Abstract
A homothymine PNA decamer bearing four lysine residues has been synthesized as a probe for the development of amperometric sensors. On one hand, the four amino groups introduced make this derivative nine times more soluble than the corresponding homothymine PNA decamer and, on the other hand, allow the stable anchoring of this molecule on Au nanostructured surface through the terminal -NH2 moieties. In particular, XPS and electrochemical investigations performed with hexylamine, as a model molecule, indicate that the stable deposition of primary amine derivatives on such a nanostructured surface is possible and involves the free electron doublet on the nitrogen atom. This finding indicates that this PNA derivative is suitable to act as the probe molecule for the development of amperometric sensors.
Thanks to the molecular probe chosen and to the use of a nanostructured surface as the substrate for the sensor assembly, the device proposed makes possible the selective recognition of the target oligonucleotide sequence with very high sensitivity.
Keywords: DNA recognition, PNA, amperometric genosensors, nanostructured surfaces, amine deposition
Introduction
Peptide nucleic acid (PNA) is one of the most interesting and versatile artificial structural mimic of nucleic acids (NA), introduced by Nielsen in 1991.1-4 It has a neutral pseudopeptide backbone replacing the negatively charged sugar-phosphate chain of NA. The backbone is made of N-(2-aminoethyl)glycine units linked in a polyamide structure, on which the nucleobases are attached to the α-nitrogen atom of the amino acid unit through methylene carbonyl residues. PNA features a high chemical stability, as well as a high resistance to cellular enzymes. Thanks to the presence of the nucleobases, which are at the same distance from each other as in DNA or RNA strands, PNA is able to recognize and bind in a very high specific and selective manner the cDNA and RNA, and generally it exhibits excellent mismatch sensitivity. This feature makes PNA exceptionally attractive for DNA recognition5 and, in particular, a lot of interest has been devoted to the use of PNA as the probe in different genosensors.6-8 Among the transduction mechanisms proposed, microgravimetry,9 optics, namely surface plasmon resonance technique,10,11 and electrochemistry9,12 have been proposed. Amperometric sensors are acknowledged to be particularly effective, also easy to use and cheap; in addition, they are characterized by short response time and can be very simply miniaturized, making them portable and suitable for in vivo detections.
Many strategies have been proposed to quantify the amount of NA finally hybridized with PNA chains on the electrode surfaces. Due to the electrochemical inertness of NA chains, the amperometric signal is collected indirectly. Among the possible strategies proposed, “label free” approaches are considered to be definitely preferable, because they do not require to chemically functionalize the NA mixture under analysis. Similar approaches are effectively feasible in the frame of amperometric sensing. The amperometric transduction may take advantage of the neutral character of PNA structure, in contrast to the negative nature of DNA chains.13-19 Before the hybridization with the target NA, a negatively-charged electroactive probe in solution, such as [Fe(CN)6]4-, can easily interact with the electrode surface to induce the electrochemical reaction: a current signal due to its reversible oxidation to [Fe(CN)6]3- is registered. On the contrary, when oligonucleotide chains are bonded to the PNA on the surface, electrostatic repulsion between DNA-PNA duplexes and the redox probe occurs, giving rise to a decrement of the signal due to Fe(III)/Fe(II) redox couple. The entity of current decrement is directly related to the number of NA chains fixed on the electrode surface and, in its turn, to the concentration of the target DNA chains in the hybridization solution.
Due to all properties described, it is evident that the neutral character of PNA structure on one hand allows the formation of stable hybrids with NA, and on the other hand assures a high sensitivity in amperometric transduction, where the difference of surface charge before and after the recognition event is highly desired.15,16 For such an application, PNA chains ought to possess functional groups suitable for their stable anchoring on the electrode surface. One of the most popular groups used to such a scope is the thiol functionality,13-17,20,21 due to its high affinity for gold surfaces, in turn very frequently used as electrode substrates. However, thiol functionalized PNA results poorly soluble in water and can rapidly oxidize in this solvent.
In principle, alternative functional groups can be used to provide for stable deposition of PNA on gold surfaces. As an example, several papers report that primary amines can be fixed on gold surfaces.22-25 However, the nature of N-Au interaction has not been completely clarified and to best of our knowledge amine-functionalized PNA chains have never been used for the development of amperometric biosensors. With this respect, amine functionalities can be very easily inserted into PNA chains, also with the possible advantage to increase the solubility of this probe molecule in water, thus facilitating the grafting process on surfaces.
The possibility of synthesizing PNA modified with additional amino groups has already been reported in the literature and has some rationale. In particular, in a research aimed at solving some of the drawbacks related to the PNA use, such as a scarce water solubility, some of the present authors have designed and synthesized new PNA monomers, dimers and oligomers, named hydrazino PNA (hydPNA),26 in which the terminal amino group of the classical aegPNA was replaced by a hydrazine moiety. PNA decamers obtained by inserting one or more hydPNA monomers exhibit increased water solubility with respect to decamers containing all aegPNA monomers, with beneficial effect for biological studies. In another context, Marchelli et al. have shown that a PNA oligomer containing three modified monomers with chiral lysines (a chiral box in which, in addition to the stereogenic centers, three additional amino groups are present) is excellent in discriminating mismatched and matched targets; moreover, the chiral box allowed the formation of the anti-parallel PNA-DNA duplex, whereas the parallel PNA-DNA duplex failed to form.27,28 Finally, the insertion of one or more lysine residues as a tail into a PNA backbone has been reported in the literature,29 and gives some beneficial effects, for example on the solubility in aqueous solutions. More recently, Ly30 has reported an interesting miniPEG modified PNA which not only exhibits a higher water solubility but also a less aggregation propensity and a decreased tendency to randomly foil if compared with the corresponding unmodified parental PNA oligomers. Appella has also shown that the presence of l-lysine residues in the γ-position on the aegPNA backbone not only increases the stability of duplexes with complementary antiparallel DNA, but, in addition, the presence of the lysine amine groups allows the support of a broad range of functional groups without modifying the ability of PNA to recognize complementary nucleic acids.31
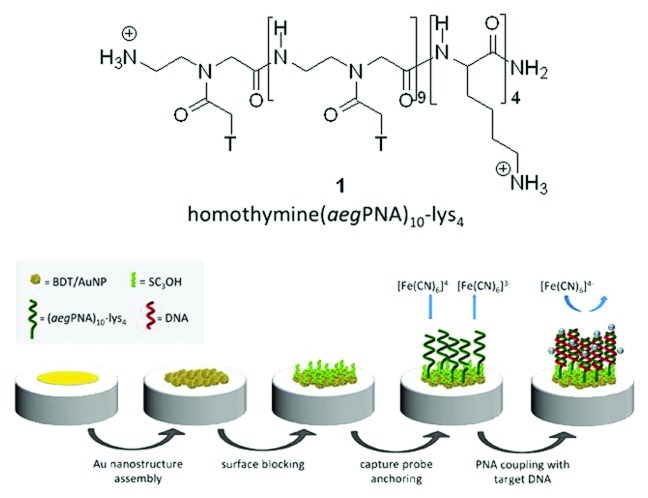
In this work we have considered the use of the homothymine PNA decamer terminated with four lysine residues (aegPNA)10-lys4 1 (Fig. 1). In addition to increase the solubility, the role of four primary amino groups is to enhance the stability of the anchoring to the Au surfaces. The peculiar oligonucleotide sequence chosen should be considered as a benchmark recognition element for NA, devoted to study the effective use of amino terminated PNA in the frame of amperometric genosensors. This terminal group constitutes a valid alternative to the usually employed thiol functionality.
