Abstract
Branching morphogenesis is a fundamental developmental process which results in amplification of epithelial surface area for exchanging molecules in organs including the lung, kidney, mammary gland and salivary gland. These complex tree-like structures are built by iterative rounds of simple routines of epithelial morphogenesis, including bud formation, extension, and bifurcation, that require constant remodeling of the extracellular matrix (ECM) and the cytoskeleton. In this review, we highlight the current understanding of the role of the ECM and cytoskeletal dynamics in branching morphogenesis across these different organs. The cellular and molecular mechanisms shared during this morphogenetic process provide insight into the development of other branching organs.
Keywords: morphodynamics, contractility, patterning, tension, actin, actomyosin
Introduction
Branching morphogenesis is a key developmental process that maximizes the surface area for efficient gas exchange or secretion of fluids across an epithelium that forms the functional architecture of organs such as the lung, kidney (ureteric bud), mammary gland and salivary gland (submandibular gland). These complex tree-like branched organs consist of two main cell populations which include the contiguous epithelium that undergoes the branching process and the surrounding mesenchyme. Spatiotemporally regulated reciprocal interactions between these cell populations are essential to build the highly ordered branching organs, yet each organ has distinct regulatory mechanisms. Although individual organs can be distinguished by the signaling pathways used for branching, they share dynamic structural components such as the extracellular matrix (ECM) and the intracellular actomyosin machinery.
A variety of ECM molecules are synthesized and secreted during morphogenesis and their distinct patterns of expression and deposition are tightly regulated for normal development. Over the past several decades, the role of the ECM has emerged from that of a passive physical material to a collection of functional and dynamic components that can serve as a physical support for tissue architecture, a reservoir for growth factors and other diffusible signals and a substratum on which to adhere through receptors such as integrins. The ECM thus provides a microenvironment that can be sensed and remodeled through both mechanical and chemical signals that influence cell behaviors including proliferation, differentiation, shape change and establishment of polarity.1 In branching organs, several ECM proteins are found at the interface between the epithelium and the mesenchyme; the composition and organization of this ECM are essential for epithelial branching morphogenesis. Moreover, multiple rounds of bud formation and bifurcation require continuous remodeling of the ECM, which is constantly in contact with cells.
Cells in 3D environments connect to ECM and neighboring cells through adhesion molecules that are physically linked to the actin cytoskeleton (Fig. 1). Filamentous actin forms a network with myosin II under the under the plasma membrane membrane and dynamic contractions of this actomyosin network empower the cellular machinery that drives migration of single cells as well as collective cell movements in multicellular systems. Although cell-ECM and cell-cell adhesions have distinct molecular compositions, both transmit forces through a similar mechanism involving dynamic F-actin; therefore, the contractile or tensile forces generated from individual cells or groups of cells can be translated into macroscopic changes in tissues and organs.2-4
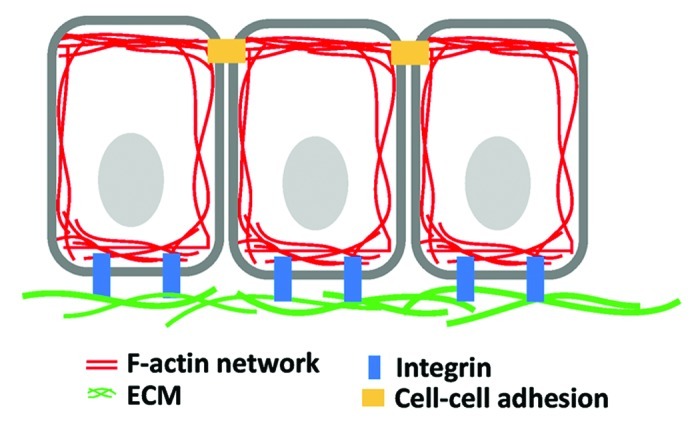
Figure 1. The epithelium. Filamentous actin cytoskeleton forms networks at the cell cortex and is connected through cell-cell adhesion and cell-matrix adhesion.
Here we focus on the role of the structural but dynamic molecules of the ECM and cytoskeleton in branching morphogenesis. We discuss the current understanding of their roles across diverse organs including the lung, kidney, mammary gland and salivary gland as they undergo similar courses of branching morphogenesis. Although different organs may use distinct molecular components to build their separate functional units, advances in our understanding of the development of each organ system will likely shed lights on possible mechanisms used to build branched architecture in others.
Branching Morphogenesis in Development of the Lung
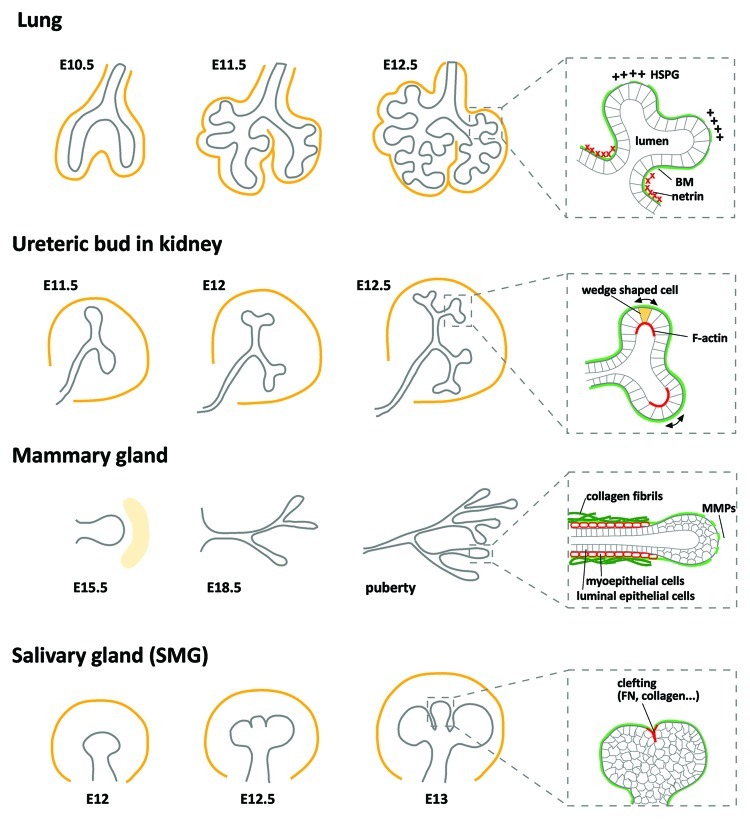
The lung serves to exchange gases between the external environment and the circulatory system. The complex tree-like structure of the lung airways is constructed through the process of branching morphogenesis. During embryonic development, the lung epithelium originates from the ventral foregut endoderm and branches into the surrounding mesenchyme to form the two main bronchi (Fig. 2). This process is followed by sequential rounds of dichotomous bud formation.
