Abstract
The maturation of reticulocytes into functional erythrocytes is a complex process requiring extensive cytoplasmic and plasma membrane remodeling, cytoskeletal rearrangements and changes to cellular architecture. Autophagy is implicated in the sequential removal of erythroid organelles during erythropoiesis, although how this is regulated during late stages of erythroid differentiation, and the potential contribution of autophagy during reticulocyte maturation, remain unclear. Using an optimized ex vivo differentiation system for human erythropoiesis, we have observed that maturing reticulocytes are characterized by the presence of one or few large vacuolar compartments. These label strongly for glycophorin A (GYPA/GPA) which is internalized from the plasma membrane; however, they also contain organellar remnants (ER, Golgi, mitochondria) and stain strongly for LC3, suggesting that they are endocytic/autophagic hybrid structures. Interestingly, we observed the release of these vacuoles by exocytosis in maturing reticulocytes, and speculate that autophagy is needed to concentrate the final remnants of the reticulocyte endomembrane system in autophagosome/endosome hybrid compartments that are primed to undergo exocytosis.
Keywords: erythropoiesis, reticulocyte maturation, autophagosome, endosome, exosome
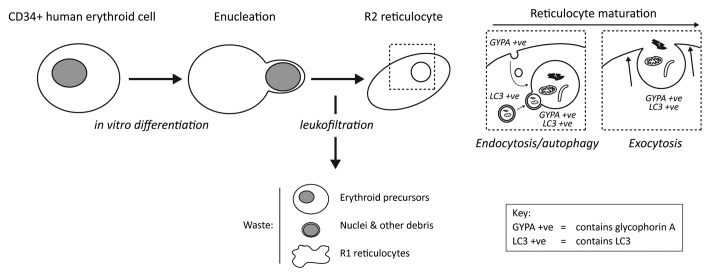
We are exploiting protocols for the in vitro expansion and differentiation of circulating adult human CD34+ stem cells to generate transfusable quantities of functional reticulocytes/erythrocytes. Using a three-stage culture system, adapted to include a leukofiltration step to eliminate nucleated cells and cellular debris (Fig. 1), we can achieve a 104-fold erythroid precursor expansion, with an enucleation rate of 55–95%. From a 24 L culture, we can recover 3 × 1010 cells after filtration, equating to around 5 mLs of packed cells. In comparison with the volume of packed erythrocytes in a unit of blood (~220 mL), this demonstrates that the objective to generate transfusable blood products from in vitro differentiation approaches is achievable, but with the need for significant scaling up. As part of this program, we have been testing the influence of autophagy during human erythroid differentiation, and will be publishing our findings in the near future; however, presently we have investigated the involvement of autophagy during the maturation of reticulocytes into functional erythrocytes in vitro.
Figure 1. Schematic of the generation of R2 reticulocytes from human CD34+ precursors. Passage of enucleated cells through a leukofilter eliminates immature forms, nuclei and other cell debris, enriching for mature R2 reticulocytes. These undergo continued remodeling involving the formation and subsequent exocytosis of large autophagic structures (LC3 positive) that also label for plasma membrane-derived GYPA.
Reticulocytes are generated following enucleation of orthochromatic erythroid cells, but they themselves then undergo a process of maturation. To begin with, while in the bone marrow, so-called R1 reticulocytes are motile and multilobular, and usually retain some membrane organelles. These are active in endocytosis and lack the stability/deformability characteristics of erythrocytes. R1 reticulocytes mature into R2 types, which are nonmotile and much more mechanically stable, and these exit the bone marrow and enter the peripheral circulation. The reticulocytes obtained following leukofiltration of our cultures contain adult hemoglobin and display oxygen binding/release and membrane deformability/stability parameters consistent with mature R2 reticulocytes. Importantly, recent data demonstrate that cultured reticulocytes can be transfused into adult human recipients, and remain functional in vivo, indicating that these cells are comparable with their in vivo counterparts. The continuing differentiation of the R1 reticulocyte to become a functional erythrocyte involves a ~20% loss of plasma membrane surface area, reduction in volume and degradation/elimination of residual cytoplasmic organelles. The endosome-exosome pathway for release of multivesicular bodies is expected to contribute to plasma membrane shedding/remodeling, meanwhile autophagy remains as a candidate pathway for the elimination of residual organelles. How these pathways are coordinated and whether they are coregulated had not been previously examined.
On close inspection, in vitro differentiated R2 reticulocytes often contain large vacuolar inclusions, typically one or two per cell. Ultrastructural analysis suggests that these vacuoles have a single limiting membrane and usually contain undegraded cytoplasmic material, including membranous structures. At the light microscope level, these inclusions are always positive for the surface marker GYPA, and in live-cell antibody uptake experiments we showed that the GYPA is internalized from the plasma membrane and gradually accumulates at the surface of the vacuolar compartment. Significantly, these compartments also label for markers of the ER, Golgi and mitochondria, suggesting that they do indeed contain cytoplasmic material. Consistent with this, they also stain for the autophagosomal marker LC3. In contrast to the staining for GYPA, which is restricted to the limiting membrane, the organelle markers and LC3 clearly decorate structures contained within the vacuolar compartment.
An intriguing feature of organelle clearance in the differentiating red blood cell is that this must take place in the background of a declining endomembrane system. This suggests that the consumption of membranes must be tightly coordinated with the biogenesis of the final population of autophagosomes, although how this is regulated remains a mystery. A related puzzle concerns the fate of these remaining autophagosomes, since data from our lab and from several others suggest that the lysosomal compartment is largely absent in reticulocytes. Exocytosis of the final bolus of cytoplasmic material seems the most likely route for elimination, and the extrusion of large vacuoles containing cytoplasmic material has previously been observed in reticulocytes. In the in vitro cultured human reticulocyte populations, we observed frequent exocytosis of these large vacuolar compartments. Indeed, analysis of human R2 reticulocytes cultured for 7 d post-filtration suggests that cells continue to lose volume over this time, and that while levels of endocytosis decline, the incidence of exocytosis of large vacuoles concomitantly increases. This suggests that one important aspect of ongoing reticulocyte maturation is the release of this residual material via exocytosis. Interestingly, we also observed plasma membrane blebbing and shedding in these cell populations, which may also contribute to the reduction in plasma membrane area and cellular volume in maturing reticuolcytes. It is formally possible that exocytosis is functionally coupled to the blebbing/shedding process in vivo, such that immediately following exocytosis a bleb is formed and subsequently shedded, further reducing cell size.
In summary, our study suggests that during the final maturation of reticulocytes, autophagosomes combine with endosomes to form large, GYPA-positive autophagic compartments that fuse with the plasma membrane to release their contents by exocytosis. This suggests an intriguing cooperation between the endocytic-exocytic and autophagic systems; however, the molecular players required for this pathway have not yet been described.
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/20648