Figure 1. Molecular structure of (aegPNA)10-lys4 1 and scheme of genosensor detection strategy.
Figure 1 also reports schematically the whole strategy of genosensor detection. In order to improve the sensitivity of the amperometric sensor, the electrode surface consisted of a gold nanostructure, realized by stably anchoring chemically synthesized AuNPs on a gold electrode through a dithiol linker, i.e., BDT. With this respect, it is important to underline that the synthesis of AuNPs has been performed in order to make them surrounded by a particularly labile encapsulating agent, such as chloride ion, that can be easily substituted by species bearing functions with higher affinity to Au. The mechanism of chemical interaction of the amine terminal group with this kind of AuNP and with the BDT/AuNP nanostructured surface has been deeply studied through spectroscopic investigations performed with C6NH2, used as the model molecule to simulate the binding mode of the (aegPNA)10-lys4 decamer primary amino groups.
Results and Discussion
Synthesis of (aegPNA)10-lys4
The synthesis of (aegPNA)10-lys4 was realized by using the MBHA resin downloaded with a lysine residue to obtain the MBHA-lys with a loading of 0.2 mmol/g. This resin was then used as starting material for the automated synthesis of (aegPNA)10-lys4 first coupling the sequence of three l-lysine aminoacids, protected as Να-t-Boc-Nε-(2-chloro-Cbz), to give MBHA-lys4 on which the PNA decamer chain was synthesized by sequentially coupling the ten aegPNA-thymine monomers. The cleavage of the (aegPNA)10-lys4 from the resin, performed in acidic conditions (TFA/TFMSA/m-cresol/thioanisole) allowed, at the same time, deprotection from the Cl-Cbz groups of the four lysine amino groups, leading to the target molecule 1.
As expected, the solubility of 1 resulted to be much higher than that of the homothymine (aegPNA)10 without the four lysine groups. In particular, we found that 1 has a solubility of 46.5 mg/mL compared with 5.0 mg/mL of (aegPNA)10. The solubilities were determined spectrophotometrically at 260 nm and calculated from the measured absorbance by Beer’s law from the absorbance of UV spectra of saturated aqueous solutions of both (aegPNA)10 and (aegPNA)10-lys4 1.
Hexylamine deposition on Au nanostructures
In order to investigate the nature of the interaction between the nitrogen terminal groups of 1 and the BDT/AuNP nanostructured surface, i.e., the possibility to fix (aegPNA)10-lys4 on this surface, a water soluble amine derivative, namely C6NH2, has been deposited as a model substrate on AuNPs and XPS spectra have been recorded.
Although the adsorption of amines on Au has been investigated in the past using different spectroscopic techniques,32-34 the mechanism of Au-N interaction has not been completely clarified. In particular, these studies suggest that amine functional groups adsorbed on metal surfaces could be present under different forms, mainly consisting of protonated (-NH3+), unprotonated (-NH2) and deprotonated (-NHx, x = 0, 1) forms. We could recently verify that C6NH2 molecules form on Au flat surfaces stable N-Au interaction involving the nitrogen free electron doublet (Terzi F. et al., unpublished results). However, when considering AuNPs, electrostatic interactions between protonated amino groups and the anionic shell surrounding the NP metal core can also occur.24,25
N1s and C1s core level spectra of C6NH2 registered in our case are reported in Figure 2. N1s core level spectrum shows a singlet located at ca. 400.4 eV, ascribed to the presence of unprotonated amino group adsorbed on Au surface;33 the spectra collected exclude the possible presence of the amine moiety under protonated form, since a singlet located at higher binding energy should be evidenced in this case.33 As to the C1s spectrum, the main peak located at ca. 284.3 eV and the broad tail at higher binding energy are compatible with the presence of alkyl chains anchored to the substrate through an amino group.35
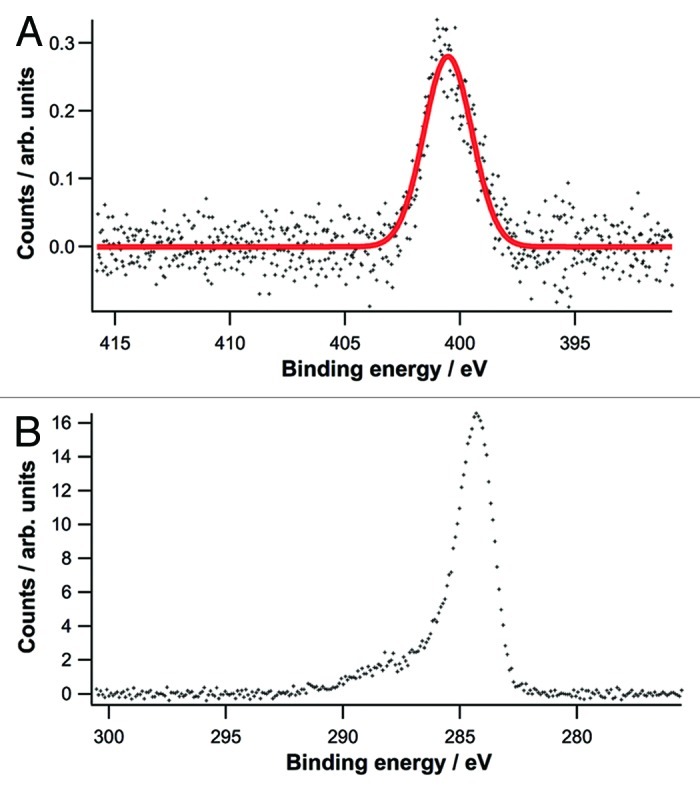
Figure 2. N1s (A) and C1s (B) core level spectra acquired at 500 eV and 385 eV photon energy.