Figure 2. Branching morphogenesis in different organs. Schematic representation of branching patterns in lung, kidney (ureteric bud; UB), mammary gland and salivary gland (submandibular gland; SMG). Branching epithelia (gray) are surrounded by mesenchyme (orange). Expanded view of each organ shows the basement membrane (BM; green) and characteristic features are highlighted in red. In the lung, the two main bronchi (E10.5) begin to form new buds (E11.5) which then undergo bifurcations (E12.5). The thinning of BM at the tip of the buds and low-sulfated HS (HSPG) at adjacent mesenchyme facilitates branching, whereas netrin around the neck prevents branching. The UB undergoes rapid branching in early morphogenesis. F-actin is concentrated in the apical domain of bifurcating buds, coincident with epithelial cells that have a wedge-like shape and an expanded basal surface (arrows). Mammary buds invade until E18.5. Extensive branching occurs postnatally during puberty with the formation of the terminal end bud (TEB). Myoepithelial cells tightly surround the duct and directly contact the ECM. MMPs degrade ECM around the tip of the TEB. SMG branching morphogenesis initiates with cleft formation. Several ECM proteins (i.e., FN and collagen) are found in the clefts.
Decades of effort to understand the mechanisms underlying lung development using transgenic mouse technology have resulted in the identification of many signals that are involved in branching morphogenesis, including fibroblast growth factors (FGFs), Sonic hedgehog (Shh), bone morphogenetic protein (BMP), Wnt, Sprouty and retinoic acid.5,6 Furthermore, characterization of stereotypical branching into modes called domain branching, planar bifurcation and orthogonal bifurcation7 revealed a possible approach to integrate the complex gene regulatory network with the geometric changes that govern lung branching morphogenesis. Repetition of these branching modes requires dynamic interactions between the epithelium and its surrounding mesenchyme. In addition to the soluble signaling molecules that initiate the branching process, remodeling of ECM and dynamic cell behaviors are essential to build the highly organized branching structure of the lung.
ECM
At the interface between the epithelium and the mesenchyme, a thin layer of ECM called basement membrane (BM) is formed that consists of collagen IV, laminins, glycoproteins and proteoglycans. The various basement membrane components are expressed in spatially distinct patterns which raises the question of whether these molecules play separate roles in controlling branching morphogenesis. In E12.5 mouse embryonic lung, both epithelium and mesenchyme express high levels of mRNA for collagen IV and laminin-γ1 chain, but considerably lower levels of laminin-α1 and -β1 chain, whereas nidogen is only produced by the surrounding mesenchyme.8 In turn, immunostaining of collagen IV, laminin-111, nidogen and fibronectin revealed a thinning of the basement membrane adjacent to the tip of newly emerging buds, as compared with non-budding regions. This thinning was found to coincide with a high proliferation rate of the epithelium.9
How do different components of the ECM regulate the cellular processes of bud outgrowth and bifurcation during lung development? The major basement membrane proteins, laminins, are heterotrimeric (α, β and γ) glycoproteins and different isoforms are expressed in distinct patterns during development. Laminin-111 is expressed only in early lung development and has received much attention for a possible function in branching of the airways. Organotypic culture of embryonic lung cells showed that laminin-111 plays a role in cell rearrangements by inducing sorting of epithelial and mesenchymal populations and by defining epithelial cell polarity.10 Furthermore, heparan sulfate proteoglycan (HSPG) binding to laminin-111 induces lumen formation in polarized epithelial cells in organotypic culture.11 Disrupting laminin-111 using a function-blocking antibody resulted in reduced growth and decreased branching morphogenesis in lung bud explants.12 Although laminin-α5 (present in both laminin-511 and -521) is also expressed in the lung from early development to adulthood, embryonic lethality of laminin-α5-null mice (Lama5−/−) at E14–17 has limited the study of the role of this protein in lung development. Nevertheless, organ culture of the lung from E12.5 showed no significant differences in branching morphogenesis between wild-type and Lama5−/− mice.13
HSPG is present in the basement membrane as well as on the cell surface, and the diverse forms of sulfated HS chains appear to have a distinct binding affinity to growth factors which consequently regulate developmental processes. In particular, HSPG has been proposed to mediate FGF10 signaling during lung branching morphogenesis. In E11.5 embryonic lung, HS is expressed both in the basement membrane and the mesenchyme. However, the distribution of different forms of HS is evident, with low-sulfated HS expressed in mesenchyme and highly sulfated HS present in basement membrane.14 The presence of low O-sulfated HS in the mesenchyme adjacent to new bud sites combined with the high binding affinity between HS and FGF10 suggests a role for HS in regulating the localization of the FGF10 signal that induces new bud formation. Decreased O-sulfation of HS in the distal mesenchyme may result in reduction of HS-FGF10 binding affinity and accelerate the diffusion of FGF10 from its source in the mesenchyme, thus providing a localized cue to induce epithelial bud formation at specific sites.
In contrast to the possible role of HSPG in facilitating FGF10 signaling at the tip of growing buds, netrin has been revealed to block FGF10 signaling around the neck region of elongating stalks, thus preventing the formation of new buds. In situ hybridization of netrin-1 and -4 combined with immunostaining of netrin-4 proteins in embryonic mouse lungs revealed that netrin is secreted by the epithelium and deposited around the neck or stalk region of the buds.15,16 The function of netrin during branching morphogenesis has been analyzed using a culture model of mesenchyme-free distal epithelium embedded in matrigel. Adding exogenous netrin into cultures that were induced to branch by FGFs inhibited the localized phosphorylation of extracellular-signal regulated protein kinase (ERK1/2) and subsequent cell shape changes, but had no effects on cell proliferation or survival.16 Taken together, these data suggest that the developing airway epithelium secretes netrin-1 and -4 to locally inhibit ERK and prevent new bud formation and thus acts like a sleeve to restrict emergence of aberrant buds.
