen IV α3α4α5, which is thought to be derived from the podocytes, as endothelial cells do not synthesize α3. Basement membranes in the other compartments of mature glomeruli are quite different: the mesangium having collagen IV α1α2α1 and Bowman’s capsule both collagens IV α1α2α1 and α5α6α5. Laminin undergoes analogous changes with maturation, transitioning from α1β1γ1 to α5β2γ1.30,37,38 There is now compelling evidence that the lack of (or mutated) matrix constituents, such as laminin α5,31 can be associated with abnormal cell differentiation; again supporting the concept of the importance of matrix-to-cell signaling. For example, polymerized laminin not only has an essential role in membrane structural integrity, but in models where laminin α5 is knocked out there was disarray of endothelial and epithelial cells that led to failed glomerulogenesis.31 Thus, the ontogeny is so complex and so dependent on the appropriate spatial orientation of multiple maturing cell types that it would be difficult to craft a methodology that depends on the insertion of the whole spectrum of cells into their correct positions in a scaffold. Hence we have chosen to use immature cells in this model, wherein the matrix guides in situ differentiation. In addition we believe this approach provides an opportunity to study ontogenesis limited to single cell lines (i.e., just the endothelial cell precursors) vs. that occurring with the more complex multi-cell line seeding (i.e., retrograde methods that could simultaneously introduce podocyte precursors). It has also not been fully elucidated how growth factors interact with this wide array of maturing cells and their associated basement membrane chemical signals. Lastly, there may be important non-chemical signals for differentiation. Our pumped perfusion apparatus methodology would not only permit the infusion of growth factors (i.e., VEGF, activin A, retinoic acid and Bmp7),39 but also allow the study of signaling from mechanical shear stress induced by pulsatile flow.40
In the present investigation we have extended our prior studies and provide further evidence of pluripotent precursor cell differentiation into endothelial cells in the scaffold’s glomeruli and vasculature. In addition to our previous gene activation, light and immunohistochemical findings, we now demonstrate glomerular labeling for murine endothelial-specific lectins and also for VEGFR2 (Flk1). The latter is a receptor now established to be integral to normal endothelial and vascular development. It identifies vascular progenitor cells that can further differentiate into endothelial and mural cells, and are known to be responsive to fluid shear stress. Specifically, signaling via the VEGFR2 (Flk1) receptor appears to be needed for migration and differentiation of endothelial lineage precursor cells and for the formation of glomerular vasculature;27,41-45 hence our interest in testing for the expression of these receptors by the cells growing in our model. Demonstration of lectin (BsLB4) positivity confirms again that the ESCs have indeed differentiated into endothelial lineage. Consistent with the hypothesis that there is remodeling of the rat scaffold, these lectin-characterized endothelial cells co-localized with the basement membrane changes, namely progressive labeling for murine-specific laminin. It was critically important to demonstrate this cross-species protein binding, i.e., progressive “murinization” of the rat scaffold, as the ultimate goal is xenotransplantation. Indeed, the ability of murine laminins to bind to rat basement membranes is supported by the literature: injection of intact mouse laminin (but not proteolytic fragments) binds to the mesangial matrices and glomerular basement membranes of newborn rats.46 Whether there would be a progressive decrease and ultimate replacement of the scaffold’s rat matrix (and associated antigenicity) will require longer incubations and were beyond the scope of these short-term experiments. Lastly, in support of our inducing normal embryologic development, the ontogeny results show that the endothelially-differentiated cells produce the immature isoforms of collagen IV, α1 and α2. This is also consistent with our demonstration of the immature laminin α1 and β1 chain isoforms; however, changes to the laminin isoforms would need to be interpreted with caution since these transitions can depend on the in vitro conditions.47
In conclusion, to succeed in regenerating kidneys using acellular scaffolds it is imperative that seeded cells not only adhere and multiply but also differentiate in a site-specific and embryologically sequenced manner so as to produce the spatially complex array of matrix constituents and multiple cell types. We have now provided further evidence that pluripotent precursor cells seeded into a decellularized scaffold vasculature differentiate into endothelial cells, which in turn follow a normal ontogeny as they remodel the laminin and collagen of their basement membranes. Methodologies that involve the differentiation of patient-derived precursor cells and subsequent remodeling of the donor scaffold hold high promise for whole organ xenotransplantation.