The same spectra reported in Figure 2 also indicate that the adsorption of C6NH2 on AuNPs does not induce side reactions, as inferred by the absence of peaks ascribed to –NOx or Au nitride species;36-38 in addition, oxygen traces are absent on the surface, indicating that no oxidation processes occur. Finally, the lack of significant shift of the Au 4f core level spectra and the absence of significant shoulder located at higher binding energy with respect to a bare Au substrate suggest that the nature of the surface is not modified by the adsorption of amines.
In order to definitively confirm the possibility to anchor primary amines on the BDT/AuNP nanostructured surface, a further set of experiments, consisting of simple electrochemical tests, have been performed. Since the spectroscopic results evidence that N-Au bond involves the free electron doublet of nitrogen atom, the deposition of C6NH2 on the Au nanostructured surface was performed in a basic medium, consisting of ammonia buffer at pH = 10. Since the probe molecule does not contain any electroactive group suitable to directly evidence the effectiveness of the deposition on the surface, the actual occurrence of the deposition process has been studied in an indirect way, i.e., in the presence of a suitable electroactive species in solution, consisting of [Fe(CN)6]4-. The access of the reversibly oxidisable FeII species, in fact, is thought to be partially or totally prevented if a partial or a total coating of C6NH2 has been formed on the electrode surface. Figure 3A shows the voltammograms recorded at different stages of C6NH2 deposition on the BDT/AuNP surface. The current signal decrease indicates a progressive lowering in the electroactive surface area, as induced by a progressive coverage with an electrically insulating molecule. This fact allows us to conclude that the primary amine groups are suitable to be anchored on such a gold nanostructured surface under these experimental conditions. Moreover, the perfect overlap of 50 subsequent scans recorded in these experimental conditions let us conclude that the anchoring of amino groups with such an Au surface is stable.
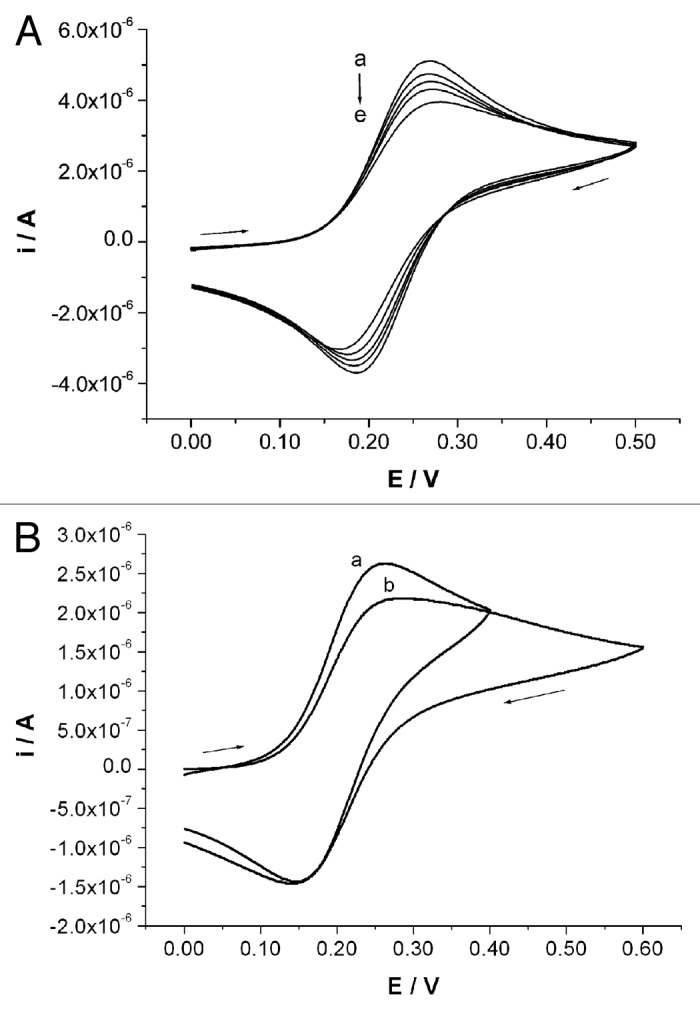
Figure 3. CV traces registered in 1 mM [Fe(CN)6]4-, 0.1 M PBS at different stages of deposition on BDT/AuNP nanostructured surface of (A) 1 mM C6NH2: (a) 0′, (b) 10′, (c) 20′, (d) 40′ and (e) 60′. (B) An amount of 0.1 mM (aegPNA)10-lys4: (a) 0′ and (b) 4 h. 0.050 V/s potential scan rate.
Development of the amperometric genosensor based on (aegPNA)10-lys4
Accordingly to the results collected for C6NH2, compound 1 was deposited on the BDT/AuNP surface by dipping the electrode in 0.1 mM solution for 4 h. Similarly to the case of C6NH2 deposition, the actual occurrence of its stable anchoring on the surface was indirectly verified by registering the decrement of CV peak currents involved in [Fe(CN)6]4- oxidation before and after PNA deposition (Fig. 3B). The slight decrement registered indicates that the deposition conditions chosen are suitable to obtain stable anchoring of the biomolecules on the electrode surface, not however forming a highly packed molecular structure. This last aspect results particularly important to allow the oligonucleotide chains to interact with PNA probes fixed on the sensor surface.
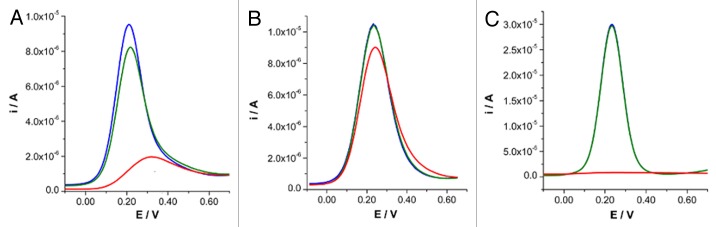
As observed in Figure 4A, the amperometric sensor obtained under these experimental conditions gives very sensitive responses when exposed to a solution containing 2 nM of the target oligonucleotide sequence. As a matter of fact, when using SQWV as the electroanalytical technique to register the [Fe(CN)6]4- oxidation, the signal decrement, proportional to the amount of DNA in solution, was quantified to amount to 80.0%. This result is particularly interesting, especially when considering that many PNA based sensors exhibit a limit of detection very close to this oligonucleotide concentration13,18,19,21 and that the PNA probe chosen for these tests only consists of a fairly short decamer, while longer PNA probes are generally chosen.14
Figure 4. SQWV signal at (aegPNA)10-lys4 modified (A) BDT/AuNP nanostructured surface, (B) BDT/AuNP nanostructured surface blocked with MESNa and (C) BDT/AuNP nanostructured surface blocked with SC3OH. Voltammetric traces are recorded in 1 mM [Fe(CN)6]4- 0.1 M PBS before (blue traces) and after the immersion in solutions containing either 3 nM of the non-complementary (green traces) or 2 nM of the complementary (red traces) oligonucleotide sequence.