Cytoskeleton
The 3D epithelial tube that lines the interior of the lung undergoes remarkable morphogenetic movements to form the tree-like branching structure. The cytoskeleton plays a decisive role in regulating epithelial cell movements during the branching process. Unlike other branching organs such as the ureteric bud, mammary gland and salivary gland, we have a very limited understanding of the cellular mechanisms that drive branching morphogenesis of the lung. Despite a lack of techniques for live imaging and the enormous focus on the role of signaling cascades during lung branching morphogenesis, several studies have attempted to incorporate a role for mechanical tension of the ECM and cytoskeleton in regulating the branching processes of the airways. As the basement membrane provides a pre-stressed substratum for adjacent epithelial and mesenchymal cells, the traction forces that cells generate through actomyosin contractions have been proposed to be important for lung epithelial branching.17,18 In organ culture, pharmacological inhibition of the Rho-associated kinase (ROCK) signaling pathway, which is known to control actomyosin contractility, significantly reduces the number of buds that form and disturbs the normal pattern of the basement membrane.18 In contrast, activating Rho with low doses of cytotoxic necrotizing factor-1 (CNF-1) enhances branching, but higher doses cause failure of branching and compaction of the tissues.17,18
A recent study has provided additional clues for the role of the cytoskeleton by demonstrating a linkage between planar cell polarity (PCP) genes and actin during lung branching morphogenesis.19 The PCP pathway is known to direct the cellular polarization orthogonal to the apical-basal axis through downstream effectors and the actin cytoskeleton, via ROCK. Mutation of PCP genes (Celsr1 and Vangl2) in mouse embryonic lungs reduces the size of the lung and induces the formation of a multilayered and disorganized epithelium by disrupting the cytoskeleton.19 Addition of CNF-1 to activate Rho signaling in explant cultures of mutant lungs partially rescued the normal branching phenotype, suggesting that the defects in cellular organization in PCP mutant lungs resulted from altered Rho activity that leads to disruption of the cytoskeleton. Furthermore, Celsr1 has been shown to localize to the neck and cleft regions of branching lung epithelium, and knockdown of Celsr1 with morpholinos blocks cleft formation and prevents bifurcation.19 Further work is needed to piece together precisely the relative roles of these various ECM and cytoskeletal proteins during lung development.
Branching Morphogenesis in Development of the Kidney
Branching morphogenesis of the developing metanephric kidney begins when the nephric duct forms the ureteric bud (UB). The primary UB then extends and invades toward the metanephric mesenchyme (MM), and begins branching in a variety of modes including terminal bifid, terminal trifid and lateral branching; nevertheless the majority of the UB branches through terminal bifurcations (Fig. 2). These UB branching processes have been uncovered using live imaging techniques in combination with explant cultures of organs from transgenic mice that express green fluorescent protein (GFP) under the Hoxb7 promoter, which is specifically expressed in the UB epithelium.20 The reciprocal inductive signals between the UB and the surrounding MM instruct the reiterative branching processes, with a large number of growth factors having been implicated as either stimulatory molecules [e.g., glial cell line-derived neurotrophic factor (GDNF) and FGFs] or inhibitory molecules (e.g., BMPs and TGFβ).21,22 Successive rounds of UB branching eventually give rise to the collecting ducts of the mature kidney.
ECM
The role of laminin in ureteric branching morphogenesis was revealed by mutant mice lacking the laminin-α5 (Lama5−/−) and laminin-γ1 (Lamc1−/−) chains. Deficiency of laminin-α5 causes attenuation of UB branching and results in unilateral or bilateral renal agenesis in about 20% of mice.23 Additionally, the laminin-332-binding receptors such as α3β1 and α6β4 integrin have been suggested to be required for UB branching. In UB cell culture, organ culture and isolated UB culture models, inhibiting both integrin-α3 and -α6 subunits significantly reduces UB branching, and thus reveals a direct role for these integrins that is independent from the mesenchyme.24 Lamc1−/− mice showed lack of basement membrane which resulted in failure of development after E5.5.25 However, selective inactivation of the Lamc1 gene in the UB revealed defects in UB growth and branching accompanied by a greatly reduced basement membrane as well as disorganized epithelial cells in the ampulla.26 Furthermore, blocking the laminin-γ1-binding site of nidogen, which serves to bridge the basement membrane networks, perturbs basement membrane assembly and branching in organ culture,27 and most mice deficient for the nidogen-binding site of the laminin-γ1 chain fail to form the UB.28
The essential role of HSPG in kidney development has been revealed using mice lacking heparin sulfate 2-sulfotransferase (Hs2st; an enzyme that catalyzes the 2-O-sulfation of uronic acids in heparan sulfate), which show renal agenesis due to defects in branching morphogenesis, not from alterations in initial UB outgrowth.29 Additional studies of Hs2st-null kidneys combined with explant culture and tissue recombination assays provided further evidence for an inductive role of MM through 2-O-sulfated HS in the developing kidney. Combining UB or MM from Hs2st-null animals with the complementary wild-type tissues permitted each to undergo branching, which indicates that the mutant tissues are intrinsically competent for branching and that it is a lack of inductive signaling through HS that prevents the progression of branching morphogenesis.30 Furthermore, inhibiting sulfation of HSPG in isolated UB and organ culture blocks budding and reduces FGF2 binding and proliferation.31 By comparison, 6-O-sulfation of HSPG has a more profound effect than 2-O-sulfation on UB branching because of the increased affinity for growth factors such as GDNF and FGF1.32
A novel ECM protein at the interface between the UB and MM, called nephronectin, was uncovered during the search for the ligand for integrin α8β1. More than 50% of mice lacking the integrin α8 subunit fail to develop ureters or kidneys without any significant defects in other organs, which results in death within days after birth.33 Even the mutant mice that did form the UB mostly failed to invade into the MM, which resulted in renal agenesis. This requirement for integrin α8β1 in kidney development led to the identification of its ligand, nephronectin, which is expressed by the ureteric bud epithelium.34 Similar to mice lacking integrin α8, nephronectin-null mice showed delays in UB invasion and frequent renal agenesis or hypoplasia.35 Interestingly, no defects in the basement membrane were found in either the nephronectin- or integrin α8-null mutants, which revealed instead a transient decrease in the expression of GDNF at E11.5 when UB invasion begins. These observations suggest a possible mechanism for the delay or inhibition of UB invasion through nephronectin-integrin α8β1 interactions in the developing kidney.35
Cytoskeleton
One potential driving force for branching morphogenesis of the UB is cell shape changes induced by contractility of the actin cytoskeleton. In both E15.5 embryonic rat UB and cultured UB explants, epithelial cells at newly forming bud regions have a wedge-like shape with a larger basal surface as compared with their apical surface, in contrast to epithelial cells along the branch that are columnar in shape (Fig. 2).36 These differences in cell shape coincide with strong localization of F-actin, myosin II and ezrin at the apical surface of the epithelium at new budding regions, suggesting a purse-string mechanism for branch initiation in the UB.36 A similar pattern of strong F-actin localization in the apical domain of the newly forming UB has been reported in cultured UB from E11.5 mouse embryos.37 However, the role of the actomyosin network during UB branching is unclear, since some investigators found that inhibiting myosin contractility by blocking ROCK reduced new bud formations37 whereas others found that this treatment induced UB branching.38
Nevertheless, the actin depolymerizing factors (ADFs), cofilin1 and destrin, appear to be important for UB branching morphogenesis. Lack of genes for both cofilin1 and destrin in mouse embryos causes failure of UB branching through accumulation of F-actin and a disorganized epithelial cell shape.39 Moreover, primary UB epithelial cells lacking cofilin1 and destrin showed impaired cell movements which may have a role in UB branching morphogenesis.39 Together these results suggest that the dynamic remodeling of F-actin plays a critical role in UB branching, likely through apically constricting epithelial cells at the sites of bud formation. More detailed live-imaging and quantitative analysis is required to reveal the mechanisms underlying intracellular dynamics of this developing tissue.