Materials and Methods
Whole kidney scaffold preparation
As previously described,9,48,49 rat kidneys were rapidly harvested and decellularized over five days using multiple sequential solutions that included Triton X-100 and SDS detergents, DNase, and deionized water rinses. All animal procedures were approved by the Institutional Animal Care and Use Committee (University of Florida) and involved 250–350 g male Sprague-Dawley rats. Briefly, during systemic heparin anticoagulation, kidneys were perfused in situ with a saline solution containing a vasodilator (nitroprusside) in order to remove the blood. Arterial and ureteral cannulae were placed. Once harvested, the kidneys underwent continuous gravity-based perfusion at a constant physiologic fluid pressure of approximately 100 mmHg. The decellularization protocol used is the following: (1) nonionic (Triton X-100, Sigma-Aldrich) and ionic (sodium dodecyl sulfate, SDS, Sigma) detergents to solubilize and disrupt cell membranes, thereby lysing cells; (2) a solution of 5 mM calcium chloride and 5 mM magnesium sulfate to activate endogenous nucleases and digest bulky nuclear material; (3) 0.0025% deoxyribonuclease 1 (DNase, Sigma) to enzymatically degrade DNA and (4) 1M sodium chloride and deionized water to osmotically disrupt cells, with the latter also used to rinse the preparation.
Embryonic stem cell seeding and tissue culture
Using the methodology previously detailed, approximately 2 × 106 murine ESCs (B5/EGFP, courtesy of Andras Nagy)50 were manually injected via the arterial cannula into the whole acellular scaffolds. The incubation media consisted of Iscove's modified Dulbecco's medium (IMDM) supplemented with 20% fetal bovine serum (Atlanta Biologicals), 2 mM L-glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin (GIBCO BRL), 0.25 μg/ml Fungizone® antimycotic (Invitrogen) and 300 μM monothioglycerol. Importantly, no growth factors were added to the media as we chose to explore only the effects from potential decellularized tissue signals. There were two incubation methods: one protocol involved transverse sectioning of the pre-seeded organ and then culturing the slices in a multi-well dish for up to 10 d at 37°C in 5% CO2. The other method kept the kidney scaffolds intact by use of an automated whole-organ perfusion system. A peristaltic pump was chosen to deliver a physiologically normal pressure profile for up to 14 d. The resultant waveform was continuously transduced and a pressure relief mechanism was incorporated so as to maintain approximately 120/80 mmHg with a periodicity of 270 to 300 beats/min. The perfusion was accomplished in a temperature-controlled incubator supplied by regulated medical-grade sterile gasses, and the CO2 content was adjusted as necessary to keep the media pH at 7.4.
Immunofluorescence microscopy and immunohistochemistry
Tissue with and without seeded ESCs was prepared and stored frozen, or fixed and embedded in paraffin blocks. Labeling experiments were performed in (at least) triplicate and summarized as follows. Cryostat sections of ESC-seeded scaffolds, 6 µm thick, were incubated with Bandeiraea simplicifolia lectin B4 (BsLB4) conjugated to fluoroscein isothicyanate (FITC) (Sigma Chemical Co.) diluted 1:50 in phosphate buffered saline (PBS). Sections were washed with PBS and coverslipped with Molecular Probes Prolong Gold antifade reagent containing DAPI (Life Technologies). Frozen sections from non-seeded scaffolds served as controls. In other cases, frozen sections were incubated with goat anti-collagen α1α2α1 (IV) (Southern Biotech) followed with chicken anti-goat IgG-AlexaFluor-488 (Molecular Probes), before coverslipping with the antifade reagent containing DAPI.
Paraffin sections from scaffolds seeded with ESCs for 4, 6, 10 and 14 d, and those that were not seeded with cells, underwent the Vector antigen retrieval method in a microwave oven following the manufacturer’s protocol (Vector Laboratories). They were then immunolabeled for 1 h with 50 µg/ml anti-laminin β1 chain monoclonal IgG 5A2,37 washed in PBS, and then treated with 50 µg/ml goat anti-rat IgG-HRP (ICN/Capple) diluted in Vector enhancer reagent. Slides were washed and incubated with Vector ImmPACT DAB color development reagent for 10 min. In other cases, paraffin sections underwent the EMS Retriever 2100 antigen unmasking method using a pressure cooker, following the manufacturer’s protocol (Electron Microscopy Sciences). Sections were then incubated for 1 h with 50 µg/ml monoclonal anti-laminin β1 IgG 5A2 or 50 µg/ml rat anti-mouse Flk1 IgG (VEGFR2) (BD PharMingen/BD Biosciences) in Vector Enhancer solution. After washing, slides were treated with goat anti-rat IgG-HRP in Vector enhancer solution for 1 h, washed, and developed with Vector ImmPACT DAB.