When the sensor was tested in the presence of 3 nM concentration of the non-complementary oligonucleotide sequence (5′-AGA-TCA-TGC-CCG-GCA-3′), a slight signal decrement of 13.7% was also registered (see Fig. 4A). This value, significantly lower than that recorded in the presence of the complementary homoadenine DNA oligonucleotide decamer, indicates that in that case the response of the sensor is actually due to the PNA-DNA hybridization occurred on the surface. On the other hand, owing to the well-known specificity of the PNA-DNA coupling5 these results suggest that the decrease of the signal is probably due to non-specific adsorption processes of DNA chains on pinholes present on the Au surface. This aspect indicates that the presence of four lysine residues on the PNA probe is not sufficient to allow the complete coverage of the Au surface, preventing the access of oligonucleotide chains to the electrode surface.
When considering amperometric genosensors based on thiol functionalized PNA, the presence of pinholes is generally avoided by depositing a thiol derivative on the electrode surface after PNA deposition.13,14,16,17,20,21 However, this strategy is not easy to adopt for amine functionalised PNA derivatives, as the attempts described hereafter demonstrate. Actually, several molecules have been tested with the aim to saturate any possible pinholes present on BDT/AuNP surface after compound 1 deposition. By considering that they have to be characterized by a low steric hindrance to allow the electrochemical process of [Fe(CN)6]4- oxidation on the surface, they have the common characteristic to possess a very short alkyl chain and a polar tail group, in order to create an hydrophilic surface suitable for the interaction with the charged redox probe. By considering the chemical nature of this particular PNA probe, that exploits amine terminal groups to anchor to the Au surface, first attempts have been performed with amine derivatives, namely 1,3-diaminopropane and N,N-bis(2-aminoethyl)ethane-1,2-diamine. Unfortunately, tests performed in the presence of the not complementary oligonucleotide sequence 5′-AGA-TCA-TGC-CCG-GCA-3′ indicate that non-specific interactions are still present.
In order to obtain a more stable saturation of the surface, thiol derivatives have been tested. By considering the different nature of the interactions between nitrogen and sulfur with Au atoms39 preliminary tests have been performed in order to test the possibility to deposit thiol derivatives on (aegPNA)10-lys4 coated Au surfaces. In this case, an amino derivative bearing a ferrocene electrochemical probe, i.e., FcCA, was used to simulate the PNA derivative. By this approach, a very simple electrochemical test allowed us to verify that 1-butanethiol completely replaces FcCA on the surface. This effect is expected, by considering the very high affinity of sulfur atoms for the Au surface.40 On the contrary, we could check that when the thiol derivative is deposited in the first deposition step, it is only partially replaced on the surface by the amino derivative. In this case, we have to underline that the deposition times chosen for thiol deposition are short enough to reasonably hypothesize that a poorly packed molecular structure is finally obtained, most probably still containing many pinholes. Figure 5 reports, as an example, the results obtained by deposition of FcCA on BDT/AuNP nanostructured surface previously modified by MESNa. The intensity of the signal, rather similar to that obtained on the BDT/AuNP surfaces not modified by MESNa41 suggests that FcCA not only saturates the holes of the thiol monolayer, but also, due to the long immersion times and to the fairly high concentration of the amino derivative, it is able to partially replace MESNa on the electrode surface.
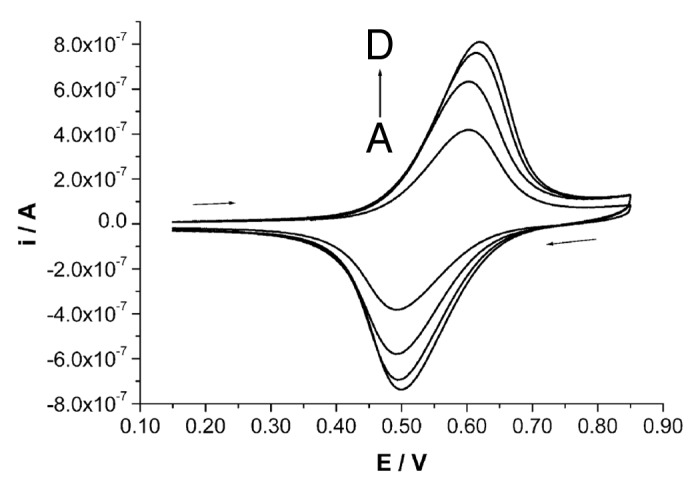
Figure 5. CV traces recorded in 50 mM TBAPF6, CHCl3 solution at a BDT/AuNP nanostructured surface first blocked with MESNa (30′ in a 5 mM water solution), at different deposition step of FcCA from 0.4 mM ethanol solution: (A) 1 h, (B) 2 h, (C) 3 and (D) 4 h. Potential scan rate is 0.050 V/s.
Among different thiol derivatives, MESNa was chosen to saturate the Au surface with the aim to prevent non-specific adsorptions of oligonucleotide chains, also thanks to the occurrence of electrostatic repulsions arising between the negatively charged sulfonic groups and the phosphonic groups on the oligonucleotide chains. As observed in Figure 4B, the absence of any signal variations as a consequence of the contact of the sensor with solutions only containing the non-complementary nucleobase sequence, is a proof of the high selectivity of PNA-DNA interaction and of the effectiveness of MESNa in blocking the Au surface to prevent non-specific interactions. On the other hand, when immersed in solutions containing 2 nM concentration of target oligonucleotide sequence, only a 12.6% of signal reduction has been observed. This effect is ascribable to the strong electrostatic repulsion exerted by external sulphonic groups of MESNa. Unfortunately, no signal improvement has been observed even at extending the PNA deposition for 15 h, with the aim to increase the number of biomolecules deposited on the sensor surface.