Branching Morphogenesis in Development of the Mammary Gland
Unlike the development of other organs, branching morphogenesis of the mammary gland takes place across distinct stages including embryonic, pubertal and adult. The embryonic mammary epithelium forms a placode and invades into the mammary mesenchyme to form the mammary buds, which remain quiescent until puberty (Fig. 2). Signaling from hormones leads to the formation of the terminal end buds (TEBs), enlarged multicellular structures that undergo elongation and bifurcation during puberty.40 The branching mammary duct is a bi-layered structure consisting of luminal epithelial cells surrounded by myoepithelial cells, which directly contact the basement membrane that separates the epithelium from the stroma. Interactions between the stroma and the epithelium are essential for normal mammary development.
ECM
At the interface between the epithelium and stroma, various ECM components are dynamically expressed in distinct regions (i.e., tip and duct) of the mammary gland over the course of development and are thought to have important roles in regulating the branching process. For instance, fibrillar collagen I is predominantly deposited around ducts, whereas collagen IV and laminins are found near the tip of buds, and the expression of these ECM proteins increases in puberty, but ceases afterward until pregnancy.41 Due to the extensive bundling of collagen and its orientation parallel to the elongating duct, the collagen fibrils have been proposed to play a mechanical role and serve as a constrained wall to prevent new bud formation and thereby act as a guidance cue for branching; these possibilities still remain to be explored and confirmed in vivo.42 Nevertheless, accumulating evidence shows increased activity of matrix-degrading proteases near the invading bud that will loosen the fibrous ECM, and thus can promote branching morphogenesis.43 Of the many matrix metalloproteinases (MMPs) and their inhibitors (TIMPs) that modify the ECM during mammary gland branching, it has been found that overexpression of MMP3/stromelysin-1 and MMP14 (MT1-MMP; membrane type-1-MMP) in mice leads to excessive side branching.44-46 Furthermore, MMP-induced fragments of fibronectin have been shown to induce epithelial cell loss that may be involved in clearing of secretory epithelium during mammary gland involution.47 Moreover, glycosaminoglycans (GAG) are thought to bind to growth factors and have been shown to accumulate specifically in the BM surrounding the ducts,48 which might serve as a reservoir for TGFβ to inhibit the formation of new branches during mammary gland development.49-51
Because the branching epithelial cells are directly in contact with ECM via transmembrane receptors like integrins, the role of β1-integrin has been explored in mammary branching morphogenesis. β1-integrin associates with numerous α-integrin subunits to bind to ECM proteins including collagens, laminins and fibronectin, and conditional deletion of β1-integrin in the luminal epithelium of mouse mammary glands revealed defects in alveolar morphogenesis that were associated with disorganized epithelial aggregates which did not clear properly.52 These altered cell-matrix adhesions coincided with decreased activity of focal adhesion kinase (FAK) and reduced proliferation, indicating the importance of β1-integrin in mammary gland development.53 Mice lacking β1-integrin in the basal epithelium provide evidence for additional roles of this integrin subunit in mammary branching, since mutant animals showed altered cell division axis, failure of segregation of different cell populations and abnormal branching pattern.54
Cytoskeleton
The behaviors of individual cells in elongating mammary epithelium have been observed through long-term live-imaging of organotypic cultures, which provided insights into the cellular dynamics that underlie the complex process of mammary branching morphogenesis. In the early stages of branching of primary mammary organoids, high rates of proliferation promoted the formation of complex cysts. The multilayered epithelium then rearranged to initiate and elongate into ducts, which consequently converted to form a bilayered polarized tissue.55 This series of morphogenetic movements required molecules that modulate the activity of the actomyosin network, including Rac, ROCK and myosin light chain kinase, suggesting an essential role for actomyosin contractility in mammary gland morphogenesis.55 Additionally, a major regulator of actin assembly, gelsolin, which is an actin-severing protein, is required for mammary branching. Mice lacking gelsolin fail to form the TEB or to elongate at puberty. However, these phenotypes were recovered when epithelial ducts from gelsolin-knockout mice were cultured in cleared fat pads obtained from wild-type animals. In complementary experiments, wild-type ductal epithelium failed to undergo normal branching in the presence of mutant stroma, providing evidence that gelsolin-regulated actin assembly is required in the mammary stroma for branching morphogenesis of the epithelium.56 Furthermore, a recent study using conditional FAK-knockout mice revealed a critical role for FAK in cell-matrix adhesions that mediate actomyosin contractility in mammary gland branching.57 FAK-deficient mammary epithelium transplanted into wild-type cleared fat pads resulted in the formation of dilated ducts and altered tissue separation between luminal and myoepithelial cells. In fact, these defects appear to be a consequence of the increased cell contractility via ROCK that inhibits branching morphogenesis in mammary organoids.57 Additionally, 3D organotypic culture of mammary epithelial tubules that mimic endogenous mammary branching revealed that activation of FAK designates new branching sites that correlate with high traction forces generated by increased actomyosin contractility.58 Altogether, these studies suggest that contractility of the cytoskeleton and adhesion to various ECM proteins play a critical role in specifying sites of branching during mammary epithelial morphogenesis.
Branching Morphogenesis in Development of the Salivary Gland [Submandibular Gland (SMG)]
The salivary gland produces saliva, thus maintaining oral physiology by providing the mouth cavity with water, electrolytes, mucus and various antibacterial compounds and enzymes. This secretory organ forms throughout embryonic development via branching morphogenesis. The salivary gland is composed of a thick spherical epithelium surrounded by mesenchyme as in other branching organs, but here branching initiates through cleft formation of the primary bud (Fig. 2). Deepening of epithelial clefts separates the primary bud into multiple smaller buds which elongate and expand to form secondary buds. Sequential rounds of bifurcation and elongation result in the extensive system of ducts present in the mature gland. Several growth factors and signaling pathways have been implicated in salivary branching morphogenesis, including mitogen-activated protein kinase (MAPK), phospholipase Cγ1 (PLCγ1) and phosphatidyl-inositol-3-kinase (PI3K) signaling stimulated from FGFs or EGF.59
ECM
Embryos lacking laminin α1, β1 or γ1 die early in development, but laminin-α5 mutant mice (Lama5−/−) survive until late in embryogenesis, thus permitting investigation of the role of this ECM protein in branching morphogenesis of the submandibular gland (SMG). At E13, SMG isolated from Lama5−/− mice shows a delay in branching morphogenesis of ~1 d, suggesting that laminin-α5 may be important for the initiation of cleft formation. However, the branching process has begun by E14.5, suggesting possible compensation from other laminin isoforms. At E17.5, the SMG shows a disorganized epithelial phenotype with disrupted lumen formation.60 Further studies using siRNA in cultured SMG suggest a reciprocal signaling between laminin-α5, FGFR and β1-integrin during branching morphogenesis.60 Moreover, a role for perlecan HS in SMG has been shown using heparanase, which cleaves and subsequently induces the biological activity of HS.61 Heparanase is found endogenously at the basement membrane as well as within the cleft of the SMG, and inhibition or addition of heparanase decreases or increases branching in cultured SMG, respectively. Heparanase specifically releases the FGF10-FGFR2b complex from the basement membrane without affecting other FGFs, resulting in increases of MAPK signaling, clefting and bud formation.