Acknowledgments
This work was supported, in part, by NIH grant P01 DK065123.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/organogenesis/article/20209
References
- 1.Little MH. Regrow or repair: potential regenerative therapies for the kidney. J Am Soc Nephrol. 2006;17:2390–401. doi: 10.1681/ASN.2006030218. [DOI] [PubMed] [Google Scholar]
- 2.Joraku A, Stern KA, Atala A, Yoo JJ. In vitro generation of three-dimensional renal structures. Methods. 2009;47:129–33. doi: 10.1016/j.ymeth.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Yokoo T, Matsumoto K, Yokote S.Potential use of stem cells for kidney regeneration. Int J Nephrol 2011; 2011:591731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atala A. Recent applications of regenerative medicine to urologic structures and related tissues. Curr Opin Urol. 2006;16:305–9. doi: 10.1097/01.mou.0000232055.20084.f6. [DOI] [PubMed] [Google Scholar]
- 5.Bhrany AD, Beckstead BL, Lang TC, Farwell DG, Giachelli CM, Ratner BD. Development of an esophagus acellular matrix tissue scaffold. Tissue Eng. 2006;12:319–30. doi: 10.1089/ten.2006.12.319. [DOI] [PubMed] [Google Scholar]
- 6.Matsunuma H, Kagami H, Narita Y, Hata K, Ono Y, Ohshima S, et al. Constructing a tissue-engineered ureter using a decellularized matrix with cultured uroepithelial cells and bone marrow-derived mononuclear cells. Tissue Eng. 2006;12:509–18. doi: 10.1089/ten.2006.12.509. [DOI] [PubMed] [Google Scholar]
- 7.Narita Y, Kagami H, Matsunuma H, Murase Y, Ueda M, Ueda Y. Decellularized ureter for tissue-engineered small-caliber vascular graft. J Artif Organs. 2008;11:91–9. doi: 10.1007/s10047-008-0407-6. [DOI] [PubMed] [Google Scholar]
- 8.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14:213–21. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 9.Ross EA, Williams MJ, Hamazaki T, Terada N, Clapp WL, Adin C, et al. Embryonic stem cells proliferate and differentiate when seeded into kidney scaffolds. J Am Soc Nephrol. 2009;20:2338–47. doi: 10.1681/ASN.2008111196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rothenpieler UW, Dressler GR. Pax-2 is required for mesenchyme-to-epithelium conversion during kidney development. Development. 1993;119:711–20. doi: 10.1242/dev.119.3.711. [DOI] [PubMed] [Google Scholar]
- 11.Shao X, Johnson JE, Richardson JA, Hiesberger T, Igarashi P. A minimal Ksp-cadherin promoter linked to a green fluorescent protein reporter gene exhibits tissue-specific expression in the developing kidney and genitourinary tract. J Am Soc Nephrol. 2002;13:1824–36. doi: 10.1097/01.ASN.0000016443.50138.CD. [DOI] [PubMed] [Google Scholar]
- 12.Narlis M, Grote D, Gaitan Y, Boualia SK, Bouchard M. Pax2 and pax8 regulate branching morphogenesis and nephron differentiation in the developing kidney. J Am Soc Nephrol. 2007;18:1121–9. doi: 10.1681/ASN.2006070739. [DOI] [PubMed] [Google Scholar]
- 13.Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233–43. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lichtenberg A, Tudorache I, Cebotari S, Ringes-Lichtenberg S, Sturz G, Hoeffler K, et al. In vitro re-endothelialization of detergent decellularized heart valves under simulated physiological dynamic conditions. Biomaterials. 2006;27:4221–9. doi: 10.1016/j.biomaterials.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 15.Chen SS, Fitzgerald W, Zimmerberg J, Kleinman HK, Margolis L. Cell-cell and cell-extracellular matrix interactions regulate embryonic stem cell differentiation. Stem Cells. 2007;25:553–61. doi: 10.1634/stemcells.2006-0419. [DOI] [PubMed] [Google Scholar]
- 16.Czyz J, Wobus A. Embryonic stem cell differentiation: the role of extracellular factors. Differentiation. 2001;68:167–74. doi: 10.1046/j.1432-0436.2001.680404.x. [DOI] [PubMed] [Google Scholar]