As an alternative molecule, SC3OH has been tested. As evidenced in Figure 4C, in this case the sensor realized combines the high selectivity induced by the saturation of the surface with a surprisingly high sensitivity. The signal decrement was in this case calculated to amount to 97.1%; similar results were obtained by repeating the procedure two further times with an estimated standard deviation of 3.4%, which demonstrates the fairly high reproducibility degree of the procedure adopted. To justify the very high signal registered, we postulate that the thiol molecules deposited on the electrode surface induced a less packed structure of PNA molecules on the surface with respect to the non-blocked surface; the capture probe finally results, more suitable to interact with complementary oligonucleotide chains in solution. The very high sensitivity induced by the presence of SC3OH allows the detection of 10 pM concentration of the target DNA sequence with a signal decrement of 35.0%, while when using unsaturated surfaces only a 4.5% signal decrement is observed. This result is particularly appealing when considering similar devices reported in the literature and that both sensitivity and detection limits can be further improved by suitably varying the electrochemical probe added to the solution.
In conclusion, we have demonstrated that an homothymine (aegPNA)10-lys4 decamer, used as a model system, is suitable for the development of effective amperometric genosensors. The four terminal amine groups of lysine residues stably anchor the PNA to the suitably nanostructured Au substrate. Therefore, the described PNA can be considered as a representative example of a class of water soluble PNA molecules, which could act as new recognition elements for DNA and RNA. In addition, we have proposed a peculiar nanostructured substrate on which to anchor such PNA residue and tested its hybridization ability on a benchmark DNA target. Since PNA consisting of mixed oligonucleotide sequences are at the basis of systems that are meaningful in an applicative context, testing more significant nucleobase sequences will represent a further development of our work. It is noteworthy that in the case of mixed oligonucleotide sequences a higher sensitivity of the detecting system can be expected, thanks to the formation of PNA-DNA duplexes rather than of (PNA)2DNA triplexes, as it happens in the case of the homothymine PNA decamer.
Materials and Methods
Reagents and apparatus
All reagents and solvents were obtained from commercial suppliers and used without further purification unless otherwise stated. N-(2-aminoethyl)-ferrocenecarboxamide (FcCA) was synthesized in 73% yield as already reported.42 AuNPs surrounded by chloride anion and possessing a mean diameter of 3.5 nm have been obtained as already reported38 and used without any further purification.
MS and HPLC-MS spectra were recorded on Advantage Thermofinnigan instruments (ESI source). ESI-HRMS spectra were recorded on Bruker Daltonics ICR-FTMS APEX II. EI-HRMS were acquired on a VG Autospec M246 spectrometer.
MALDI-TOF analyses were executed on a Bruker Omniflex spectrometer.
Vials with PTFE frits were used as reactor for solid phase synthesis. Automated solid phase syntheses were performed with peptide synthesizer “ABI433A,” equipped with Synthassist 2.0 software for peptide synthesis, according to the Applied Biosystems ABI 433A Peptide Synthesis user’s manual. MBHA resin in 3 mL reaction vessel was used.
HPLC spectra of PNA oligomers were obtained with a HPLC AGILENT 1100 Series, using reverse-phase analytical column DISCOVERY® BIO WIDE PORE C18 (25 cm × 4.6 mm, 5 μm) and a semi-preparative column DISCOVERY® BIO WIDE PORE C18 (25 cm × 10 mm, 10 μm).
Electrochemical tests were performed with an Autolab PGSTAT30 (Ecochemie) potentiostat/galvanostat, equipped with a Metrohm 663 VA stand and FRA module for impedance spectroscopy measurements. The single compartment electrochemical cell consisted of a 2 mm diameter Au disk working electrode (Metrohm), suitably modified as reported hereafter, a glassy carbon rod auxiliary electrode (Metrohm) and an aqueous Ag/AgCl, 3 M KCl reference electrode (Amel). Unless otherwise specified, all the potential values given are referred to this reference electrode.
Photoemission spectroscopy tests were performed in the ultra-high vacuum (UHV) end station of the BEAR beam line at the synchrotron radiation laboratory (Elettra).43,44 Photoemission measurements from the Au 4f, C 1s, O 1s and N 1s core levels were performed at normal emission with a hemispherical deflection analyzer (66 mm mean radius) driven at constant pass energy, with 500 meV energy resolution. The value of the photon energy (hν = 184 eV for Au 4f, hν = 385 eV for C 1s, hν = 400 eV, for N 1s, hν = 630 eV for O 1s) was chosen in order to maximize the surface sensitivity and increase the photoabsorption cross section, with respect to conventional Al and Mg Kα excitation sources. The emission lines from the Au 4f levels were acquired at each photon energy and were taken as an energy reference for the alignment of the spectra on the binding energy scale (Au 4f7/2 = 84.0 eV). Care was taken to minimize X-ray induced damage by applying a low intensity photon flux and by monitoring the time evolution of the spectra in subsequent scans.
Synthesis of the homothymine (aegPNA)10-lys4 1
Homothymine (aegPNA)10-lys4 decamer 1 was prepared, as reported below, using solid phase synthetic protocol, starting from MBHA resin downloaded with a lysine unit on which, using the Applied Biosystems ABI 433A Peptide Synthesizer, three additional lysine units were sequentially loaded to give MBHA-lys4. Then, the PNA decamer was built on this resin following the reported procedure3 using Applied Biosystems ABI 433A Peptide Synthesizer and according to the procedure described in the user’s manual.
Downloading of MBHA resin with lysine: preparation of MBHA-lys resin
MBHA resin 0.5 g (1 g, 0.63 mmol/g, 0.63 mmol) was poured into a 10 mL teflon vial and swelled in excess of DCM shaking at room temperature for 30 min. The resin was treated with a solution of DIPEA (5% in DCM) shaking for 3 min, then washed twice with DCM (5 mL) and dried by suction for 1 min. A solution of Boc-Lys(2-Cl-Cbz)-OH (0.2 mmol), DIPEA (2 eq, 0.4 mmol, 51.7 mg, 70 μL) in NMP (2 mL) and a solution of HBTU (0.2 mmol, 75.9 mg) in NMP (2 mL) were mixed together, shaked for 2 min and added to the resin. The coupling reaction was allowed to proceed at room temperature for 4h under shaking, the resin was then filtered and washed with NMP (3 × 2 mL) and treated with 5 mL of the capping solution Ac2O/Py/NMP (1:25:25). The resin was washed with NMP (2 × 2 mL), DCM (2 × 2 mL), DIPEA 5% in DCM (2 mL), MeOH (2 mL), DCM (3 × 2 mL) and dried after suction, thus giving the MBHA-Lys resin.