Among the several types of collagens that are present in the salivary gland, collagen I and III are found at the cleft points of branching SMG in E12–13 mice.62,63 A role for these interstitial collagens in cleft formation has been revealed by degrading collagen with collagenase or inhibiting collagenase activity in explant cultures. The initiation of SMG cleft formation is completely blocked with collagenase treatment, whereas inhibiting collagenase enhances the number of clefts by approximately two-fold,64,65 thus suggesting an essential role for collagen III in initiation of clefting in SMG branching morphogenesis. In addition to collagen III, the ECM protein fibronectin (FN) is required during cleft formation of the branching SMG.66 Microdissection of SMG convincingly demonstrated a marked increase in FN synthesis in clefts as compared with bud epithelium. New cleft formation is inhibited by siRNA against FN, anti-FN antibodies or antibodies against integrin-α5 and -α6, whereas exogenous FN induces the formation of additional clefts. Furthermore, FN fibrils accumulate locally in narrow clefts as adjacent epithelial cells decrease cell-cell adhesion and increase cell-matrix adhesion. While these studies were the first to show the spatiotemporally distinct expression of FN that directly controls the initiation and formation of the cleft, a later study provided insight into the dynamic assembly of FN during SMG branching morphogenesis. In pulse-chase experiments using fluorescently labeled proteins, FN accumulated at the base of clefts and ingressed as the clefts deepened, while new FN assembled behind the existing FN.67 Thus, locally assembled and deposited ECM proteins appear to provide a guidance cue for symmetry-breaking of the spherical bud and may consequently direct the later steps in branching morphogenesis.
Cytoskeleton
How do cells move to build the branched structure of the salivary gland? Although branching morphogenesis appears to be a highly organized process, analysis of fluorescently labeled epithelial cells in the developing SMG revealed unexpected rapid and random movements of individual cells,67 with epithelial cells along the bud seeming to move more actively than those at the cleft.68 To further dissect the cellular mechanisms involved in cleft initiation and progression, the role of ROCK-mediated actomyosin contractility has been examined in cultured mouse SMG. When the activity of ROCK or myosin II is inhibited, cultures of SMG epithelium with or without mesenchyme can initiate the formation of clefts by making a shallow deepening of the bud, but fail to complete bud formation.69 In turn, the progression of bud formation requires ROCK-mediated cell proliferation once actomyosin contractility has reached a certain threshold, called a “checkpoint,” which then promotes FN fibril assembly and stabilizes the cleft. Thus, SMG branching is proposed to undergo these separate processes for cleft initiation and progression, with ROCK-mediated actomyosin contractility playing a role in determining the transition point. Furthermore, the focal adhesion complexes that simultaneously link the actin cytoskeleton, ECM and integrins are regulated by actomyosin contractility and have been shown to play a key role in the mechanochemical checkpoint.70
Perspective and Conclusions
Different organ systems are built by branching morphogenesis, which is comprised of shared morphological steps including bud initiation, outgrowth and bifurcation. Nonetheless, each organ has characteristic features that may imply distinct control mechanisms during morphogenesis. The lung, for instance, undergoes stereotypic branching that results in a defined branching pattern across individuals within a species; the kidney (ureteric bud) is surrounded by thick mesenchyme and branching progresses without a specific pattern; the mammary gland has an additional layer of cells around the stalk called the myoepithelium; salivary bud is filled with epithelium that forms clefts to initiate a bifurcation (Fig. 2). However, currently it is unclear whether these differences between branching organs arise from specific needs for organ functions, or how these differences are integrated to build a complicated but similarly branched structure. In this review, we focused on the essential mechanical elements, the ECM and cytoskeleton, that direct branching morphogenesis.
Studies of ECM molecules over different branching organs indicate that the major mechanisms to facilitate the branching process are both the controlled assembly and the modification of ECM proteins (i.e., HSPG) at distinct regions of the tree (i.e., budding or non-budding regions). In addition, accumulating evidence shows the physical role of the ECM that is deposited in the cleft or non-budding regions, which can serve as a tractive substratum to initiate or stabilize bud formations. Although continuing efforts to elucidate the role of the ECM in branching morphogenesis have identified a list of molecules that are involved in this process, much of our understanding is limited to the localization of these molecules at discrete developmental stages. In addition to the rigorous effort of identifying important ECM molecules, it is necessary to follow the dynamics of ECM remodeling that occurs in branching morphogenesis in vivo. Critical questions include the following: what are the dynamics of ECM assembly in the sequential steps of branching morphogenesis? What are the consequences of changing ECM microenvironments? How does the temporal localization or degradation of ECM alter the chemical and mechanical signaling? What are the feedback mechanisms that regulate the iterative branching process?
Recent advances in live imaging and quantitative image analysis have considerably enhanced our understanding of the cellular mechanisms that drive morphogenesis. Although only a few model systems have been explored, surprisingly similar dynamics of the actomyosin network, for example, flowing of subcellular contractile foci and pulsatile contractions, have been reported to drive morphogenesis in different tissues.4 Future research will undoubtedly explore the numerous remaining questions concerning the role of cytoskeletal dynamics during branching morphogenesis, including, for instance, how localized cellular forces generated through actomyosin contraction shape the developing epithelial tube.
Acknowledgments
Work from the authors’ lab was supported in part by the NIH (GM083997, HL110335, and CA128660), the David and Lucile Packard Foundation, the Alfred P. Sloan Foundation and Susan G. Komen for the Cure. C.M.N. holds a Career Award at the Scientific Interface from the Burroughs Wellcome Fund.
