Abstract
Autophagy allows the elimination of superfluous or damaged macromolecules or organelles. Genetic evidence indicates that autophagy plays essential functions during differentiation. The differentiation of human blood monocytes into macrophages is a caspase-dependent process triggered by colony stimulating factor1 (CSF1/CSF-1). We have established, using pharmacological inhibitors, siRNA approaches and Atg7−/− mice, that autophagy is required for proper CSF1/CSF-1-driven differentiation of human and murine monocytes and acquisition of phagocytic functions. Collectively, these findings highlight an essential role of autophagy during monocyte differentiation and acquisition of macrophage functions. Deciphering the complex interplay between caspase and autophagy that occurs during this process will undoubtedly bring new insights in our understanding of monocyte differentiation.
Keywords: caspase, autophagy, differentiation, primary monocytes, CSF1/CSF-1, Atg7−/− mice
Autophagy is the conserved catabolic process by which the lysosomes degrade superfluous or damaged cell material, including macromolecules (proteins, lipids, nucleotides) and organelles (mitochondria, peroxisomes, endoplasmic reticulum). There are at least three different types of autophagy, including macroautophagy, chaperone-mediated autophagy and microautophagy. Macroautophagy, usually referred to as autophagy, is initiated by the formation of a double-membrane vesicle called an autophagosome that fuses with the lysosome to become an autolysosome in which the autophagosomal content is degraded by lysosomal enzymes including cathepsins. Cells often use this process to promote cell survival in adverse conditions such as extracellular signal or nutrient deprivation.
The analysis of systemic and tissue-specific knockout models of Atg genes in mice has led to significant increase of our knowledge regarding the functions of autophagy in mammalian development and differentiation. Thus, autophagy has been shown to be important for pre-implantation development, survival during neonatal starvation, and maturation of various hematopoietic cell lineages, namely the erythroid and lymphoid ones, yet its role in macrophagic differentiation has remained so far unexplored.
To address the implication of autophagy in macrophagic differentiation, we used human peripheral blood monocytes that can be ex vivo differentiated into M2-polarized macrophages by exposure to CSF1/CSF-1, a process that requires limited activation of CASP8 and CASP3. We demonstrate that autophagy is induced during macrophagic differentiation of monocytes. We describe that engagement of the CSF1/CSF-1 receptor in monocytes induces typical autophagic structures, including phagophores and autophagosomes, accumulation of LC3-II, and increased expression of SQSTM1/p62, and a dramatic increase in cathepsin activities, reflecting the final stages of the autophagy process. Importantly, we also establish using bafilomycin A1 that the occurrence of autophagy reflects increased lysosomal flux, since LC3-II and SQSTM1 accumulation increases in the presence of this inhibitor. Next, we also analyzed the implication of autophagy in other types of myeloid differentiation and showed that this process is activated in monocytes exposed to GM-CSF (differentiation in M1-polarized macrophages) or CSF1/CSF-1 + RANK-ligand (osteoclastic differentiation), but not during dendritic differentiation upon GM-CSF + IL4 treatment. In agreement with our findings, a recent study confirmed induction of autophagy during monocyte differentiation by GM-CSF.
To gain insights into the mechanism of autophagy induction by CSF1/CSF-1, we analyzed the potential role of ULK1, a kinase that initiates autophagy during macrophagic differentiation. We show that CSF1/CSF-1 increased the expression and phosphorylation status of ULK1, thus contributing to increased induction of autophagy, and that knockdown of Ulk1 with specific siRNA impaired differentiation. To further determine the role of autophagy in the process of macrophage differentiation, we investigated the consequence of inhibiting autophagy by pharmacological inhibitors, siRNA approaches and Atg7−/− mice. Importantly, we were able to demonstrate that in the absence of ATG7, differentiation of monocytes into macrophages is severely impaired as is the acquisition of specific macrophagic functions, including the ability of macrophages to phagocyte bacteria.
Mechanistic connections between apoptosis and autophagy have been recently highlighted. Most of these interactions reflect apoptosis altering autophagy, whereas less is known at the molecular level about how autophagy interferes with apoptosis. Thus, an important question that arises from our data concerns the mechanistic connections between caspase activation and autophagy, more specifically when caspase activation does not induce apoptosis as it is the case in most of the differentiation processes.
Of note, a mechanistic interplay between the apoptotic and autophagic machinery has been already reported. For instance, Fas-associated death domain (FADD) protein and CFLAR/FLICE-inhibitory protein (FLIP) have both been shown to interfere with the autophagic machinery. Indeed, it has been reported that in certain circumstances ATG5 is able to activate the DISC (death inducing signaling complex) through a direct interaction with FADD, and that CFLAR can inhibit the interaction between ATG3 and LC3 that is essential for autophagy induction.