- 17.Lelongt B, Ronco P. Role of extracellular matrix in kidney development and repair. Pediatr Nephrol. 2003;18:731–42. doi: 10.1007/s00467-003-1153-x. [DOI] [PubMed] [Google Scholar]
- 18.Philp D, Chen SS, Fitzgerald W, Orenstein J, Margolis L, Kleinman HK. Complex extracellular matrices promote tissue-specific stem cell differentiation. Stem Cells. 2005;23:288–96. doi: 10.1634/stemcells.2002-0109. [DOI] [PubMed] [Google Scholar]
- 19.Badylak SF. Regenerative medicine and developmental biology: the role of the extracellular matrix. Anat Rec B New Anat. 2005;287:36–41. doi: 10.1002/ar.b.20081. [DOI] [PubMed] [Google Scholar]
- 20.Baharvand H, Azarnia M, Parivar K, Ashtiani SK. The effect of extracellular matrix on embryonic stem cell-derived cardiomyocytes. J Mol Cell Cardiol. 2005;38:495–503. doi: 10.1016/j.yjmcc.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 21.Battista S, Guarnieri D, Borselli C, Zeppetelli S, Borzacchiello A, Mayol L, et al. The effect of matrix composition of 3D constructs on embryonic stem cell differentiation. Biomaterials. 2005;26:6194–207. doi: 10.1016/j.biomaterials.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Boudreau N, Sympson CJ, Werb Z, Bissell MJ. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science. 1995;267:891–3. doi: 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sorokin L, Ekblom P. Development of tubular and glomerular cells of the kidney. Kidney Int. 1992;41:657–64. doi: 10.1038/ki.1992.101. [DOI] [PubMed] [Google Scholar]
- 24.Oliver J, Al-Awqati Q. Development of vascular elements during renal organogenesis. Kidney Int. 2000;57:2167–8. doi: 10.1046/j.1523-1755.2000.00071.x. [DOI] [PubMed] [Google Scholar]
- 25.Desjardins M, Bendayan M. Ontogenesis of glomerular basement membrane: structural and functional properties. J Cell Biol. 1991;113:689–700. doi: 10.1083/jcb.113.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robert B, Abrahamson DR. Control of glomerular capillary development by growth factor/receptor kinases. Pediatr Nephrol. 2001;16:294–301. doi: 10.1007/s004670000534. [DOI] [PubMed] [Google Scholar]
- 27.Abrahamson DR, Leardkamolkarn V. Development of kidney tubular basement membranes. Kidney Int. 1991;39:382–93. doi: 10.1038/ki.1991.50. [DOI] [PubMed] [Google Scholar]
- 28.Abrahamson DR. Development of kidney glomerular endothelial cells and their role in basement membrane assembly. Organogenesis. 2009;5:275–87. doi: 10.4161/org.7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Couchman JR, Abrahamson DR, McCarthy KJ. Basement membrane proteoglycans and development. Kidney Int. 1993;43:79–84. doi: 10.1038/ki.1993.14. [DOI] [PubMed] [Google Scholar]
- 30.Tunggal P, Smyth N, Paulsson M, Ott MC. Laminins: structure and genetic regulation. Microsc Res Tech. 2000;51:214–27. doi: 10.1002/1097-0029(20001101)51:3<214::AID-JEMT2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 31.Miner JH, Li C. Defective glomerulogenesis in the absence of laminin alpha5 demonstrates a developmental role for the kidney glomerular basement membrane. Dev Biol. 2000;217:278–89. doi: 10.1006/dbio.1999.9546. [DOI] [PubMed] [Google Scholar]
- 32.Miner JH. Organogenesis of the kidney glomerulus: focus on the glomerular basement membrane. Organogenesis. 2011;7:75–82. doi: 10.4161/org.7.2.15275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abrahamson DR, Hudson BG, Stroganova L, Borza DB, St John PL. Cellular origins of type IV collagen networks in developing glomeruli. J Am Soc Nephrol. 2009;20:1471–9. doi: 10.1681/ASN.2008101086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.St John PL, Abrahamson DR. Glomerular endothelial cells and podocytes jointly synthesize laminin-1 and -11 chains. Kidney Int. 2001;60:1037–46. doi: 10.1046/j.1523-1755.2001.0600031037.x. [DOI] [PubMed] [Google Scholar]