Glossary
Abbreviations:
- ADF
actin depolymerizing factor
- BM
basement membrane
- BMP
bone morphogenetic protein
- CNF
cytotoxic necrotizing factor
- E
embryonic day
- ECM
extracellular matrix
- EGF
epidermal growth factor
- ERK
extracellular-signal regulated protein kinase
- FAK
focal adhesion kinase
- FGF
fibroblast growth factor
- FN
fibronectin
- GAG
glycosaminoglycan
- GDNF
glial cell line-derived neurotrophic factor
- GFP
green fluorescent protein
- HS
heparin sulfate
- HSPG
heparan sulfate proteoglycan
- MAPK
mitogen-activated protein kinase
- MM
metanephric mesenchyme
- MMP
matrix metalloproteinase
- PCP
planar cell polarity
- PLC
phospholipase C
- PI3K
phosphatidyl-inositol-3-kinase
- ROCK
Rho-associated kinase
- Shh
Sonic hedgehog
- SMG
submandibular gland
- TEB
terminal end bud
- TIMP
tissue inhibitor of metalloproteinases
- TGF
transforming growth factor
- UB
ureteric bud
Footnotes
Previously published online: www.landesbioscience.com/journals/organogenesis/article/19813
References
- 1.Rozario T, DeSimone DW. The extracellular matrix in development and morphogenesis: a dynamic view. Dev Biol. 2010;341:126–40. doi: 10.1016/j.ydbio.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maruthamuthu V, Aratyn-Schaus Y, Gardel ML. Conserved F-actin dynamics and force transmission at cell adhesions. Curr Opin Cell Biol. 2010;22:583–8. doi: 10.1016/j.ceb.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin AC. Pulsation and stabilization: contractile forces that underlie morphogenesis. Dev Biol. 2010;341:114–25. doi: 10.1016/j.ydbio.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 4.Gorfinkiel N, Blanchard GB. Dynamics of actomyosin contractile activity during epithelial morphogenesis. Curr Opin Cell Biol. 2011;23:531–9. doi: 10.1016/j.ceb.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Warburton D, Schwarz M, Tefft D, Flores-Delgado G, Anderson KD, Cardoso WV. The molecular basis of lung morphogenesis. Mech Dev. 2000;92:55–81. doi: 10.1016/S0925-4773(99)00325-1. [DOI] [PubMed] [Google Scholar]
- 6.Morrisey EE, Hogan BLM. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev Cell. 2010;18:8–23. doi: 10.1016/j.devcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Metzger RJ, Klein OD, Martin GR, Krasnow MA. The branching programme of mouse lung development. Nature. 2008;453:745–50. doi: 10.1038/nature07005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas T, Dziadek M. Expression of collagen α 1(IV), laminin and nidogen genes in the embryonic mouse lung: implications for branching morphogenesis. Mech Dev. 1994;45:193–201. doi: 10.1016/0925-4773(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 9.Mollard R, Dziadek M. A correlation between epithelial proliferation rates, basement membrane component localization patterns, and morphogenetic potential in the embryonic mouse lung. Am J Respir Cell Mol Biol. 1998;19:71–82. doi: 10.1165/ajrcmb.19.1.3158. [DOI] [PubMed] [Google Scholar]
- 10.Schuger L, O’Shea KS, Nelson BB, Varani J. Organotypic arrangement of mouse embryonic lung cells on a basement membrane extract: involvement of laminin. Development. 1990;110:1091–9. doi: 10.1242/dev.110.4.1091. [DOI] [PubMed] [Google Scholar]
- 11.Schuger L, Skubitz APN, Gilbride K, Mandel R, He L. Laminin and heparan sulfate proteoglycan mediate epithelial cell polarization in organotypic cultures of embryonic lung cells: evidence implicating involvement of the inner globular region of laminin β 1 chain and the heparan sulfate groups of heparan sulfate proteoglycan. Dev Biol. 1996;179:264–73. doi: 10.1006/dbio.1996.0256. [DOI] [PubMed] [Google Scholar]
- 12.Schuger L, O’Shea S, Rheinheimer J, Varani J. Laminin in lung development: effects of anti-laminin antibody in murine lung morphogenesis. Dev Biol. 1990;137:26–32. doi: 10.1016/0012-1606(90)90004-3. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen NM, Miner JH, Pierce RA, Senior RM. Laminin α 5 is required for lobar septation and visceral pleural basement membrane formation in the developing mouse lung. Dev Biol. 2002;246:231–44. doi: 10.1006/dbio.2002.0658. [DOI] [PubMed] [Google Scholar]
- 14.Izvolsky KI, Shoykhet D, Yang Y, Yu Q, Nugent MA, Cardoso WV. Heparan sulfate-FGF10 interactions during lung morphogenesis. Dev Biol. 2003;258:185–200. doi: 10.1016/S0012-1606(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 15.Dalvin S, Anselmo MA, Prodhan P, Komatsuzaki K, Schnitzer JJ, Kinane TB. Expression of Netrin-1 and its two receptors DCC and UNC5H2 in the developing mouse lung. Gene Expr Patterns. 2003;3:279–83. doi: 10.1016/S1567-133X(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Stein E, Oliver T, Li Y, Brunken WJ, Koch M, et al. Novel role for Netrins in regulating epithelial behavior during lung branching morphogenesis. Curr Biol. 2004;14:897–905. doi: 10.1016/j.cub.2004.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore KA, Huang S, Kong Y, Sunday ME, Ingber DE. Control of embryonic lung branching morphogenesis by the Rho activator, cytotoxic necrotizing factor 1. J Surg Res. 2002;104:95–100. doi: 10.1006/jsre.2002.6418. [DOI] [PubMed] [Google Scholar]
- 18.Moore KA, Polte T, Huang S, Shi B, Alsberg E, Sunday ME, et al. Control of basement membrane remodeling and epithelial branching morphogenesis in embryonic lung by Rho and cytoskeletal tension. Dev Dyn. 2005;232:268–81. doi: 10.1002/dvdy.20237. [DOI] [PubMed] [Google Scholar]
- 19.Yates LL, Schnatwinkel C, Murdoch JN, Bogani D, Formstone CJ, Townsend S, et al. The PCP genes Celsr1 and Vangl2 are required for normal lung branching morphogenesis. Hum Mol Genet. 2010;19:2251–67. doi: 10.1093/hmg/ddq104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watanabe T, Costantini F. Real-time analysis of ureteric bud branching morphogenesis in vitro. Dev Biol. 2004;271:98–108. doi: 10.1016/j.ydbio.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 21.Costantini F. Renal branching morphogenesis: concepts, questions, and recent advances. Differentiation. 2006;74:402–21. doi: 10.1111/j.1432-0436.2006.00106.x. [DOI] [PubMed] [Google Scholar]
- 22.Nigam SK, Shah MM. How does the ureteric bud branch? J Am Soc Nephrol. 2009;20:1465–9. doi: 10.1681/ASN.2008020132. [DOI] [PubMed] [Google Scholar]