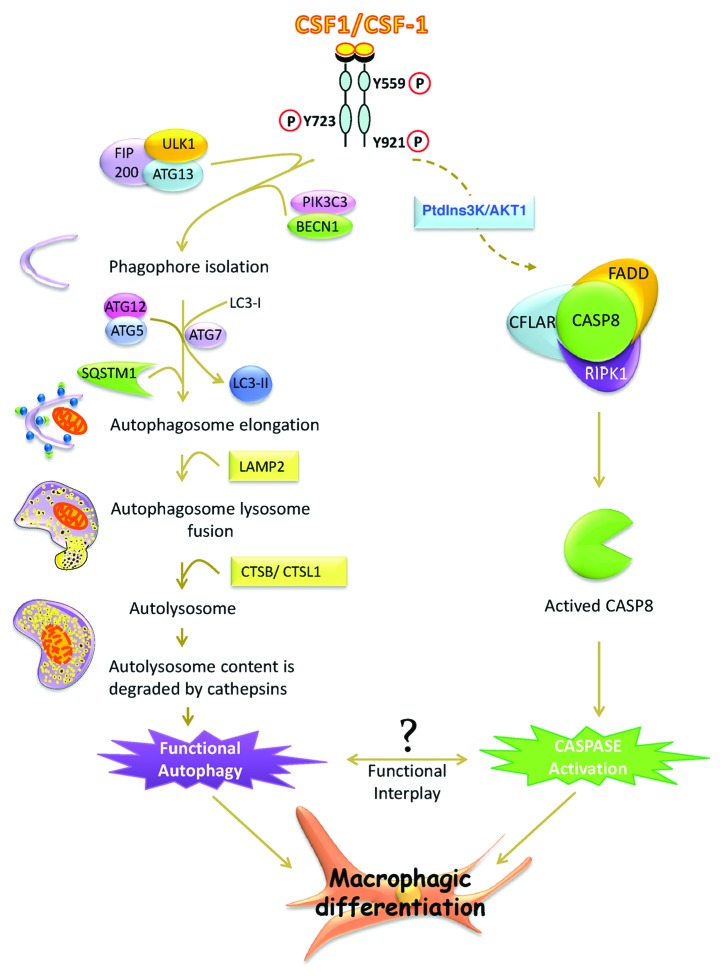
In addition, ATG proteins including ATG3, ATG4, ATG6, ATG7, ATG9 have been shown to be substrates for caspases during induction of apoptosis. Along these lines we have also reported that some autophagy proteins are cleaved by caspases during macrophagic differentiation but the precise role that these cleavages exert during differentiation still remains to be determined. Moreover, the activation of CASP8 that occurs during macrophagic differentiation of monocytes participates in a multimolecular complex including RIPK1/RIP1, and CFLAR, in the absence of any death receptor, which is the likely reason why this process occurs without apoptosis (Fig. 1). Importantly, we showed that ATG5 and SQSTM1 are present in the same subcellular compartment than CASP8, FADD, CFLAR and RIPK1, suggesting that initiation of autophagy and differentiation occurs likely in the same molecular complex. It has been recently established that SQSTM1, an adaptor protein that directs ubiquitinated substrates toward autophagic degradation, can regulate the activation of CASP8 through direct interaction. Of note, SQSTM1 is also present in the same subcellular compartment as the above-mentioned proteins.
Figure 1. A schematic molecular view of the pathway involved in macrophagic differentiation. Engagement of the CSF1/CSF-1 tyrosine kinase receptor induces autophagy and caspase activation that are both essential for macrophagic differentiation. The FIP200-ULK1-ATG13 and PIK3C3/VPS34-BECN1 complexes are necessary to trigger the initial steps of autophagy. After phagophore initiation, the ATG12–ATG5 complex along with ATG7, mediates the conversion of LC3-I to LC3-II, favoring autophagosome elongation. The fusion of autophagosomes with lysosomes is facilitated by overexpression of LAMP2. The autolysosome protein contents are next degraded by CTSB/cathepsin B and CTSL1/cathepsin L. All steps are needed to trigger functional autophagy and proper macrophagic differentiation. Engagement of the CSF1/CSF-1 receptor also activates the PtdIns3K-AKT1 pathway and induces CASP8 activation within the CFLAR-FADD-RIPK1 multimolecular complex, the activation of which is essential to promote macrophagic differentiation. The complex functional interplay between autophagy and caspase activation that orchestrates macrophagic differentiation still remains to be elucidated.
Therefore, differentiation of monocytes into macrophages represents a unique model to decipher the complex crosstalk between caspase activation and autophagy in the absence of apoptosis. Further studies are needed to identify the exquisite interplay between caspase activation and induction of autophagy and to decipher the connections between caspase, cathepsin and autophagy regulation during macrophagic, and more generally hematopoietic, cell differentiation.
Although the molecular mechanisms linking CSF1/CSF-1 receptor to autophagy induction still need to be investigated, we have reported for the first time that autophagy is required for proper differentiation of monocytes into macrophages and acquisition of phagocytic functions.
Acknowledgments
Our research was founded by grants from INSERM, Université de Nice Sophia Antipolis, Ligue Nationale Contre le Cancer (Equipe Labelisée 2011-2013), Region PACAC, INCA (PL2011-0249) and Fondation de France.
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/20367