Synthesis of the homothymine (aegPNA)10-lys4 1
Starting from the MBHA-lys resin, first three lysine aminoacids were loaded, following the procedure reported above, in order to obtain the MBHA-lys4. Then, the ten aegPNA monomers were coupled using the standard solid phase synthetic protocol for PNA synthesis, using the automated Applied Biosystems ABI 433A Peptide Synthesizer. At the end of the synthesis, the (aegPNA)10-lys4 was cleaved from the resin as follow: the resin was washed with TFA (2 × 200 μL) and then shaken for 1 h with a solution of TFA/TFMSA/thioanisole/m-cresol 6:2:1:1 (500 μL). The reaction mixture was then filtered and the resin washed with TFA (4 × 200 μL), collecting the filtrate which was concentrated under nitrogen flow. Et2O (5 mL) was added to the residue to precipitate PNA. Centrifugation of the slurry gave a white solid, which was washed with Et2O (8 × 5 mL) and dried to afford (aegPNA)10-lys4 1. The cleaved PNA was then purified by reverse phase HPLC (gradient: from water/CH3CN 95:5 to CH3CN 100% in 60 min. tR: 10 min) and characterized by MALDI-TOF: m/z 3192.7 (M+).
Genosensor assembly
For the preparation of the genosensors, the bare Au surface was previously cleaned with piranha solution and carefully washed with an abundant flux of water. The surface was then mechanically scraped with 1 and 0.3 µm alumina powder, and then rinsed with ultra-pure water (18 MΩ cm resistivity) in an ultrasonic bath for 5 min. The procedure was completed by repeatedly cycling the electrode potential between -0.20 and +1.15 V vs. saturated Hg/HgSO4 (Amel) at 0.1 V/s potential scan rate, in 0.5 M H2SO4 solution, until a repeatable cyclic voltammogram typical of a clean Au electrode, was recorded. The nanostructured surface was obtained by a two-step procedure. The Au surface was first modified by dipping the electrode in a 10 mM 1,4-benzenedimethanethiol (Aldrich, 98% pure), ethanol solution, for 30 min. In the following step, the dithiol modified electrode was dipped in the AuNP solution for 30 min.
Compound 1 was deposited on the Au nanostructured surface by dipping the electrode in a 0.1 mM, 0.1 M ammonia buffer solution (pH = 10) for 4 h, at room temperature. The electrode was then carefully rinsed with abundant ultrapure water and immersed into a 0.1 M PBS containing either 2 nM concentration of the target oligonucleotide sequence, namely 5′-AAA-AAAA-AAA-3′, or 3 nM of a non-complementary sequence, namely 5′-AGA-TCA-TGC-CCG-GCA-3′, both acquired from Primm, Milan. Hybridization was let to proceed at 25°C for 1 h. The electrode surfaces were finally washed with ultrapure water.
The selectivity of the genosensor was improved by depositing different blocking species either after or before deposition of compound 1. In the former case, 1,3-diaminopropane or N,N-bis(2-aminoethyl)ethane-1,2-diamine have been tested, while in the second case either MESNa or SC3OH have been deposited on the bare BDT/AuNP nanostructured surface. In any case, the blocking species have been deposited from 1 mM solutions for 60 min, and the electrode was carefully washed with abundant water in order to remove molecules not stably fixed on the electrode surface. The possible deposition of amino derivatives on a thiol modified surface was tested by depositing FcCA (1 mM ethanol solution) on a MESNa modified BDT/AuNP surface; the presence of ferrocene redox groups fixed on the surface, i.e., the actual occurrence of amine deposition, was verified by recording CV tests in 0.1 M TBAPF6, CHCl3 solvent media.
The response of the sensor was tested by registering SQWV signals (amplitude: 50 mV; frequency: 20 Hz; step potential: 5 mV) in 1 mM K4Fe(CN)6 in 0.1 M PBS, before and after the hybridization procedure either with the target or with the non-complementary oligonucleotide sequence. In order to remove molecules not stably fixed on the electrode surface, the electrode was rotated for 2 min in PBS at 500 rpm before each voltammetric measurement.
XPS and electrochemical investigations of C6NH2 adsorption
AuNPs surrounded by C6NH2 have been obtained by adding the amine derivative (0.4 mM) on a freshly prepared AuNP solution at pH 10 (NaOH 0.1 mM). Samples for XPS analyses have been prepared according to a procedure previously reported.45 All measurements were performed at room temperature.
The adsorption of C6NH2 on BDT/AuNP nanostructured surface was obtained by immersing the electrode in a 0.4 mM C6NH2, 0.1 M ammonium buffer solution (pH = 10). After each deposition step, the electrode was carefully washed with abundant water in order to remove molecules not stably fixed on the electrode surface. Electrochemical investigations of the C6NH2 modified electrodes have been collected in a 1 mM K4Fe(CN)6, 0.1 M PBS, by recording CV scans at 0.05 V/s.
Acknowledgments
E.L. and S.M. wish to thank the Ministero dell'Istruzione, dell'Università e della Ricerca (MIUR, Rome) and University of Milan, for the financial support from PRIN 2007 (2007F9TWKE_002) and PRIN 2009 (20093N774P_003). C.B. wish to thank CNR-PM.P06.003. Authors also thank Dr Vijaia Amareshwar and Mrs Silvia Sonzini for some experimental work. C.Z. and R.S. acknowledge the MIUR for the financial support from PRIN 2009: “Electrochemical sensors based on thin films of nanostructured functional materials.”