- 23.Miner JH, Li C. Defective glomerulogenesis in the absence of laminin α5 demonstrates a developmental role for the kidney glomerular basement membrane. Dev Biol. 2000;217:278–89. doi: 10.1006/dbio.1999.9546. [DOI] [PubMed] [Google Scholar]
- 24.Zent R, Bush KT, Pohl ML, Quaranta V, Koshikawa N, Wang Z, et al. Involvement of laminin binding integrins and laminin-5 in branching morphogenesis of the ureteric bud during kidney development. Dev Biol. 2001;238:289–302. doi: 10.1006/dbio.2001.0391. [DOI] [PubMed] [Google Scholar]
- 25.Smyth N, Vatansever HS, Murray P, Meyer M, Frie C, Paulsson M, et al. Absence of basement membranes after targeting the LAMC1 gene results in embryonic lethality due to failure of endoderm differentiation. J Cell Biol. 1999;144:151–60. doi: 10.1083/jcb.144.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang D-H, McKee KK, Chen Z-L, Mernaugh G, Strickland S, Zent R, et al. Renal collecting system growth and function depend upon embryonic γ1 laminin expression. Development. 2011;138:4535–44. doi: 10.1242/dev.071266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ekblom P, Ekblom M, Fecker L, Klein G, Zhang HY, Kadoya Y, et al. Role of mesenchymal nidogen for epithelial morphogenesis in vitro. Development. 1994;120:2003–14. doi: 10.1242/dev.120.7.2003. [DOI] [PubMed] [Google Scholar]
- 28.Willem M, Miosge N, Halfter W, Smyth N, Jannetti I, Burghart E, et al. Specific ablation of the nidogen-binding site in the laminin γ1 chain interferes with kidney and lung development. Development. 2002;129:2711–22. doi: 10.1242/dev.129.11.2711. [DOI] [PubMed] [Google Scholar]
- 29.Bullock SL, Fletcher JM, Beddington RSP, Wilson VA. Renal agenesis in mice homozygous for a gene trap mutation in the gene encoding heparan sulfate 2-sulfotransferase. Genes Dev. 1998;12:1894–906. doi: 10.1101/gad.12.12.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shah MM, Sakurai H, Sweeney DE, Gallegos TF, Bush KT, Esko JD, et al. Hs2st mediated kidney mesenchyme induction regulates early ureteric bud branching. Dev Biol. 2010;339:354–65. doi: 10.1016/j.ydbio.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steer DL, Shah MM, Bush KT, Stuart RO, Sampogna RV, Meyer TN, et al. Regulation of ureteric bud branching morphogenesis by sulfated proteoglycans in the developing kidney. Dev Biol. 2004;272:310–27. doi: 10.1016/j.ydbio.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 32.Shah MM, Sakurai H, Gallegos TF, Sweeney DE, Bush KT, Esko JD, et al. Growth factor-dependent branching of the ureteric bud is modulated by selective 6-O sulfation of heparan sulfate. Dev Biol. 2011;356:19–27. doi: 10.1016/j.ydbio.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Müller U, Wang D, Denda S, Meneses JJ, Pedersen RA, Reichardt LF. Integrin α8β1 is critically important for epithelial-mesenchymal interactions during kidney morphogenesis. Cell. 1997;88:603–13. doi: 10.1016/S0092-8674(00)81903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brandenberger R, Schmidt A, Linton J, Wang D, Backus C, Denda S, et al. Identification and characterization of a novel extracellular matrix protein nephronectin that is associated with integrin α8β1 in the embryonic kidney. J Cell Biol. 2001;154:447–58. doi: 10.1083/jcb.200103069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Linton JM, Martin GR, Reichardt LF. The ECM protein nephronectin promotes kidney development via integrin α8β1-mediated stimulation of Gdnf expression. Development. 2007;134:2501–9. doi: 10.1242/dev.005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyer TN, Schwesinger C, Bush KT, Stuart RO, Rose DW, Shah MM, et al. Spatiotemporal regulation of morphogenetic molecules during in vitro branching of the isolated ureteric bud: toward a model of branching through budding in the developing kidney. Dev Biol. 2004;275:44–67. doi: 10.1016/j.ydbio.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 37.Michael L, Sweeney DE, Davies JA. A role for microfilament-based contraction in branching morphogenesis of the ureteric bud. Kidney Int. 2005;68:2010–8. doi: 10.1111/j.1523-1755.2005.00655.x. [DOI] [PubMed] [Google Scholar]
- 38.Meyer TN, Schwesinger C, Sampogna RV, Vaughn DA, Stuart RO, Steer DL, et al. Rho kinase acts at separate steps in ureteric bud and metanephric mesenchyme morphogenesis during kidney development. Differentiation. 2006;74:638–47. doi: 10.1111/j.1432-0436.2006.00102.x. [DOI] [PubMed] [Google Scholar]
- 39.Kuure S, Cebrian C, Machingo Q, Lu BC, Chi X, Hyink D, et al. Actin depolymerizing factors cofilin1 and destrin are required for ureteric bud branching morphogenesis. PLoS Genet. 2010;6:e1001176. doi: 10.1371/journal.pgen.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gjorevski N, Nelson CM. Integrated morphodynamic signalling of the mammary gland. Nat Rev Mol Cell Biol. 2011;12:581–93. doi: 10.1038/nrm3168. [DOI] [PubMed] [Google Scholar]
- 41.Keely PJ, Wu JE, Santoro SA. The spatial and temporal expression of the α 2 β 1 integrin and its ligands, collagen I, collagen IV, and laminin, suggest important roles in mouse mammary morphogenesis. Differentiation. 1995;59:1–13. doi: 10.1046/j.1432-0436.1995.5910001.x. [DOI] [PubMed] [Google Scholar]
- 42.Ingman WV, Wyckoff J, Gouon-Evans V, Condeelis J, Pollard JW. Macrophages promote collagen fibrillogenesis around terminal end buds of the developing mammary gland. Dev Dyn. 2006;235:3222–9. doi: 10.1002/dvdy.20972. [DOI] [PubMed] [Google Scholar]
- 43.Alcaraz J, Mori H, Ghajar CM, Brownfield D, Galgoczy R, Bissell MJ. Collective epithelial cell invasion overcomes mechanical barriers of collagenous extracellular matrix by a narrow tube-like geometry and MMP14-dependent local softening. Integr Biol (Camb) 2011;3:1153–66. doi: 10.1039/c1ib00073j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sympson CJ, Talhouk RS, Alexander CM, Chin JR, Clift SM, Bissell MJ, et al. Targeted expression of stromelysin-1 in mammary gland provides evidence for a role of proteinases in branching morphogenesis and the requirement for an intact basement membrane for tissue-specific gene expression. J Cell Biol. 1994;125:681–93. doi: 10.1083/jcb.125.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Witty JP, Wright JH, Matrisian LM. Matrix metalloproteinases are expressed during ductal and alveolar mammary morphogenesis, and misregulation of stromelysin-1 in transgenic mice induces unscheduled alveolar development. Mol Biol Cell. 1995;6:1287–303. doi: 10.1091/mbc.6.10.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ha H-Y, Moon H-B, Nam M-S, Lee J-W, Ryoo Z-Y, Lee T-H, et al. Overexpression of membrane-type matrix metalloproteinase-1 gene induces mammary gland abnormalities and adenocarcinoma in transgenic mice. Cancer Res. 2001;61:984–90. [PubMed] [Google Scholar]