- 35.Daniel TO, Abrahamson D. Endothelial signal integration in vascular assembly. Annu Rev Physiol. 2000;62:649–71. doi: 10.1146/annurev.physiol.62.1.649. [DOI] [PubMed] [Google Scholar]
- 36.Khoshnoodi J, Pedchenko V, Hudson BG. Mammalian collagen IV. Microsc Res Tech. 2008;71:357–70. doi: 10.1002/jemt.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abrahamson DR, Irwin MH, St John PL, Perry EW, Accavitti MA, Heck LW, et al. Selective immunoreactivities of kidney basement membranes to monoclonal antibodies against laminin: localization of the end of the long arm and the short arms to discrete microdomains. J Cell Biol. 1989;109:3477–91. doi: 10.1083/jcb.109.6.3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miner JH, Sanes JR. Collagen IV alpha 3, alpha 4, and alpha 5 chains in rodent basal laminae: sequence, distribution, association with laminins, and developmental switches. J Cell Biol. 1994;127:879–91. doi: 10.1083/jcb.127.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim D, Dressler GR. Nephrogenic factors promote differentiation of mouse embryonic stem cells into renal epithelia. J Am Soc Nephrol. 2005;16:3527–34. doi: 10.1681/ASN.2005050544. [DOI] [PubMed] [Google Scholar]
- 40.Liu H, Lin J, Roy K. Effect of 3D scaffold and dynamic culture condition on the global gene expression profile of mouse embryonic stem cells. Biomaterials. 2006;27:5978–89. doi: 10.1016/j.biomaterials.2006.05.053. [DOI] [PubMed] [Google Scholar]
- 41.Haigh JJ, Gerber HP, Ferrara N, Wagner EF. Conditional inactivation of VEGF-A in areas of collagen2a1 expression results in embryonic lethality in the heterozygous state. Development. 2000;127:1445–53. doi: 10.1242/dev.127.7.1445. [DOI] [PubMed] [Google Scholar]
- 42.Haigh JJ. Role of VEGF in organogenesis. Organogenesis. 2008;4:247–56. doi: 10.4161/org.4.4.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eremina V, Quaggin SE. The role of VEGF-A in glomerular development and function. Curr Opin Nephrol Hypertens. 2004;13:9–15. doi: 10.1097/00041552-200401000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Eremina V, Baelde HJ, Quaggin SE. Role of the VEGF–a signaling pathway in the glomerulus: evidence for crosstalk between components of the glomerular filtration barrier. Nephron, Physiol. 2007;106:32–7. doi: 10.1159/000101798. [DOI] [PubMed] [Google Scholar]
- 45.Hirashima M. Regulation of endothelial cell differentiation and arterial specification by VEGF and Notch signaling. Anat Sci Int. 2009;84:95–101. doi: 10.1007/s12565-009-0026-1. [DOI] [PubMed] [Google Scholar]
- 46.Wang R, Moorer-Hickman D, St John PL, Abrahamson DR. Binding of injected laminin to developing kidney glomerular mesangial matrices and basement membranes in vivo. J Histochem Cytochem. 1998;46:291–300. doi: 10.1177/002215549804600302. [DOI] [PubMed] [Google Scholar]
- 47.St John PL, Wang R, Yin Y, Miner JH, Robert B, Abrahamson DR. Glomerular laminin isoform transitions: errors in metanephric culture are corrected by grafting. Am J Physiol Renal Physiol. 2001;280:F695–705. doi: 10.1152/ajprenal.2001.280.4.F695. [DOI] [PubMed] [Google Scholar]
- 48.Brendel K, Hjelle J, Meezan E. Proximal Renal Tubule Preparations Isolated from Rat or Rabbit Kidneys without the Use of Collagenase. Toxicology Methods. 1993;3:87–99. [Google Scholar]
- 49.Meezan E, Brendel K, Nagle R. Acellular Perfused Kidney—Model For Basement-Membrane Permeability. Federation Proceedings . 1977;36:521. [Google Scholar]
- 50.Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci U S A. 1993;90:8424–8. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]