Glossary
Abbreviations:
- PNA
peptide nucleic acids
- NA
nucleic acids
- ON
oligonucleotide
- Cl-Cbz
2-chlorobenzyloxycarbonyl
- MBHA
4-methylbenzhydrylamine hydrochloride salt
- TFA
trifluoroacetic acid
- TFMSA
trifluoromethanesulfonic acid
- AuNP
gold nanoparticle
- BDT
1,4-benzenedimethanethiol
- C6NH2
1-hexylamine
- PBS
phosphate buffer solution
- CV
cyclic voltammetry
- SQWV
square wave voltammetry
- FcCA
N-(2-aminoethyl)-ferrocenecarboxamide
- MESNa
sodium mercaptoethanesulphonate
- SC3OH
3-mercaptopropan-1-ol
- TBAPF6
tetrabuthylammonium hexafuorophosphate
- Ac2O
acetic anhydride
- Boc
tert-butoxycarbonyl
- CH3CN
acetonitrile
- DCM
dichloromethane
- DIPEA
diisopropyl ethyl amine
- NMP
N-methylpyrrolidone
- HBTU
O-benzotriazole-N,N,N′,N′-tetramethyl-uronium-hexafluoro-phosphate
- Py
pyridine
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/artificialdna/article/20777
References
- 1.Nielsen PE, Egholm M, Berg RH, Buchardt O. Sequence-selective recognition of DNA by strand displacement with a thymine-substituted polyamide. Science. 1991;254:1497–500. doi: 10.1126/science.1962210. [DOI] [PubMed] [Google Scholar]
- 2.Uhlman E, Peyman A, Breipohl G, Will DW. PNA: Synthetic polyamide nucleic acids with unusual binding properties. Angew Chem Int Ed. 1998;37:2796–823. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2796::AID-ANIE2796>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 3.Nielsen PE, ed. Peptide Nucleic Acids: Protocols and Applications. 2. Wymondham: Horizon Bioscience, 2004. [Google Scholar]
- 4.Koppelhus U, Nielsen PE. Cellular delivery of peptide nucleic acid (PNA) Adv Drug Deliv Rev. 2003;55:267–80. doi: 10.1016/S0169-409X(02)00182-5. [DOI] [PubMed] [Google Scholar]
- 5.Egholm M, Buchardt O, Christensen L, Behrens C, Freier SM, Driver DA, et al. PNA hybridizes to complementary oligonucleotides obeying the Watson-Crick hydrogen-bonding rules. Nature. 1993;365:566–8. doi: 10.1038/365566a0. [DOI] [PubMed] [Google Scholar]
- 6.Wang J. DNA biosensors based on peptide nucleic acid (PNA) recognition layers. A review. Biosens Bioelectron. 1998;13:757–62. doi: 10.1016/S0956-5663(98)00039-6. [DOI] [PubMed] [Google Scholar]
- 7.Sforza S, Corradini R, Tedeschi T, Marchelli R. Food analysis and food authentication by peptide nucleic acid (PNA)-based technologies. Chem Soc Rev. 2011;40:221–32. doi: 10.1039/b907695f. [DOI] [PubMed] [Google Scholar]
- 8.Singh RP, Oh BK, Choi JW. Application of peptide nucleic acid towards development of nanobiosensor arrays. Bioelectrochemistry. 2010;79:153–61. doi: 10.1016/j.bioelechem.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Lucarelli F, Tombelli S, Minunni M, Marrazza G, Mascini M. Electrochemical and piezoelectric DNA biosensors for hybridisation detection. Anal Chim Acta. 2008;609:139–59. doi: 10.1016/j.aca.2007.12.035. [DOI] [PubMed] [Google Scholar]
- 10.Endo T, Kerman K, Nagatani N, Takamura Y, Tamiya E. Label-free detection of peptide nucleic acid-DNA hybridization using localized surface plasmon resonance based optical biosensor. Anal Chem. 2005;77:6976–84. doi: 10.1021/ac0513459. [DOI] [PubMed] [Google Scholar]
- 11.D’Agata R, Corradini R, Ferretti C, Zanoli L, Gatti M, Marchelli R, et al. Ultrasensitive detection of non-amplified genomic DNA by nanoparticle-enhanced surface plasmon resonance imaging. Biosens Bioelectron. 2010;25:2095–100. doi: 10.1016/j.bios.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 12.Degefa TH, Kwak J. Electrochemical impedance sensing of DNA at PNA self assembled monolayer. J Electroanal Chem. 2008;612:37–41. doi: 10.1016/j.jelechem.2007.09.004. [DOI] [Google Scholar]
- 13.Aoki H, Tao H. Gene sensors based on peptide nucleic acid (PNA) probes: relationship between sensor sensitivity and probe/target duplex stability. Analyst. 2005;130:1478–82. doi: 10.1039/b507121f. [DOI] [PubMed] [Google Scholar]
- 14.Aoki H, Umezawa Y. Trace analysis of an oligonucleotide with a specific sequence using PNA-based ion-channel sensors. Analyst. 2003;128:681–5. doi: 10.1039/b300465a. [DOI] [PubMed] [Google Scholar]
- 15.Fang Z, Kelley SO. Direct electrocatalytic mRNA detection using PNA-nanowire sensors. Anal Chem. 2009;81:612–7. doi: 10.1021/ac801890f. [DOI] [PubMed] [Google Scholar]
- 16.Steichen M, Decrem Y, Godfroid E, Buess-Herman C. Electrochemical DNA hybridization detection using peptide nucleic acids and [Ru(NH3)6]3+ on gold electrodes. Biosens Bioelectron. 2007;22:2237–43. doi: 10.1016/j.bios.2006.10.041. [DOI] [PubMed] [Google Scholar]
- 17.Hejazi MS, Pournaghi-Azar MH, Ahour F. Electrochemical detection of short sequences of hepatitis C 3a virus using a peptide nucleic acid-assembled gold electrode. Anal Biochem. 2010;399:118–24. doi: 10.1016/j.ab.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 18.Fang B, Jiao S, Li M. Qu Yuan, Jiang X. Label-free electrochemical detection of DNA using ferrocene-containing cationic polythiophene and PNA probes on nanogold modified electrodes. Biosens Bioel. 2008;23:1175–9. doi: 10.1016/j.bios.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 19.Le Floch F, Ho H, Harding-Lepage P, Bédard M, Neagu-Plesu R, Leclerc M. Ferrocene-functionalized cationic polythiophene for the label-free electrochemical detection of DNA. Adv Mater (Deerfield Beach Fla) 2005;17:1251–4. doi: 10.1002/adma.200401474. [DOI] [Google Scholar]
- 20.Aoki H, Tao H. Label- and marker-free gene detection based on hybridization-induced conformational flexibility changes in a ferrocene-PNA conjugate probe. Analyst. 2007;132:784–91. doi: 10.1039/b704214k. [DOI] [PubMed] [Google Scholar]
- 21.Won BY, Yoon HC, Park HG. Enzyme-catalyzed signal amplification for electrochemical DNA detection with a PNA-modified electrode. Analyst. 2008;133:100–4. doi: 10.1039/b712638g. [DOI] [PubMed] [Google Scholar]