- 47.Schedin P, Strange R, Mitrenga T, Wolfe P, Kaeck M. Fibronectin fragments induce MMP activity in mouse mammary epithelial cells: evidence for a role in mammary tissue remodeling. J Cell Sci. 2000;113:795–806. doi: 10.1242/jcs.113.5.795. [DOI] [PubMed] [Google Scholar]
- 48.Silberstein GB, Daniel CW. Glycosaminoglycans in the basal lamina and extracellular matrix of the developing mouse mammary duct. Dev Biol. 1982;90:215–22. doi: 10.1016/0012-1606(82)90228-7. [DOI] [PubMed] [Google Scholar]
- 49.Silberstein GB, Flanders KC, Roberts AB, Daniel CW. Regulation of mammary morphogenesis: evidence for extracellular matrix-mediated inhibition of ductal budding by transforming growth factor-β 1. Dev Biol. 1992;152:354–62. doi: 10.1016/0012-1606(92)90142-4. [DOI] [PubMed] [Google Scholar]
- 50.Nelson CM, Vanduijn MM, Inman JL, Fletcher DA, Bissell MJ. Tissue geometry determines sites of mammary branching morphogenesis in organotypic cultures. Science. 2006;314:298–300. doi: 10.1126/science.1131000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pavlovich AL, Boghaert E, Nelson CM. Mammary branch initiation and extension are inhibited by separate pathways downstream of TGFβ in culture. Exp Cell Res. 2011;317:1872–84. doi: 10.1016/j.yexcr.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Naylor MJ, Li N, Cheung J, Lowe ET, Lambert E, Marlow R, et al. Ablation of β1 integrin in mammary epithelium reveals a key role for integrin in glandular morphogenesis and differentiation. J Cell Biol. 2005;171:717–28. doi: 10.1083/jcb.200503144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li N, Zhang Y, Naylor MJ, Schatzmann F, Maurer F, Wintermantel T, et al. Beta1 integrins regulate mammary gland proliferation and maintain the integrity of mammary alveoli. EMBO J. 2005;24:1942–53. doi: 10.1038/sj.emboj.7600674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taddei I, Deugnier M-A, Faraldo MM, Petit V, Bouvard D, Medina D, et al. Beta1 integrin deletion from the basal compartment of the mammary epithelium affects stem cells. Nat Cell Biol. 2008;10:716–22. doi: 10.1038/ncb1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ewald AJ, Brenot A, Duong M, Chan BS, Werb Z. Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev Cell. 2008;14:570–81. doi: 10.1016/j.devcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crowley MR, Head KL, Kwiatkowski DJ, Asch HL, Asch BB. The mouse mammary gland requires the actin-binding protein gelsolin for proper ductal morphogenesis. Dev Biol. 2000;225:407–23. doi: 10.1006/dbio.2000.9844. [DOI] [PubMed] [Google Scholar]
- 57.van Miltenburg MHAM, Lalai R, de Bont H, van Waaij E, Beggs H, Danen EHJ, et al. Complete focal adhesion kinase deficiency in the mammary gland causes ductal dilation and aberrant branching morphogenesis through defects in Rho kinase-dependent cell contractility. FASEB J. 2009;23:3482–93. doi: 10.1096/fj.08-123398. [DOI] [PubMed] [Google Scholar]
- 58.Gjorevski N, Nelson CM. Endogenous patterns of mechanical stress are required for branching morphogenesis. Integr Biol (Camb) 2010;2:424–34. doi: 10.1039/c0ib00040j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patel VN, Rebustini IT, Hoffman MP. Salivary gland branching morphogenesis. Differentiation. 2006;74:349–64. doi: 10.1111/j.1432-0436.2006.00088.x. [DOI] [PubMed] [Google Scholar]
- 60.Rebustini IT, Patel VN, Stewart JS, Layvey A, Georges-Labouesse E, Miner JH, et al. Laminin α5 is necessary for submandibular gland epithelial morphogenesis and influences FGFR expression through β1 integrin signaling. Dev Biol. 2007;308:15–29. doi: 10.1016/j.ydbio.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Patel VN, Knox SM, Likar KM, Lathrop CA, Hossain R, Eftekhari S, et al. Heparanase cleavage of perlecan heparan sulfate modulates FGF10 activity during ex vivo submandibular gland branching morphogenesis. Development. 2007;134:4177–86. doi: 10.1242/dev.011171. [DOI] [PubMed] [Google Scholar]
- 62.Hardman P, Spooner BS. Localization of extracellular matrix components in developing mouse salivary glands by confocal microscopy. Anat Rec. 1992;234:452–9. doi: 10.1002/ar.1092340315. [DOI] [PubMed] [Google Scholar]
- 63.Nakanishi Y, Nogawa H, Hashimoto Y, Kishi J, Hayakawa T. Accumulation of collagen III at the cleft points of developing mouse submandibular epithelium. Development. 1988;104:51–9. doi: 10.1242/dev.104.1.51. [DOI] [PubMed] [Google Scholar]
- 64.Fukuda Y, Masuda Y, Kishi J, Hashimoto Y, Hayakawa T, Nogawa H, et al. The role of interstitial collagens in cleft formation of mouse embryonic submandibular gland during initial branching. Development. 1988;103:259–67. doi: 10.1242/dev.103.2.259. [DOI] [PubMed] [Google Scholar]
- 65.Nakanishi Y, Sugiura F, Kishi J-I, Hayakawa T. Collagenase inhibitor stimulates cleft formation during early morphogenesis of mouse salivary gland. Dev Biol. 1986;113:201–6. doi: 10.1016/0012-1606(86)90122-3. [DOI] [PubMed] [Google Scholar]
- 66.Sakai T, Larsen M, Yamada KM. Fibronectin requirement in branching morphogenesis. Nature. 2003;423:876–81. doi: 10.1038/nature01712. [DOI] [PubMed] [Google Scholar]
- 67.Larsen M, Wei C, Yamada KM. Cell and fibronectin dynamics during branching morphogenesis. J Cell Sci. 2006;119:3376–84. doi: 10.1242/jcs.03079. [DOI] [PubMed] [Google Scholar]
- 68.Kadoya Y, Yamashina S. Cellular dynamics of epithelial clefting during branching morphogenesis of the mouse submandibular gland. Dev Dyn. 2010;239:1739–47. doi: 10.1002/dvdy.22312. [DOI] [PubMed] [Google Scholar]
- 69.Daley WP, Gulfo KM, Sequeira SJ, Larsen M. Identification of a mechanochemical checkpoint and negative feedback loop regulating branching morphogenesis. Dev Biol. 2009;336:169–82. doi: 10.1016/j.ydbio.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Daley WP, Kohn JM, Larsen M. A focal adhesion protein-based mechanochemical checkpoint regulates cleft progression during branching morphogenesis. Dev Dyn. 2011;240:2069–83. doi: 10.1002/dvdy.22714. [DOI] [PMC free article] [PubMed] [Google Scholar]