- 22.Słojkowska R, Jurkiewicz-Herbich M. Adsorption study of amino acids on a polycrystalline gold electrode. Colloids Surf A Physicochem Eng Asp. 2001;178:325–36. doi: 10.1016/S0927-7757(00)00738-X. [DOI] [Google Scholar]
- 23.Łuczak T. A comparative study of adsorption of aliphatic amines at a gold electrode. Collect Czech Chem Commun. 2005;70:2027–37. doi: 10.1135/cccc20052027. [DOI] [Google Scholar]
- 24.Orza A, Olenic L, Prunneanu S, Pogacean F, Biris AS. Morphological and electrical characteristics of amino acid-AuNP nanostructured two-dimensional ensembles. Chem Phys. 2010;373:295–9. doi: 10.1016/j.chemphys.2010.06.001. [DOI] [Google Scholar]
- 25.Zhong Z, Patskovskyy S, Bouvrette P, Luong JHT, Gedanken A. The surface chemistry of Au colloids and their interactions with functional amino acids. J Phys Chem B. 2004;108:4046–52. doi: 10.1021/jp037056a. [DOI] [Google Scholar]
- 26.Cerea P, Giannini C, Dall'Angelo S, Licandro E, Maiorana S, Marchelli R. Synthesis of hydrazino-peptide nucleic acid monomers and dimers as new PNA backbone building blocks. Tetrahedron. 2007;63:4108–19. doi: 10.1016/j.tet.2007.02.102. [DOI] [Google Scholar]
- 27.Sforza S, Corradini R, Ghirardi S, Dossena A, Marchelli R. DNA binding of a D-lysine-based chiral PNA: direction control and mismatch recognition. Eur J Org Chem. 2000;16:2905–13. doi: 10.1002/1099-0690(200008)2000:16<2905::AID-EJOC2905>3.0.CO;2-D. [DOI] [Google Scholar]
- 28.Sforza S, Tedeschi T, Corradini R, Dossena A, Marchelli R. Direction control in DNA binding of chiral D-lysine-based peptide nucleic acid (PNA) probed by electrospray mass spectrometry. Chem Commun (Camb) 2003:1102–3. doi: 10.1039/b212718k. [DOI] [PubMed] [Google Scholar]
- 29.Vernille JP, Kovell LC, Schneider JW. Peptide nucleic acid (PNA) amphiphiles: synthesis, self-assembly, and duplex stability. Bioconjug Chem. 2004;15:1314–21. doi: 10.1021/bc049831a. [DOI] [PubMed] [Google Scholar]
- 30.Sahu B, Sacui I, Rapireddy S, Zanotti KJ, Bahal R, Armitage BA, et al. Synthesis and characterization of conformationally preorganized, (R)-diethylene glycol-containing γ-peptide nucleic acids with superior hybridization properties and water solubility. J Org Chem. 2011;76:5614–27. doi: 10.1021/jo200482d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Englundand EA, Appella DH. γ-substituted peptide nucleic acids constructed from L-lysine are a versatile scaffold for multifunctional display. Angew Chem Int Ed. 2007;46:1414–8. doi: 10.1002/anie.200603483. [DOI] [PubMed] [Google Scholar]
- 32.Kamenetska M, Dell'Angela M, Widawsky JR, Kladnik G, Verdini A, Cossaro A, et al. Structure and energy level alignment of tetramethyl benzenediamine on Au(111) J Phys Chem C. 2011;115:12625–30. doi: 10.1021/jp202555d. [DOI] [Google Scholar]
- 33.Adenier A, Chehimi MM, Gallardo I, Pinson J, Vilà N. Electrochemical oxidation of aliphatic amines and their attachment to carbon and metal surfaces. Langmuir. 2004;20:8243–53. doi: 10.1021/la049194c. [DOI] [PubMed] [Google Scholar]
- 34.Thornburg DM, Madix RJ. Cleavage of NH bonds by active oxygen on Ag(110) Surf Sci. 1990;226:61–76. doi: 10.1016/0039-6028(90)90154-Z. [DOI] [Google Scholar]
- 35.Zharnikov M. High-resolution X-ray photoelectron spectroscopy in studies of self-assembled organic monolayers. J Electron Spectrosc. 2010;178–179:380–93. doi: 10.1016/j.elspec.2009.05.008. [DOI] [Google Scholar]
- 36.Lee SH, Lin WC, Kuo CH, Karakachian M, Lin YC, Yu BY, et al. Photooxidation of amine-terminated self-assembled monolayers on gold. J Phys Chem C. 2010;114:10512–9. doi: 10.1021/jp101426h. [DOI] [Google Scholar]
- 37.Iqbal P, Critchley K, Attwood D, Tunnicliffe D, Evans SD, Preece JA. Chemical manipulation by X-rays of functionalized thiolate self-assembled monolayers on Au. Langmuir. 2008;24:13969–76. doi: 10.1021/la802244a. [DOI] [PubMed] [Google Scholar]
- 38.Siller L, Alves L, Brieva AC, Butenko YU, Hunt MRC. Gold nitride: preparation and properties. Top Catal. 2009;52:1604–10. doi: 10.1007/s11244-009-9281-6. [DOI] [Google Scholar]
- 39.Chen F, Li X, Hihath J, Huang Z, Tao N. Effect of anchoring groups on single-molecule conductance: comparative study of thiol-, amine-, and carboxylic-acid-terminated molecules. J Am Chem Soc. 2006;128:15874–81. doi: 10.1021/ja065864k. [DOI] [PubMed] [Google Scholar]
- 40.Finklea HO. Organized monolayers of thiols of electochemistry and related molecules on electrodes. In: Bard AJ, Rubinstein I, eds. Electroanalytical Chemistry. New York: M. Dekker, 1996:109–335. [Google Scholar]
- 41.Zanardi C, Terzi F, Baldoli C, Licandro E, Seeber R. Development of a gold nanostructured surface for amperometric genosensors. J Nanopart Res. 2012 Submitted. [Google Scholar]
- 42.Liu J, He P, Yan J, Fang X, Peng J, Liu K, et al. An organometallic super-gelator with multiple-stimulus responsive properties. Adv Mater (Deerfield Beach Fla) 2008;20:2508–11. doi: 10.1002/adma.200703195. [DOI] [Google Scholar]
- 43.Nannarone S, Borgatti F, De Luisa A, Doyle BP, Gazzadi GC, Giglia A, et al. The BEAR beamline at Elettra. AIP Conf Proc. 2004;705:450–3. doi: 10.1063/1.1757831. [DOI] [Google Scholar]
- 44.Pasquali L, De Luisa A, Nannarone S. The UHV Experimental chamber for optical measurements (reflectivity and absorption) and angle resolved photoemission of the BEAR beamline at ELETTRA. AIP Conf Proc. 2004;705:1142–5. doi: 10.1063/1.1758001. [DOI] [Google Scholar]
- 45.Terzi F, Pasquali L, Montecchi M, Nannarone S, Viinikanoja A, Ääritalo T, et al. New insights on the interaction between thiophene derivatives and Au surfaces. The case of 3,4-ethylenedioxythiophene and the relevant polymer. J Phys Chem C. 2011;115:17836–44. doi: 10.1021/jp203219b. [DOI] [Google Scholar]